Abstract
We prospectively investigated the association between a change of serum vascular endothelial growth factor (VEGF) level after transcatheter arterial chemoembolization (TACE) and hepatocellular carcinoma (HCC) patient prognosis. The study involved 147 patients with unresectable HCC treated at the National Cancer Center, Korea, between July and December 2005. Serum samples were collected from each patient before TACE, and 1–2 days and 1 month after TACE. Serum VEGF concentrations were measured using an enzyme‐linked immunosorbent assay (ELISA). The loge(VEGF/platelets) increased transiently 1–2 days after TACE and declined thereafter. Frequency of previous TACE did not correlate with loge(VEGF/platelets). This study found that loge(VEGF/platelets) 1–2 days after TACE, but not loge(VEGF/platelets) at baseline, was strongly correlated with vascular or nodal invasion and AJCC (American Joint Committee on Cancer)/UICC (International Union Against Cancer) stage, and was significantly greater in men. Relative changes in serum VEGF/platelet levels 1–2 days after TACE (ΔVEGF) > 0.5 were directly correlated with tumor size, vascular invasion and modified UICC and AJCC/UICC stage (P < 0.05 for each). Additionally, ΔVEGF > 0.5 was significantly correlated with newly developed extrahepatic metastases one and six months after TACE (P = 0.005 and 0.003, respectively). Progression free survival of patients with ΔVEGF > 0.5 was significantly worse (P < 0.001) and ΔVEGF > 0.5 was an independent prognostic factor for PFS (hazard ratio, 3.111; P < 0.001). This study showed that a high increment in serum VEGF level 1–2 days after TACE in HCC patients was associated with distant metastasis and unfavorable outcomes. (Cancer Sci 2008; 99: 2037–2044)
Hepatocellular carcinoma (HCC) is a hypervascular tumor and radiologic identification of an arterial enhanced pattern has been included in the non‐invasive criteria of HCC diagnosis.( 1 , 2 ) Angiogenesis and the production of angiogenic factors are essential for tumor growth, invasion and metastasis.( 3 ) One cause of unfavorable prognosis in HCC patients is the high incidence of early microvascular invasion and the presence of microscopic venous invasion was also predictive of recurrence and survival after surgical resection or liver transplantation.( 4 , 5 ) Therefore, strategies that reduce angiogenesis and vascular invasion may lead to beneficial outcomes for patients with HCC, especially in advanced stage tumors.( 3 )
Vascular endothelial growth factor (VEGF) is a primary driving force for physiological and pathological angiogenesis( 6 ) and overexpression of VEGF has been observed in HCC.( 7 , 8 ) The concentration of circulating VEGF was found to correlate with advanced HCC tumor stage, with the highest level observed in patients with metastases( 9 ) and also could be a significant predictive factor of response to treatment and other clinical outcomes of HCC.( 10 , 11 , 12 ) VEGF polymorphism might be significant genetic markers for HCC prognosis.( 13 ) Therefore, inhibition of VEGF pathway represents a potential therapeutic target in HCC, and several antiangiogenic agents have entered clinical studies in HCC.( 14 , 15 )
Because most HCC patients are diagnosed at advanced stages in Korea,( 16 ) transcatheter arterial chemoembolization (TACE) is considered a key modality for palliative treatment in these HCC patients.( 2 ) TACE consists primarily of directly targeted chemotherapy and embolization of arteries feeding the tumors, inevitably resulting in a hypoxic insult to HCC and surrounding liver tissues. Central tumor hypoxia was found to up‐regulate proangiogenic growth factors, which are potent mediators of tumor angiogenesis.( 6 , 17 , 18 ) Therefore, expression of circulating or tissue VEGF was enhanced after TACE in patients or animals with HCC( 19 , 20 , 21 , 22 ) and there could be some probability of adverse effects of TACE in HCC patients. To date, however, there have been no large, prospective studies on the prognostic significance of TACE‐induced change in serum VEGF concentration of HCC patients, which could provide a rationale of the adjuvant therapy of antiangiogenic agents after TACE. This prospective study was designed to investigate the effect of TACE on serum VEGF concentration and, in particular, the clinical and prognostic significance of altered serum VEGF concentration after a single session of TACE in 147 HCC patients.
