Abstract
Gastric cancer displays different biological behaviors according to histological differentiation. The different biological behavior might involve the activation of distinct signaling pathways necessary for the growth and survival of cancer cells in gastric cancer. We investigated the differentiation‐related signal interaction between Hedgehog and Wnt pathways in gastric cancer cells. Differentiation of gastric cancer cells was induced by sodium butyrate. The sonic Hedgehog (SHH) signal expressions were increased during cellular differentiation. In contrast, the expression of Wnt signaling was decreased during differentiation. Ectopic expression of glioma‐associated oncogene‐1 (GLI1) increased the level of secreted frizzled related protein‐1 (SFRP1) transcript, whereas inhibition of GLI1 reduced the level of SFRP1 transcript. ChIP assay showed that GLI1 induced the transcriptional regulation of SFRP1 gene expression. Ectopic expression of GLI1 decreased the nuclear β‐catenin staining, but the inhibition of GLI1 induced the reversal of nuclear β‐catenin overexpression. Ectopic expression of β‐catenin also decreased the expression of GLI1 in the butyrate treated cancer cells. SHH and GLI1 immunoexpression was greater in well differentiated gastric cancer tissues compared to poorly differentiated tissues, and nuclear β‐catenin immunoexpression was lower in well differentiated compared to poorly differentiated tissues. The SHH and Wnt pathways are differentially involved according to gastric cancer cell differentiation. (Cancer Sci 2009)
Abbreviations
- ALP
alkaline phosphatase, Brm, Brahma
- ATRA
all‐trans retinoic acid
- Brm
Brahma
- CEA
carcinoembryonic antigen
- GLI1
glioma‐associated oncogene‐1
- Hh
Hedgehog
- NaBU
sodium butyrate
- PTC
patched
- SFRP1
secreted frizzled related protein
- SHH
sonic Hedgehog
- TER
transepithelial electrical resistance
There are several histological classifications of gastric carcinoma according to cellular and glandular differentiation, growth, or invasion patterns. Generally, gastric cancers are believed to have different biologic behaviors according to histological types. For example, histologically poorly differentiated gastric cancers, according to World Health Organization classification, have more aggressive behaviors than histologically well or moderately differentiated gastric cancers. Thus, histological type is one of the determining factors in the clinical therapeutic approach. The different histological types of gastric cancer might involve the activation of distinct signaling(s) necessary for the growth and survival of cancer cells. Many studies have revealed the differences in molecular alterations as different histological types in gastric cancer,( 1 , 2 , 3 , 4 , 5 ) however, there have been few studies into signaling pathways as histological differentiations in gastric cancer.
The Hh and Wnt pathways are representative signalings related to cancer development. In Hh signaling, secreted Hh molecules (SHH, Indian Hedgehog, and Desert Hedgehog) bind to PTC1 and PTC2 receptors, alleviating PTC‐mediated suppression of Smoothened, a putative seven‐transmembrane protein. Smoothened signaling triggers a cascade of intracellular events, leading to activation of a pathway through GLI‐dependent transcription.( 6 ) In Wnt signaling, the signal‐transducing components of the Wnt receptor are members of the low‐density lipoprotein receptor‐related protein and Frizzled protein families.( 6 ) In the absence of signal stimulation, β‐catenin protein is destabilized by a cytoplasmic complex containing the proteins Axin, adenomatous polyposis coli, and glycogen synthase kinase‐3β.( 6 ) Wnt signaling stabilizes β‐catenin, which acts as a transcriptional co‐activator by associating with the TCF/LEF family of transcription factors.( 6 )
These pathways are necessary for the growth and survival of cancer cells. Activation of Hh or Wnt signaling has been implicated in the development of gastric cancer in many studies.( 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ) However, the association between the activation of these signals and the histological growth types of gastric cancer has been inconsistent. 16 , 18 , 19 , 20 The discrepancy is probably due to the different proportion of cancer stages in each study that complicated efforts to detect a correlation with specific signaling pathways. Because the histological types or differentiation of gastric cancer can be altered by tumor invasion, morphological and phenotypic shifts could occur in the process of tumor progression.( 21 , 22 ) Furthermore, a molecular link between Hh and Wnt signaling has been reported.( 18 , 23 , 24 , 25 ) In this study, we intend to investigate the activity and interplay of Hh and Wnt signaling during gastric cancer cell differentiation in vitro.
Materials and Methods
Tumor samples and immunohistochemistry. Stored surgical specimens obtained from 20 patients with gastric cancer were used. All cases were provided by the Gastrointestinal Tumor Working Group Tissue Bank, Yonsei University Medical Center (Seoul, Korea) between December 1996 and December 2004. Authorization for the use of these tissues for research purposes was obtained from the Institutional Review Board of Yonsei University Health System. Ten of the specimens were well differentiated adenocarcinoma, and the remainder were solid poorly differentiated adenocarcinoma.
Tissue sections in microslides were deparaffinized with xylene, dehydrated in serial dilutions of alcohol, and immersed in 3% H2O2. Following antigen retrieval in citrate buffer (pH 6.0), the tissue sections were incubated with protein blocking agent (Immunotech [Coulter], Marseille, France) to block non‐specific antibody binding for 20 min at room temperature and then incubated overnight at 4°C with primary polyclonal goat antibodies against GLI1 (clone H‐300) (SC‐20687; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β‐catenin (clone E‐5) (SC‐7963; Santa Cruz Biotechnology). After washing with PBS three times, the sections were incubated with a biotinylated secondary antibody (goat antirabbit IgG; Immunotech) and streptavidin conjugated to horseradish peroxidase (Immunotech) for 20 min at room temperature, followed by a PBS wash. The chromogen was developed for 5 min with liquid 3,3′‐diaminobenzidine (Immunotech). Slides were then counterstained with Meyer’s hematoxylin. Expression status was quantified by scoring both the positive rate and intensity of GLI1 and β‐catenin staining. The positive rate of expression was determined by counting the number of tissues with positively stained cancer cells in 20 specimens (10 well differentiated, 10 solid poorly differentiated). The intensity of GLI1 and β‐catenin staining was scored as: 0, no detectable nuclear staining; 1, weak nuclear staining; and 2, strong nuclear staining.( 26 ) To compare well differentiated with solid poorly differentiated adenocarcinoma by the intensity of GLI1 and β‐catenin staining, the number of positively stained cancer cells in relation to total of 100 cells was multiplied by the intensity score (0, 1, or 2), and then added up.