Materials and Methods
Patients. Between July and December 2005, 147 patients were diagnosed with unresectable or inoperable HCC at the National Cancer Center Hospital (Goyang, South Korea) and indicated for TACE. This study was approved by the Institutional Review Board at our institute. The diagnosis of HCC and the indication of TACE were based on the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center.( 23 ) Inclusion criteria included age over 18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate liver function (Child–Pugh classification A or B) and adequate renal function (serum Cr ≤ 1.4 mg/dL).
Before study entry, written informed consent was obtained from all participants, and each patient underwent baseline laboratory examinations, including liver function tests, creatinine level, prothrombin time, α‐fetoprotein (α‐FP) level, complete blood count, and assays for viral hepatitis.
Sample collection and serum VEGF measurement. Serum samples were prospectively collected from each patient before TACE (baseline), and 1–2 days and 1 month after TACE. TACE was performed in all patients within 1 week of baseline examinations. Venous blood samples were drawn into a serum separator tube and centrifuged at 1800 g for 10 min, and the serum samples were stored at –80°C. Serum VEGF concentrations were quantitatively measured using an enzyme‐linked immunosorbent assay (ELISA) kit (Quantikine Human VEGF Immunoassay; R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.( 13 ) The VEGF concentration in each sample was measured in duplicate by an investigator blinded to the clinical information.
Baseline serum VEGF concentrations of the HCC patients significantly correlated with their baseline platelet counts (r = 0.632, P < 0.005; Fig. 1). Platelets are the main transporters of circulating VEGF, and serum VEGF concentrations showed high correlations with platelet counts.( 24 ) Thus, serum VEGF concentrations were corrected for variation in platelet counts, and the VEGF/platelets ratios were logarithmically transformed for parametric statistical analyzes because of skewed distribution. To analyze the data, we used loge(VEGF/platelets) values.
Figure 1.
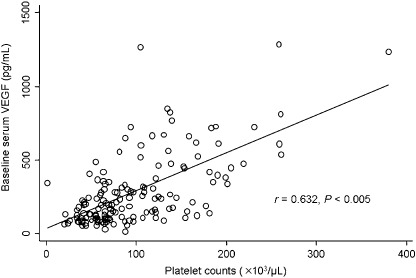
Scatter plot showing the correlation between serum vascular endothelial growth factor (VEGF) levels and platelet counts. Baseline serum VEGF levels of the patients significantly correlated with their baseline platelet counts.
In addition, the relative change in serum VEGF/platelets ratio from baseline until 1–2 days after a single session of TACE (ΔVEGF) was defined as:
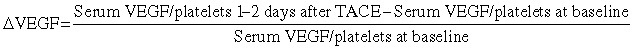 |
TACE methodology. Details of the TACE procedure performed in our institution have been described previously.( 25 ) Briefly, a solution containing 20–60 mg of doxorubicin hydrochloride and 2–20 mL of iodized oil (lipiodol) with absorbable gelatin sponge particles was infused through the catheter. The dosages of doxorubicin and lipiodol were determined for each patient based on tumor characteristics and underlying liver functions.
Follow‐up assessment. Tumor characteristics, modified International Union Against Cancer (UICC; Union Internationale Contre le Cancer) TNM stage( 26 ) and the American Joint Committee on Cancer (AJCC)/UICC TNM (tumor, node, metastases) stage( 27 ) were initially evaluated in all patients by multiphasic spiral computed tomography (CT) scan and dynamic contrast‐enhanced magnetic resonance imaging (MRI), if necessary. CT scans covered the area from the hilum of the lung to the whole pelvis. Follow‐up after TACE generally included monthly laboratory monitoring, including liver function tests and α‐FP level, chest X‐ray and CT scan; patients showing a complete remission were evaluated every 2–3 months.
Therapeutic effect of TACE was assessed according to the pattern of lipiodol retention in the target tumors that could reflect tumor necrosis.( 28 ) As assessed by CT scan, lipiodol uptake was considered compact if the oily contrast medium was distinctly visible and well scattered throughout all viable target tumors, and was considered non‐compact in all other cases.( 29 ) Intrahepatic tumor status was evaluated in terms of the pattern of lipiodol retention and the tumor extent. Tumor improvement was defined as a compact uptake of lipiodol in viable tumors, or a ≥30% decrease in the sum of the longest diameter of viable tumors despite non‐compact lipiodol uptake; tumor aggravation was defined as a ≥20% increase in the sum of the longest diameter of viable tumors without compact lipiodol uptake, or the appearance of new tumors regardless of lipiodol labeling; tumor stabilization was defined as intermediate between tumor improvement and aggravation.
New nodal invasion was defined as the appearance of enlarged nodes in patients without preexisting nodal invasion; all others were regarded as ‘stable’ nodal invasion. New extrahepatic metastasis was defined as a metastasis at sites other than preexisting sites, or de novo development in patients without preexisting metastasis; all others were regarded as ‘stable’ extrahepatic metastasis.
Following TACE, patients were treated individually according to the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center.( 23 ) In particular, repetition of TACE was tailored to tumor response and liver tolerance. TACE was repeated every 1–2 months on demand if a viable but responsive tumor was observed.
Statistical analysis. The data were analyzed using STATA software v9.1 (StataCorp LP, College Station, TX, USA). Correlation between serum VEGF level and platelet count was assessed by Pearson's correlation coefficient. The χ2 test or Fisher's exact test, if indicated, was used to assess categorical variables. In addition, Student's t‐test or ANOVA test, and Wilcoxon rank‐sum test or Kruskal–Wallis test were used to assess continuous variables. All data for continuous variables were expressed as the means±standard deviations. The Kaplan–Meier method was used to estimate progression free survival (PFS) and overall survival (OS) curves, and survival curves were compared using the log‐rank test. Prognostic relevance of each variable to PFS was analyzed using the Cox proportional hazards models. All variables with a P‐value <0.2 in univariate analysis were subjected to multivariate analysis to assess their values as independent predictors of PFS. P‐values <0.05 were considered statistically significant for all comparisons.
Results
Patient characteristics. The median age of the enrolled patients was 60 years (range, 40–76 years) and approximately 80% were men (Table 1). HCC etiology was related to hepatitis B in 105 patients (71.4%), hepatitis C in 16 (10.9%), and other causes in 26 (17.7%). The majority of patients had tolerable hepatic function, and over half of patients had tumors ≤5 cm in size and ≤2 tumors. According to modified UICC staging system, 15 patients (10.2%) had stage I, 52 (35.4%) had stage II, 55 (37.4%) had stage III, 14 (9.5%) had stage IVa and 11 (7.5%) had stage IVb tumors, whereas according to AJCC/UICC staging system, 54 patients (36.7%) had stage I, 45 (30.6%) had stage II, 35 (23.8%) had stage IIIA, 4 (2.7%) had stage IIIC and 9 (6.2%) had stage IV tumors. One hundred and seven patients (72.8%) previously underwent one or more sessions of TACE.
Table 1.
Baseline characteristics of 147 patients with hepatocellular carcinoma (HCC)
| Characteristics | n = 147 † | |
|---|---|---|
| Age (years) ‡ | <60 | 68 (46.3) |
| ≥60 | 79 (53.7) | |
| Sex | Male | 118 (80.3) |
| Female | 29 (19.7) | |
| Etiology | HBV | 105 (71.4) |
| HCV | 16 (10.9) | |
| Alcohol | 15 (10.2) | |
| Others | 11 (7.5) | |
| Child–Pugh class | A | 92 (62.6) |
| B | 55 (37.4) | |
| Serum α‐FP (ng/mL) § | <400 | 108 (73.5) |
| ≥400 | 39 (26.5) | |