Cell culture and induction of differentiation. AGS (ATCC CRL 1739, poorly differentiated) and MKN‐45 (KCLB 80103, poorly differentiated) gastric cancer cells were maintained in RPMI‐1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco) and 1% penicillin–streptomycin sulfate (Gibco). All cultures were maintained in a 37°C incubator supplemented with 5% CO2. Exponentially growing cells were trypsinized and seeded into 10 cm2 cell culture Petri dishes at a density of 5 × 106 cells/cm2. When cells reached 50–70% confluency, as determined by microscopic examination, medium was renewed and drugs were added from concentrated stock solutions. For drug‐induced cell differentiation, NaBU (Sigma, Munich, Germany) and ATRA (Sigma) was used at a final concentration of 1–3 μM for 48 h.
Alkaline phosphatase activity was determined as a marker for differentiation. The expression of one of the ALP isoenzymes was stronger in well differentiated gastric carcinomas than in poorly differentiated carcinomas.( 27 ) For ALP activity, cell lysates were assayed using 7 μM p‐nitrophenylphosphate as substrate and 2‐amino‐2‐methylpropan‐1‐ol as solvent. To determine ALP activity, the product (p‐nitrophenol) produced per minute was measured and normalized for cellular protein.( 28 ) CEA was also detected by RT‐PCR and Western blot analysis as a marker for differentiation.( 22 , 29 ) Furthermore, the expression of Brm, which is lost in poorly differentiated gastric cancer in vivo,( 30 ) was analyzed by RT‐PCR and Western blot analysis in gastric cancer cells after NaBU treatment. A tendency toward a Brm decrease was more prominent in poorly differentiated than in well differentiated gastric cancers.( 30 )
Transepithelial electrical resistance. AGS and MKN‐45 gastric cancer cells were cultured and seeded on 12‐mm cell cultured inserts as previously reported.( 31 ) The cell monolayer integrity was checked by measuring the TER using a Transwell (Corning, NY, USA). The TER value of cell monolayers was measured before and after adding NaBU (1–3 μM), and the effect of NaBU is expressed as the relative TER value to the value in control.
RT‐PCR. Total RNA was extracted from cultured cells using an RNeasy mini kit (Qiagen, Tokyo, Japan). PCR was carried out with a PCR Maxi kit (iNtRON, Sungnam, Korea) according to the manufacturer’s instructions. Amplification conditions included denaturation at 95°C for 5 min, followed by: 30 cycles of 30 s each at 95, 57, and 72°C for Shh; 32 cycles of 45 s each at 95, 55, and 72°C for CEA; 32 cycles of 45 s each at 95, 58, and 72°C for Brm; 30 cycles of 30 s each at 95, 55, and 72°C for SFRP1; and 18 cycles of 30 s each at 95, 60, and 72°C for β‐actin. PCR products were separated in 1.5% agarose gels. The oligonucleotide primers used for RT‐PCR were as follows: human Shh, 5′‐GAGATGTCTGCTGCTAGTCC‐3′ and 5′‐GTTTCTGGAGATCTTCCCTT‐3′; CEA, 5′‐CCAGAACGTCACCCAGAATG‐3′ and 5′‐GGTTCAGATTTTCCCCTGGA‐3′; Brm, 5′‐CTGCAAGAGCGGGAATACAGACTTCAGGCCCG‐3′ and 5′‐GGCTGCCTGGGCTTGCTTGTGCTCCCAAACC‐3′; SFRP1, 5′‐TCATGCAGTTCTTCGGCTTC‐3′ and 5′‐CCAACTTCAGGGGCTTCTTC‐3′; and β‐actin, 5′‐TTGCCGACAGGATGCAGAAGA‐3′ and 5′‐AGGTGGACAGCGAGGCCAGGAT‐3′.
Western blot analysis. Prepared cells were harvested after washing with PBS. Collected cells were lysed with buffer (50 mM Tris‐Cl [pH 7.5], 150 mM NaCl, 1 mM EDTA [pH 8.0], 1% Triton X‐100, 1 mM PMSF, 1 mM Na3VO4, and protease inhibitor cocktail [Roche Molecular Biochemicals, Indianapolis, IN, USA]). Fractionation was carried out by sequential extraction of cytosolic and nuclear proteins in non‐ionic detergent for analysis of β‐catenin as described previously.( 32 ) Cells were lysed for 10 min on ice then quick‐spun for 15 s to collect cytosolic lysate. Pellets were washed twice with cytoplasmic lysis buffer, then lysed with nuclear lysis buffer (50 mM HEPES [pH 7.9], 250 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1% NP‐40, 0.1% glycerol, 10 mM NaF, 10mM Na3VO4, 1 mM DTT, 1× protease inhibitors, and 0.5 mM PMSF) for 30 min on ice. The lysates were spun for 20 min at 12 000 g at 4°C to collect nuclear lysates.
The same amount of protein was boiled at 95°C after adding SDS sample buffer (62.5 mM Tris‐Cl [pH 6.8], 2% SDS, 10% glycerol, β‐mercaptoethanol, and 0.002% bromophenol blue). Samples were loaded in: 12% SDS‐PAGE gels for SHH and SFRP; 10% SDS‐PAGE gels for CEA and β‐catenin; 8% SDS‐PAGE gels for PTC1, GLI1; and 6% SDS‐PAGE gels for Brm, then transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ, USA).
Rabbit anti‐SHH (SC‐9024), anti‐GLI1 (SC‐20687), anti‐PTC1 (SC‐6149), anti‐Brm (SC‐6449), anti‐SFRP (SC‐7425), anti‐β‐catenin (SC‐7963; all Santa Cruz Biotechnology), and anti‐CEA (07‐296; Upstate Biotechnology, Lake Placid, NY, USA) were used as the primary labeling antibodies and the appropriate HRP‐conjugated antibodies (Santa Cruz Biotechnology) were used as secondary antibodies. An ECL detection system (ECL‐Plus; iNtRON) was used for detection according to the manufacturer’s protocol.