| Tumor size (cm) | ≤2 | 31 (21.1) |
| 2–5 | 62 (42.2) | |
| 5–10 | 36 (24.5) | |
| >10 | 18 (12.2) | |
| Tumor number | 1 | 63 (42.9) |
| 2 | 27 (18.4) | |
| 3–4 | 22 (14.9) | |
| ≥5 | 35 (23.8) | |
| Tumor type | Well‐defined | 83 (56.5) |
| Poorly defined | 64 (43.5) | |
| Portal vein invasion | Yes | 23 (15.6) |
| No | 124 (84.4) | |
| Hepatic vein invasion | Yes | 2 (1.4) |
| No | 145 (98.6) | |
| Vascular invasion ¶ | Yes | 24 (16.3) |
| No | 123 (83.7) | |
| Modified UICC stage | I | 15 (10.2) |
| II | 52 (35.4) | |
| III | 55 (37.4) | |
| IVa | 14 (9.5) | |
| IVb | 11 (7.5) | |
| AJCC/UICC stage | I | 54 (36.7) |
| II | 45 (30.6) | |
| IIIA | 35 (23.8) | |
| IIIB | 0 (0.0) | |
| IIIC | 4 (2.7) | |
| IV | 9 (6.2) | |
| Number of prior TACE | None | 40 (27.2) |
| 1–2 | 50 (34.1) | |
| 3–4 | 32 (21.7) | |
| ≥5 | 25 (17.0) | |
| Laboratory parameters | Platelet count (103/µL) | 84 (40–380) |
| Albumin (g/dL) | 3.8 (2.7–4.9) | |
| Total bilirubin (mg/dL) | 1.0 (0.3–2.5) | |
| AST (IU/L) | 47 (19–191) | |
| ALT (IU/L) | 33.5 (8–151) | |
| PT (INR) | 1.17 (0.84–1.55) |
HBV indicates hepatitis B virus; HCV, hepatitis C virus; α‐FP, α‐fetoprotein; UICC, International Union Against Cancer; AJCC, American Joint Committee on Cancer; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IU, International unit; PT, prothrombin time; INR, international normalized ratio.
Values in the column are represented as number of patients (%) or median (range).
Range, 40–76 years (median, 60 years).
Range, 2.1–120,551.0 ng/mL (median, 25.2 ng/mL).
Vascular invasion was defined as positive when either portal vein or hepatic vein invasion was present, which was radiologically observed.
Serum VEGF/platelets level before and after TACE. We found that serum VEGF levels were significantly higher 1–2 days after TACE than at baseline (289.62 ± 243.08 pg/mL versus 326.26 ± 271.11 pg/mL, P < 0.001). One month after TACE, serum VEGF levels declined to baseline levels (317.31 ± 292.92 pg/mL, P = NS compared with 1–2 days after TACE; Fig. 2a), significantly lower than on days 1–2 (P = 0.024; Fig. 3). On the contrary, platelet counts were significantly elevated 1–2 days after TACE (94.0 ± 52.4 × 103/µL) compared with baseline (99.5 ± 59.8 × 103/µL, P = 0.005) and then platelet counts were thoroughly recovered 1 month after TACE (107.3 ± 60.0 × 103/µL, P < 0.001), significantly greater than on days 1–2 (P = 0.001; Fig. 2b).
Figure 2.
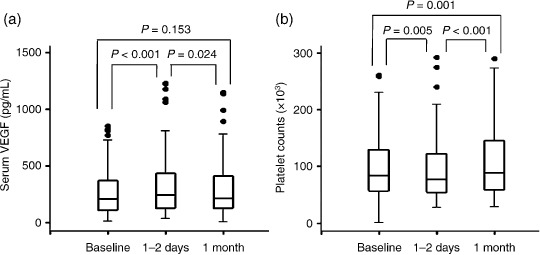
Box plots showing the comparison of serum vascular endothelial growth factor (VEGF) levels and platelet counts before and after transcatheter arterial chemoembolization (TACE). Serum VEGF levels were significantly higher 1–2 days after TACE than at baseline. One month after TACE, serum VEGF levels declined to baseline levels. Instead, platelet counts increased significantly 1–2 days after TACE (94.0 ± 52.4 × 103/µL) compared to baseline (99.5 ± 59.8 × 103/µL), although they were recovered 1 month after TACE (107.3 ± 60.0 × 103/µL) beyond expectation. P‐value was calculated using Wilcoxon rank‐sum test.
Figure 3.