Vectors. pcDNA3.1/SRα‐GLI1 and pcDNA3.1 were kindly provided by Dr Ishii (Tsukuba Life Science Center, Ibaraki, Japan). pSG5‐HA/β‐catenin and pSG5‐HA were kindly provided by Professor Kim (Department of Biochemistry and Molecular Biology, Yonsei University College of Medicine). pTopflash and pFopflash were also kindly provided by Professor Ryu (National Research Laboratory of Tumor Virology and Department of Biochemistry, Yonsei University). Cells were plated onto six‐well plates 24 h before transfection and transfected with 2 μg plasmid using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were treated with NaBU for 24 h then transfected. Forty‐eight hours after transfection, cells were harvested and subjected to further examination.
RNA interference. Small interfering RNA against GLI1 and negative‐control siRNA were kindly provided by Dr Ishii (Tsukuba Life Science Center). Cells were transfected with 100 nM siRNA using TransIT‐TKO transfection reagent (Mirus Bio, Madison, WI, USA) according to the manufacturer’s instructions. Forty‐eight hours after transfection, cells were harvested and subjected to further evaluation.
Luciferase reporter assay. Cells grown in six‐well tissue culture plates were transfected with 2 μg pTopflash and pFopflash, 2 μg pcDNA3.1 (internal control), 2 or 3 μg gene expression plasmid, and 50 μg Renilla TK‐plasmid. Luciferase assays were carried out 48 h after transfection using a Dual‐Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
ChIP assay. ChIP analysis was done using a ChIP assay kit (Upstate Biotechnology) according to the manufacturer’s instructions. AGS cells were used, and immunoprecipitation was done overnight at 4°C with 10 μL of the sample used as the “input,” or 1 μg of GLI1 or the negative control mouse IgG, positive control anti‐RNA polymerase beads. After reverse crosslinking, DNA was purified with QIAquick PCR purification kit (Qiagen, Valencia, CA, USA) from the immunoprecipitates. PCR was carried out with 35 cycles of 96°C for 30 s, 55°C for 45 s, and 72°C for 45 s using the following primers flanking the putative GLI‐binding sites in the human SFRP1 promoter: SFRP1 sense, 5′‐GTTGGAGCTGTTTGCTGTGA‐3′; SFRP1 antisense, 5′‐ATGTTTTGGCTTTCCACACC‐3′.
Statistical analysis. Statistical significance was evaluated using Student’s t‐test and Pearson’s χ2‐test for immunohistochemistry, Student’s t‐test for luciferase activity, and a one‐way ANOVA with multiple comparison test for densitometry analysis. P < 0.05 was considered statistically significant. To confirm the induction results, experiments were repeated at least three times. All statistical analyses were carried out using SPSS 12.0 software (SPSS, Chicago, IL, USA).
Results
Hedghog and Wnt signaling in human tissues. To investigate Hh and Wnt signal expression in human tissues, immunohistochemical staining was carried out for 20 paraffin‐embedded surgical specimens: 10 well differentiated and 10 solid poorly differentiated adenocarcinomas. All specimens were obtained from patients with American Joint Committee on Cancer TNM stage II or III.
The positive rate of nuclear GLI1 expression was higher in well differentiated adenocarcinomas than in poorly differentiated adenocarcinomas (100%vs 70%, respectively) (Table 1, Fig. 1a,b). The intensity of nuclear GLI1 staining was significantly higher in well differentiated adenocarcinomas than in poorly differentiated adenocarcinomas (P < 0.005; Table 1). In contrast, the positive rate of nuclear β‐catenin expression was higher in poorly differentiated adenocarcinomas than in well differentiated adenocarcinomas (60%vs 30%) (Table 1, Fig. 1c,d). The intensity of nuclear β‐catenin staining was significantly higher in poorly differentiated adenocarcinomas than in well differentiated adenocarcinomas (P < 0.005; Table 1).
Table 1.
Expression of nuclear glioma‐associated oncogene‐1 (GLI1) and nuclear β‐catenin in gastric cancer
| GLI1 | β‐catenin | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive rate† | P* | Intensity‡ | P** | Positive rate† | P* | Intensity‡ | P** | |
| WD | 100% (10/10) | 0.060 | 79.8 ± 28.6 | 0.015 | 30% (3/10) | 0.178 | 5.5 ± 12.6 | 0.016 |
| PD | 70% (7/10) | 33.0 ± 45.5 | 60% (6/10) | 67.5 ± 72.9 | ||||
*Pearson’s χ2‐test. **Student’s t‐test. †The positive rate of expression was determined by counting the number of tissues to have positively stained cancer cells in 10 specimens of each differentiated cancers. ‡The intensity was scored as 0, no detectable nuclear staining; 1, weak nuclear staining; or 2, strong nuclear staining. The number of positively stained cancer cells in relation to a total of a hundred cells was multiplied by the intensity score (0, 1 or 2), and then was added up (mean ± SD). PD, poorly differentiated gastric cancers; WD, well differentiated gastric cancers.
Figure 1.
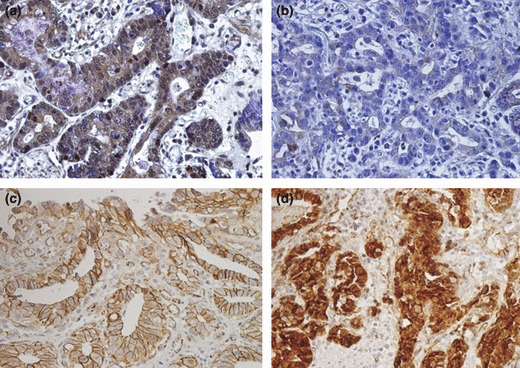
Immunohistochemical staining for glioma‐associated oncogene‐1 (GLI1; a,b), and β‐catenin (c,d) in well differentiated (WD; a,c) and poorly differentiated (PD; b,d) gastric carcinoma tissues. Nuclear GLI1 staining revealed stronger expression in WD than PD tissues. In contrast, the expression of nuclear β‐catenin was stronger in PD than WD tissues. Magnification, ×400.
To investigate the correlation between Wnt and Hh signaling, gastric cancers were divided into two groups based on the intensity of nuclear GLI1 staining (mean intensity of total gastric specimens 56.4).( 18 ) The low GLI1 group (<57.0, n = 9) showed a significantly higher intensity of nuclear β‐catenin staining than the high GLI1 group (≥57.0, n = 11) (P = 0.004; Table 2).
Table 2.
Inverse correlation between nuclear glioma‐associated oncogene‐1 (GLI1) and β‐catenin staining in gastric cancer
| No. of cases | Intensity of β‐catenin (mean ± SD) | P* | |
|---|---|---|---|
| Intensity of GLI1 < 57.0† | 9 | 76.1 ± 71.9 | 0.004 |
| Intensity of GLI1 ≥ 57.0† | 11 | 4.1 ± 12.0 |
*Student’s t‐test. †Gastric cancers were divided into two groups based on the mean intensity of nuclear GLI1 staining of total gastric specimens (56.4).
These findings suggest that Hh signaling components are expressed in well differentiated adenocarcinomas at a higher level than in poorly differentiated adenocarcinomas. The Wnt pathway showed the reverse result.
In vitro differentiation of gastric cancer cells. Poorly differentiated gastric cancer cell lines (AGS and MKN‐45) were treated with NaBU and ATRA, well‐known differentiation‐inducing agents. In this study, increased ALP activity was noted in NaBU‐treated AGS and MKN‐45 cells (Fig. 2a). Maximal ALP activity was found in gastric cancer cells treated with 2 μM NaBU, consistent with a previous study.( 33 ) ALP activity was also increased in gastric cancer cells after treatment with another differentiation‐inducing agent, ATRA (Fig. 2b). The expression of CEA and Brm increased in gastric cancer cells after NaBU treatment (Fig. 3). These findings suggest that gastric cancer cells can be differentiated by differentiation‐inducing agents. Furthermore, differentiated gastric cancer cells overexpressed Brm, a valid marker for well differentiated gastric cancer in human tissues (Fig. 3c,d).
Figure 2.
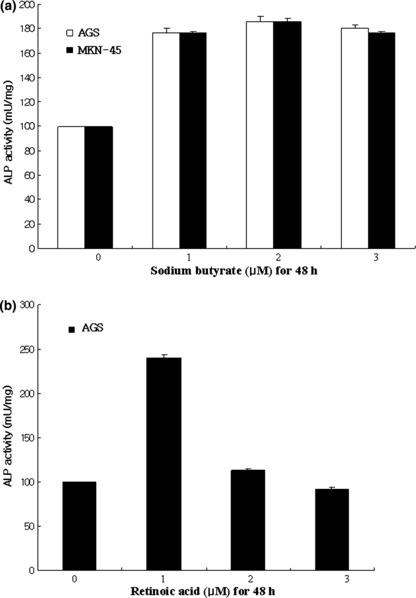
Increased alkaline phosphatase (ALP) activity by differentiation. (a) Subconfluent AGS and MKN‐45 gastric cancer cells were treated with sodium butyrate (0–3 μM) for 48 h. ALP activity was increased in AGS and MKN‐45 cells. Error bars were calculated from three experiments. (b) Subconfluent AGS cells were treated with all‐trans retinoic acid (0–3 μM) for 48 h. ALP activity was increased after 1 μM retinoid acid treatment. Error bars were calculated from three experiments.
Figure 3.
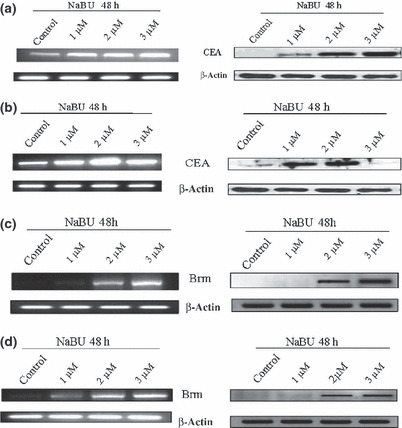
Overexpression of carcinoembryonic antigen (CEA) and Brahma (Brm) by sodium butyrate treatment (NaBU) in AGS (a,c) and MKN‐45 (b,d) gastric cancer cells. RT‐PCR (left column) and Western blot (right column) were carried out with β‐actin as internal standard.
Transepithelial electrical resistance was also measured to validate the differentiation of gastric cancer cells after differentiating agent. TER is believed to be correlated with the paracellular permeability of the cell monolayer.( 34 ) Confluent differentiated colon cancer cells adhere to each other by proteinaceous tight junctions, thereby creating an electrically resistant monolayer. Thus, to follow the differentiation status of the colon cancer cell lines, TER measurements were used.( 35 ) In the present study, TER was measured in AGS and MKN‐45 gastric cancer cells after differentiation (Fig. 4). TER was increased in gastric cancer cells after NaBU treatment.
Figure 4.
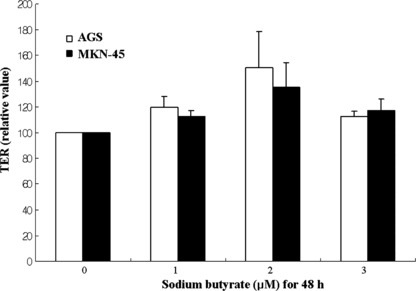
Transepithelial electrical resistance (TER) during gastric cancer cell differentiation. TER values are presented as relative to the values at control. TER was increased in AGS and MKN‐45 cells. Error bars were calculated from three experiments.
Hedghog signaling in gastric cancer cell differentiation. To evaluate the Hh signaling during gastric cancer cell differentiation, it was first examined whether the expression of SHH changed in gastric cancer cells after NaBU treatment, using RT‐PCR and Western blot analyses. NaBU was used at a concentration of 2 μM, because gastric cancer cells were maximally differentiated in 2 μM of NaBU in this study.
The expression of SHH increased in AGS and MKN‐45 cells during differentiation by NaBU (Fig. 5a,b). Ligand‐dependent Hh signal pathway activation in gastric cancer cells after NaBU treatment, according to GLI1 and PTC1, was also evaluated using Western blotting. Overexpression of GLI1 and PTC1 proteins was noted in both gastric cancer cell lines after NaBU treatment (Fig. 5c,d). These data suggest that the Hh pathway is enhanced during gastric cancer cell differentiation.
Figure 5.