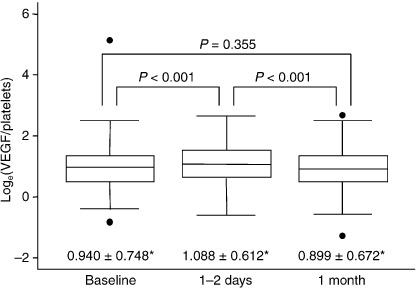
Box plots showing the comparison of loge(vascular endothelial growth factor [VEGF]/platelets) before and after after transcatheter arterial chemoembolization (TACE). Loge(VEGF/platelets) values were significantly higher 1–2 days after TACE than at baseline. One month after TACE, loge(VEGF/platelets) fell to baseline levels, significantly lower than on days 1–2. P‐value was calculated using Student's t‐test. (*means ± standard deviations)
In addition, loge(VEGF/platelets) values were significantly higher 1–2 days after TACE than at baseline (1.088 ± 0.612 versus 0.940 ± 0.748, P < 0.001). Thereafter, loge(VEGF/platelets) fell to baseline levels on 1 month of TACE (0.899 ± 0.672, P = NS compared with baseline), significantly lower than on days 1–2 (P < 0.001; Fig. 3).
The number of prior TACE sessions was not significantly associated with loge(VEGF/platelets), either at baseline (Fig. 4a) or 1–2 days after TACE (Fig. 4b).
Figure 4.
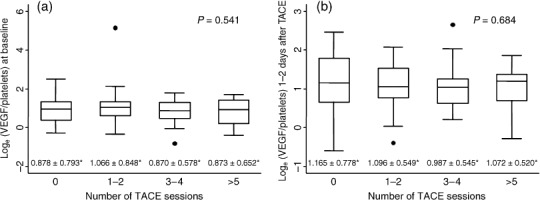
Box plots showing the effect of previous number of after transcatheter arterial chemoembolization (TACE) sessions on loge(vascular endothelial growth factor [VEGF] /platelets) (a) at baseline and (b) 1–2 days after TACE. The number of prior TACE sessions had no effect on loge(VEGF/platelets), either at baseline or 1–2 days after TACE. P‐values were calculated using ANOVA test. (*means ± standard deviations)
Association between serum VEGF level and HCC characteristics. Table 2 shows the association between serum VEGF levels before and after TACE and various clinical parameters. Serum VEGF levels at baseline were significantly greater in patients with Child–Pugh class A (P = 0.008), and serum VEGF levels 1–2 days after TACE were significantly greater in men (P = 0.019) and patients with Child–Pugh class A (P < 0.001) or vascular invasion (P = 0.015) and also, both of them had a significant correlation with tumor size (P = 0.007 and P = 0.008, respectively) and tumor number (P = 0.010 and P = 0.004, respectively).
Table 2.
Association between serum vascular endothelial growth factor (VEGF) levels and clinical parameters
| Characteristics | Serum VEGF (pg/mL) | ||||
|---|---|---|---|---|---|
| Baseline † | P‐value* | Day 1–2 after TACE* | P‐value* | ||
| Age (years) | <60 | 312.71 ± 292.67 | 0.287 | 351.34 ± 324.79 | 0.300 |
| ≥60 | 269.75 ± 190.16 | 304.68 ± 214.35 | |||
| Sex | Male | 306.94 ± 253.65 | 0.081 | 346.51 ± 277.62 | 0.068 |
| Female | 219.17 ± 181.23 | 243.87 ± 228.97 | |||
| Etiology | HBV | 290.58 ± 248.34 | 0.940 | 326.77 ± 280.37 | 0.971 |
| Others | 287.24 ± 232.30 | 324.99 ± 249.68 | |||
| Child–Pugh class | A | 325.59 ± 253.73 | 0.020 | 368.40 ± 267.96 | 0.014 |
| B | 229.46 ± 213.02 | 255.78 ± 263.89 | |||
| Serum α‐FP (ng/mL) | <400 | 272.78 ± 222.11 | 0.148 | 305.21 ± 244.81 | 0.105 |
| ≥400 | 339.68 ± 294.66 | 388.84 ± 333.54 | |||
| Tumor size (cm) | ≤2 | 252.31 ± 203.83 | <0.001 | 260.63 ± 200.84 | <0.001 |
| 2–5 | 272.83 ± 206.39 | 306.76 ± 241.61 | |||
| 5–10 | 233.98 ± 178.94 | 290.77 ± 223.41 | |||