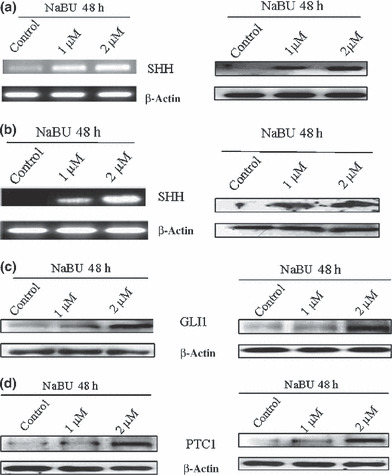
Overexpression of sonic Hedgehog (SHH; a,b), glioma‐associated oncogene‐1 (GLI1; c) and Patched‐1 (PTC1; d) by sodium butyrate (NaBU) treatment for 48 h. RT‐PCR (left column) and Western blot (right column) for SHH were carried out in AGS (a) and MKN‐45 (b) gastric cancer cells. Western blot for GLI1 and PTC1 was done in AGS (left column) and MKN‐45 (right column) cells.
Wnt signaling in gastric cancer cell differentiation. To evaluate the Wnt signaling in gastric cancer cell differentiation, the expression of nuclear β‐catenin proteins was assessed by Western blotting after sequential extraction of cytosolic and nuclear proteins from cells.
The expression of nuclear β‐catenin decreased without a change in cytoplasmic β‐catenin during differentiation in both gastric cancer cell lines (Fig. 6). These results show that the activity of Wnt signaling decreased in gastric cancer cell differentiation.
Figure 6.
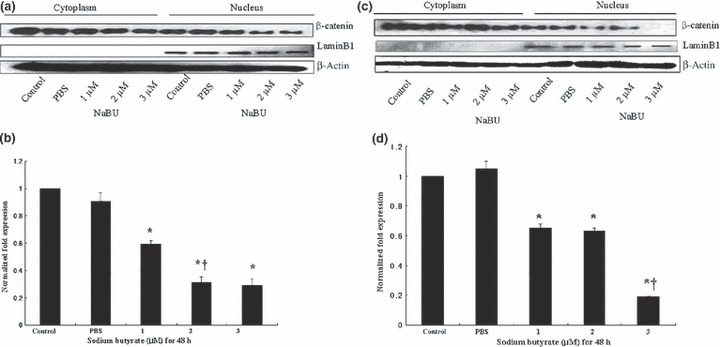
Decreased nuclear β‐catenin expression by sodium butyrate (NaBU) treatment in AGS (a,b) and MKN‐45 (c,d) gastric cancer cells. Western blot for β‐catenin was carried out after sequential extraction of cytosolic and nuclear proteins from cells in non‐ionic detergent. Lamin B1 (a nuclear protein) and β‐actin were used as internal standards. Protein expression levels of nuclear β‐catenin were analyzed by densitometry analysis (b,d). Densitometry data presented in graphical form are “fold change” compared with control after normalization with respective loading control (Lamin B1). Similar results were obtained in three independent experiments. Statistical significance was determined by one‐way ANOVA with multiple comparison test. *P < 0.05, statistically significant difference compared with control/PBS. †P < 0.05, statistically significant difference from nuclear β‐catenin of NaBU previous dose‐treated group.
Regulation of Wnt signaling by enhanced Hh signaling in gastric cancer cell differentiation. In the present study, ligand‐dependent Hh signal activation and Wnt signal suppression were found in gastric cancer cell differentiation. To examine the mechanism of the inverse expression patterns between Hh and Wnt signaling during gastric cancer cell differentiation, the expression of SFRP in NaBU‐treated gastric cancer cells was analyzed. SFRP1 is an antagonist of Wnt and a transcriptional target of Hh signaling.( 18 , 23 ) A previous study reported that GLI1 suppressed Wnt signaling through SFRP.( 23 ) In the present study, the expression of SFRP increased in NaBU‐treated cancer cells, according to RT‐PCR and Western blot analyses (Fig. 7a,b). Furthermore, GLI1 regulated the expression of SFRP. Ectopically overexpressed GLI1 increased SFRP1 transcription and expression (Fig. 7c). When NaBU was added, overexpressed GLI1 also increased SFRP1 transcription and expression. Thus, maximal expression of SFRP1 was found when GLI1 was increased by the vector and NaBU (Fig. 7c). ChIP assay was used to assess whether GLI1, increased by the vector or NaBU, was involved in binding the SFRP1 promoter. As shown in Figure 7(d), the regulation by GLI1 involved direct binding to the SFRP1 promoter. Overexpression of SFRP1 after NaBU treatment was abolished by a siRNA against GLI1 (Fig. 7e).
Figure 7.
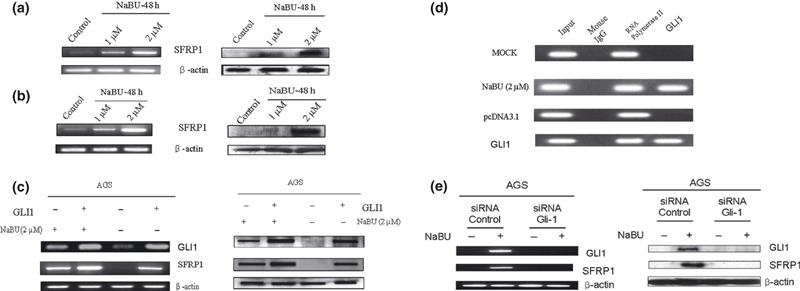
Regulation of secreted frizzled related protein‐1 (SFRP1) transcript by glioma‐associated oncogene‐1 (GLI1) in gastric cancer cells. (a,b) Overexpression of SFRP1 by sodium butyrate (NaBU) treatment for 48 h in AGS (a) and MKN‐45 (b) cells. RT‐PCR (left column) and Western blot (right column) analyses for SFRP1 were carried out with β‐actin as the internal standard. (c) Increased GLI1 expression by GLI1 vector or 2 μM NaBU increased the expression of SFRP1 in RT‐PCR (left column) and Western blot (right column) analyses. The maximal expression of SFRP1 was found when GLI1 was overexpressed by vector and NaBU. (d) ChIP analysis of SFRP1 promoter region in AGS cells. Overexpressed GLI1 by NaBU or GLI1 vector was directly binding to the SFRP1 promoter region. Mouse IgG was used as the negative control and anti‐RNA polymerase beads were used as the positive control. (e) Decreased expression of SFRP1 by siRNA against GLI1. The increased expression of SFRP1 by Hedgehog signal activation after NaBU treatment was significantly decreased by siRNA against GLI1 in RT‐PCR (left column) and Western blot (right column) analyses.
To test whether increased GLI1 expression could decrease Wnt signaling, Western blotting was used to analyze the nuclear pools of β‐catenin after GLI1 overexpression by the vector or NaBU treatment. Ectopically overexpressed GLI1 by vector decreased nuclear β‐catenin protein (Fig. 8a). When NaBU was added, the increased GLI1 due to Hh signal activation also reduced the nuclear β‐catenin protein. Thus, nuclear β‐catenin decreased the most when GLI1 was overexpressed by the vector and NaBU treatment (Fig. 8a). In addition, when the Topflash or Fopflash reporter assay was used, TCF activity was decreased in transfected AGS cells with the GLI1 expression plasmid (Fig. 8b). When GLI1 was suppressed by siRNA, the decreased nuclear β‐catenin by NaBU recovered (Fig. 8c). These findings suggest that increased GLI1 in differentiated gastric cancer cells, through activated Hh signaling, suppressed the Wnt signaling pathway through SFRP overexpression.
Figure 8.
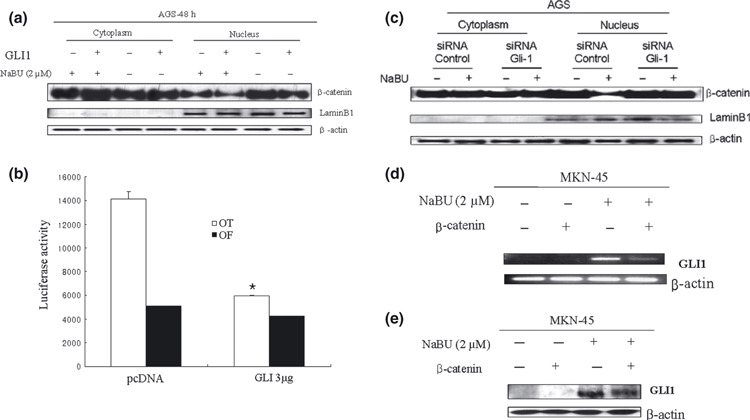
The inverse correlation between Hedgehog and Wnt signal pathways in gastric cancer cells. (a) Overexpressed glioma‐associated oncogene‐1 (GLI1) suppressed nuclear β‐catenin in AGS cells. Western blot for β‐catenin was carried out after sequential extraction of cytosolic and nuclear proteins from cells in non‐ionic detergent. Lamin B1 (a nuclear protein) and β‐actin were used as internal standards. Increased GLI1 expression by GLI1 vector or sodium butyrate (NaBU) decreased nuclear β‐catenin proteins. The most decreased nuclear β‐catenin proteins were found when GLI1 was overexpressed by vector and NaBU. (b) AGS cells were transfected with Topflash (OT) or Fopflash (OF), a control, and the indicated amount of a GLI1 vector. Transfection with GLI1 vector decreased TCF transcription factor activity compared with control (bars, SD; *P < 0.001 vs control). (c) Decreased nuclear β‐catenin after NaBU treatment was recovered by siRNA against GLI1 in AGS cells. (d) Increased expression of GLI1 by NaBU treatment was decreased after β‐catenin overexpression by the vector in MKN‐45 cells in RT‐PCR (left column) and Western blot (right column) analyses.
To validate the inverse correlation between Hh and Wnt pathways, Western blotting was used to evaluate the Hh signal changes after β‐catenin overexpression by the vector during gastric cancer cell differentiation. Increased GLI1 protein by NaBU treatment was decreased after β‐catenin overexpression by the vector (Fig. 8d,e).
Discussion
Several histopathological classifications of gastric cancers exist, such as the World Health Organization, Lauren, Ming, and Japanese systems. These classifications divide gastric cancers based on grades of cellular and glandular differentiation, and growth and invasion patterns that influence intrinsic biologic characteristics of the tumor.( 5 ) Generally, the more differentiated gland pattern is believed to have a less aggressive biological behavior. Thus, the clinical therapeutic approach is different according to the histological type. For example, local endoscopic treatment is not generally accepted in undifferentiated‐type gastric cancers because undifferentiated‐type gastric cancer has more lymph node metastasis compared to differentiated‐type gastric cancer. The different grades of glandular differentiation of gastric cancer might involve different predominant molecular signaling pathways for different biological behavior.
Several studies have sought to analyze signal changes according to histological classification in human gastric cancer tissues. However, these studies have shown inconsistent results.( 16 , 18 , 19 , 20 ) These discrepancies could be the result of the different extents of cancer progression or migration in the studies, and/or failure to take into account the interactions between signaling. We investigated two pathways, Hh and Wnt signaling, in gastric cancer cells in vitro to examine changes purely according to histological differentiation, minimizing the effect of different cancer stages. An in vitro differentiation induction of human gastric cancer cell lines is similar to that used in colon cancer studies.( 22 , 29 , 33 , 36 )
In vitro differentiation of gastric cancer cells might reflect the molecular conditions of in vivo histological differentiation in gastric cancer, based on previous biomorphological and molecular studies.( 22 , 27 , 30 , 37 ) When evaluating morphological changes after applying differentiation‐inducing agents under electron microscopy, numerous microvilli, more desmosomes with tightly cohesive clusters, and intercellular lumens along cell junctions were observed, mimicking primitive gland formation.( 22 ) In addition, when cancer cells were heterotransplanted into SCID mice with or without differentiation‐inducing agents, the tumor originating from gastric cancer cells grown in the presence of the differentiation‐inducing agent showed numerous well developed gland formations, the lumina of which were lined by many microvilli and filled with secretions.( 22 ) These results suggest that gastric cancer cells treated with differentiation‐inducing agents might have the characteristics of the gland formation growth pattern, as seen in histopathologically differentiated‐type gastric cancers in vivo. Furthermore, several studies have revealed that molecular markers for cancer cell differentiation, by inducing agents in vitro, such as ALP or Brm, were correlated with histologically differentiated‐type gastric cancer tissues.( 27 , 30 , 37 ) The expression of one of the ALP isoenzymes was stronger in well differentiated than in poorly differentiated gastric cancer tissues.( 27 ) Brm is also an important factor for determining the differentiation status of gastric cancers.( 30 ) A tendency toward a Brm decrease was more prominent in poorly differentiated than in well differentiated gastric cancers.( 30 ) The present study showed increased ALP and Brm expression during gastric cancer cell differentiation by NaBU. The present study also showed increased TER in gastric cancer cells after NaBU, suggestive of biomorphological change by tight junctions.
These findings suggest that ligand‐dependent Hh signal activation and inverse Wnt signal suppression by Hh signaling occurred in gastric cancer cell differentiation. These results also suggest that the Hh signaling might be activated predominantly in gastric cancer tissues that have a more gland formation‐like growth pattern. In contrast, the Wnt signaling might be activated predominantly in gastric cancer tissues that have a less gland formation‐like growth pattern. Immunohistochemical analysis of gastric cancer specimens in our study showed stronger expression of Hh signaling in well differentiated tissues than in poorly differentiated cancer tissues.
Immunohistochemistry on specimens was limited to stage II and III gastric cancers to exclude any effect of stage on the signals. Specimens for immunohistochemical studies were limited to intestinal type gastric cancer (limited to solid poorly differentiated adenocarcinoma). Poorly differentiated adenocarcinoma in stage I early cancers possibly contains diffuse‐type origin of gastric cancer, which might shift signet ring cell carcinoma during the progression. Diffuse‐type gastric cancer has different molecular characteristics compared to intestinal type or histological subtype in diffuse‐type gastric cancer.( 1 , 2 , 3 , 4 , 5 ) For example, recent studies reveal that Hh and Wnt signal pathways are highly activated in diffuse‐type gastric cancer.( 7 , 8 , 38 , 39 ) However, signet ring cell carcinoma, a subtype in diffuse‐type gastric cancer, has no activated Hh or Wnt signal pathways.( 7 , 18 )
According to our study, the inverse correlation between Hh and Wnt signaling was the result of SFRP1, acting through GLI1. GLI1 bound directly to the SFRP1 promoter region, consistent with the results of He et al. ( 23 ) However, Yanai et al. ( 18 ) reported a different result because the promoter region of the SFRP1 gene was methylated in AGS cells. Thus, siRNA against GLI1 was used to investigate whether overexpression of SFRP1 in gastric cancer cell differentiation was derived from demethylation by a differentiation‐inducing agent, not by GLI1 binding to SFRP1. When GLI1 was suppressed by siRNA, SFRP1 was also suppressed, regardless of NaBU treatment. It was also shown that GLI1 was directly bound to the SFRP1 promoter region, based on the ChIP assay. These results suggest that the transcription of SFRP1 was increased by GLI1 binding to the SFRP1 promoter region during gastric cancer cell differentiation. Increased expression of SFRP1 then suppressed the Wnt pathway. That is, crosstalk between the Hh and Wnt pathways occurred in gastric cancer cell differentiation.
The crosstalk between Hh and Wnt pathways though SFRP1 is helpful to explain the previous findings.( 39 ) Although Kurayoshi et al. ( 39 ) reported that Wnt‐5a was activated frequently in diffuse‐type gastric cancer, nuclear β‐catenin accumulation is not found at significant level in this type of gastric cancer. The inhibition of nuclear β‐catenin accumulation by SFRP1 acting though GLI1 is a possible mechanism, based on our study. According to our results, a Wnt signal inhibitor, SFRP1, was transcriptionally activated by a primary Hh signal transducer GLI1 in diffuse‐type gastric cancer derived cell line MKN‐45. Thus, SFRP1 increased by GLI1 might suppress the β‐catenin pathway. In spite of that, Wnt‐5a stimulated cell migration and invasion in the gastric cancer cells through focal adhesion complexes.( 39 ) Therefore, other signaling molecules can have crucial roles in gastric cancer aggressiveness.
The present study suggests that the predominant signaling might be dissimilar between well differentiated glandular type and poorly differentiated glandular type gastric cancers. However, variable signaling might be involved in clinically presenting gastric cancers, such as signals related to carcinogenesis, progression, and migration. Despite that, the existence of baseline signals related to carcinogenesis according to histological types in gastric cancer could be important in the investigation of signaling pathways related to progression or migration.
In conclusion, the activation of the Hh pathway and suppression of the Wnt pathway by Hh signaling occurred during gastric cancer cell differentiation. If these differentiation‐specific signal changes are analyzed combined with signals related to the process of tumor progression or migration, they could great assist in discovering the molecular heterogeneity of gastric cancers.
Acknowledgments
This research was supported by the Yonsei University College of Medicine, Internal Medicine Research Grant 2007. This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF‐2007‐313‐E00213).
References
- 1. Tahara E, Semba S, Tahara H. Molecular biological observations in gastric cancer. Semin Oncol 1996; 23: 307–15. [PubMed] [Google Scholar]
- 2. Nardone G. Review article: molecular basis of gastric carcinogenesis. Aliment Pharmacol Ther 2003; 17 (Suppl 2): 75–81. [DOI] [PubMed] [Google Scholar]
- 3. Chong JM, Fukayama M, Hayashi Y et al. Microsatellite instability in the progression of gastric carcinoma. Cancer Res 1994; 54: 4595–7. [PubMed] [Google Scholar]
- 4. Craanen ME, Blok P, Dekker W, Offerhaus GJ, Tytgat GN. Chronology of p53 protein accumulation in gastric carcinogenesis. Gut 1995; 36: 848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer 1998; 1: 31–50. [DOI] [PubMed] [Google Scholar]
- 6. Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001; 411: 349–54. [DOI] [PubMed] [Google Scholar]
- 7. Ma X, Chen K, Huang S et al. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis 2005; 26: 1698–705. [DOI] [PubMed] [Google Scholar]
- 8. Fukaya M, Isohata N, Ohta H et al. Hedgehog signal activation in gastric pit cell and in diffuse‐type gastric cancer. Gastroenterology 2006; 131: 14–29. [DOI] [PubMed] [Google Scholar]
- 9. Yanai K, Nagai S, Wada J et al. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol 2007; 95: 55–62. [DOI] [PubMed] [Google Scholar]
- 10. Horii A, Nakatsuru S, Miyoshi Y et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res 1992; 52: 3231–3. [PubMed] [Google Scholar]
- 11. Sasaki Y, Morimoto I, Kusano M et al. Mutational analysis of the beta‐catenin gene in gastric carcinomas. Tumour Biol 2001; 22: 123–30. [DOI] [PubMed] [Google Scholar]
- 12. Tong JH, To KF, Ng EK et al. Somatic beta‐catenin mutation in gastric carcinoma – an infrequent event that is not specific for microsatellite instability. Cancer Lett 2001; 163: 125–30. [DOI] [PubMed] [Google Scholar]
- 13. Clements WM, Wang J, Sarnaik A et al. beta‐Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 2002; 62: 3503–6. [PubMed] [Google Scholar]
- 14. Yuasa Y. Control of gut differentiation and intestinal‐type gastric carcinogenesis. Nat Rev Cancer 2003; 3: 592–600. [DOI] [PubMed] [Google Scholar]
- 15. Tamura G. Alterations of tumor suppressor and tumor‐related genes in the development and progression of gastric cancer. World J Gastroenterol 2006; 12: 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyazawa K, Iwaya K, Kuroda M et al. Nuclear accumulation of beta‐catenin in intestinal‐type gastric carcinoma: correlation with early tumor invasion. Virchows Arch 2000; 437: 508–13. [DOI] [PubMed] [Google Scholar]
- 17. Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta‐catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology 2001; 39: 141–9. [DOI] [PubMed] [Google Scholar]
- 18. Yanai K, Nakamura M, Akiyoshi T et al. Crosstalk of hedgehog and Wnt pathways in gastric cancer. Cancer Lett 2008; 263: 145–56. [DOI] [PubMed] [Google Scholar]
- 19. Wang LH, Choi YL, Hua XY et al. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod Pathol 2006; 19: 675–83. [DOI] [PubMed] [Google Scholar]
- 20. Kim B, Koo H, Yang S et al. TC1(C8orf4) correlates with Wnt/beta‐catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res 2006; 12: 3541–8. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura T, Yao T, Kabashima A, Nishiyama K, Maehara Y, Tsuneyoshi M. Loss of phenotypic expression is related to tumour progression in early gastric differentiated adenocarcinoma. Histopathology 2005; 47: 357–67. [DOI] [PubMed] [Google Scholar]
- 22. Choe G, Kim WH, Park JG, Kim YI. Effect of suramin on differentiation of human stomach cancer cell lines. J Korean Med Sci 1997; 12: 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He J, Sheng T, Stelter AA et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP‐1. J Biol Chem 2006; 281: 35598–602. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Deng W, Lobo‐Ruppert SM, Ruppert JM. GLI1 acts through Snail and E‐cadherin to promote nuclear signaling by beta‐catenin. Oncogene 2007; 26: 4489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeda O, Kondo M, Fujita T et al. Enhancement of GLI1‐transcriptional activity by beta‐catenin in human cancer cells. Oncol Rep 2006; 16: 91–6. [PubMed] [Google Scholar]
- 26. Ohki K, Kumamoto H, Ichinohasama R, Sato T, Takahashi N, Ooya K. PTC gene mutations and expression of SHH, PTC, SMO, and GLI‐1 in odontogenic keratocysts. Int J Oral Maxillofac Surg 2004; 33: 584–92. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe H, Tokuyama H, Ohta H et al. Expression of placental alkaline phosphatase in gastric and colorectal cancers. An immunohistochemical study using the prepared monoclonal antibody. Cancer 1990; 66: 2575–82. [DOI] [PubMed] [Google Scholar]
- 28. Buras RR, Shabahang M, Davoodi F et al. The effect of extracellular calcium on colonocytes: evidence for differential responsiveness based upon degree of cell differentiation. Cell Prolif 1995; 28: 245–62. [DOI] [PubMed] [Google Scholar]
- 29. Ribiczey P, Tordai A, Andrikovics H et al. Isoform‐specific up‐regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium 2007; 42: 590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamamichi N, Inada K, Ichinose M et al. Frequent loss of Brm expression in gastric cancer correlates with histologic features and differentiation state. Cancer Res 2007; 67: 10727–35. [DOI] [PubMed] [Google Scholar]
- 31. Cottet S, Corthesy‐Theulaz I, Spertini F, Corthesy B. Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Ras‐associated proteins in cellular signaling. J Biol Chem 2002; 277: 33978–86. [DOI] [PubMed] [Google Scholar]
- 32. Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3‐dependent nuclear translocation of beta‐catenin is required for TGF‐beta1‐induced proliferation of bone marrow‐derived adult human mesenchymal stem cells. Genes Dev 2006; 20: 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hung MW, Tsai LC, Lin YL, Chen YH, Chang GG, Chang TC. Differential regulation of placental and germ cell alkaline phosphatases by glucocorticoid and sodium butyrate in human gastric carcinoma cell line TMK‐1. Arch Biochem Biophys 2001; 388: 45–54. [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto K, Kawagishi H, Nakayama T, Shimizu M. Effect of capsianoside, a diterpene glycoside, on tight‐junctional permeability. Biochim Biophys Acta 1997; 1323: 281–90. [DOI] [PubMed] [Google Scholar]
- 35. Marvin‐Guy LF, Duncan P, Wagniere S et al. Rapid identification of differentiation markers from whole epithelial cells by matrix‐assisted laser desorption/ionisation time‐of‐flight mass spectrometry and statistical analysis. Rapid Commun Mass Spectrom 2008; 22: 1099–108. [DOI] [PubMed] [Google Scholar]
- 36. Gelebart P, Kovacs T, Brouland JP et al. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J Biol Chem 2002; 277: 26310–20. [DOI] [PubMed] [Google Scholar]
- 37. Itoh T, Miyake K, Iijima S. Differentiation‐specific expression of chromatin remodeling factor BRM. Biochem Biophys Res Commun 2008; 366: 827–33. [DOI] [PubMed] [Google Scholar]
- 38. Ohta H, Aoyagi K, Fukaya M et al. Cross talk between hedgehog and epithelial‐mesenchymal transition pathways in gastric pit cells and in diffuse‐type gastric cancers. Br J Cancer 2009; 100: 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurayoshi M, Oue N, Yamamoto H et al. Expression of Wnt‐5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–48. [DOI] [PubMed] [Google Scholar]