| >10 | 523.01 ± 384.15 | 577.44 ± 411.25 | |||
| Tumor number | 1 | 310.65 ± 252.73 | 0.004 | 334.00 ± 268.54 | 0.002 |
| 2 | 178.33 ± 127.60 | 220.27 ± 155.98 | |||
| 3–4 | 217.76 ± 146.82 | 233.47 ± 152.60 | |||
| ≥5 | 382.80 ± 297.59 | 452.43 ± 346.61 | |||
| Tumor type | Well‐defined | 298.89 ± 232.51 | 0.600 | 335.99 ± 264.04 | 0.622 |
| Poorly defined | 277.60 ± 257.50 | 313.65 ± 281.60 | |||
| Vascular invasion | Yes | 392.32 ± 338.57 | 0.023 | 483.25 ± 301.42 | 0.002 |
| No | 269.58 ± 215.92 | 295.63 ± 233.97 | |||
| Lymph node invasion | Yes | 438.70 ± 232.99 | 0.125 | 480.19 ± 309.82 | 0.156 |
| No | 283.28 ± 421.45 | 319.71 ± 268.63 | |||
| Extrahepatic metastasis | Yes | 439.78 ± 473.02 | 0.056 | 461.30 ± 254.62 | 0.123 |
| No | 279.83 ± 219.83 | 317.45 ± 457.46 | |||
| Modified UICC stage | I | 267.24 ± 218.36 | 0.352 | 266.50 ± 214.38 | 0.182 |
| II | 304.41 ± 218.00 | 332.75 ± 253.50 | |||
| III | 253.72 ± 228.22 | 286.40 ± 247.86 | |||
| IVa | 301.90 ± 221.43 | 416.87 ± 323.41 | |||
| IVb | 414.14 ± 426.99 | 461.08 ± 409.28 | |||
| AJCC/UICC stage | I | 300.80 ± 222.79 | 0.186 | 328.10 ± 254.31 | 0.286 |
| II | 236.55 ± 181.64 | 267.23 ± 222.13 | |||
| IIIA | 309.10 ± 263.73 | 363.24 ± 298.42 | |||
| IIIC | 227.38 ± 90.49 | 338.21 ± 142.32 | |||
| IV | 439.78 ± 473.02 | 461.30 ± 457.46 | |||
| Lipiodol uptake | Compact | 237.28 ± 171.18 | 0.239 | 260.97 ± 209.74 | 0.187 |
| Noncompact | 300.35 ± 253.47 | 339.64 ± 280.89 | |||
HBV indicates hepatitis B virus; α‐FP, α‐fetoprotein; UICC, International Union Against Cancer; AJCC, American Joint Committee on Cancer.
P‐values for each clinical variable relative to serum VEGF calculated using Wilcoxon rank‐sum test or Kruskal‐Wallis test.
Values in the columns are represented as means ± standard deviations.
Association between serum VEGF/platelets level and HCC characteristics. At baseline, loge(VEGF/platelets) did not correlate significantly with any characteristics of patients. In contrast, loge(VEGF/platelets) 1–2 days after TACE showed a significant direct correlation with vascular (P = 0.005) or nodal (P = 0.008) invasion and AJCC/UICC stage (P = 0.044), and was significantly greater in men than in women (P = 0.034). The pattern of lipiodol retention was not significantly associated with loge(VEGF/platelets) either at baseline or 1–2 days after TACE (Table 3).
Table 3.
Association between loge(vascular endothelial growth factor [VEGF]/platelets) and ΔVEGF > 0.5 and clinical parameters
| Characteristics | Loge(VEGF/platelets) | ΔVEGF > 0.5 | |||||
|---|---|---|---|---|---|---|---|
| Baseline † | P‐value* | Day 1–2 after TACE † | P‐value* | n = 147 ‡ | P‐value** | ||
| Age (years) | <60 | 0.957 ± 0.875 | 0.796 | 1.065 ± 0.649 | 0.676 | 15/68 (22.1) | 0.916 |
| ≥60 | 0.928 ± 0.623 | 1.107 ± 0.581 | 18/79 (22.8) | ||||
| Sex | Male | 0.980 ± 0.771 | 0.186 | 1.140 ± 0.588 | 0.034 | 29/118 (24.6) | 0.159 |
| Female | 0.775 ± 0.631 | 0.872 ± 0.670 | 4/29 (13.8) | ||||
| Etiology | HBV | 1.003 ± 0.762 | 0.101 | 1.122 ± 0.636 | 0.280 | 22/105 (21.0) | 0.492 |
| Others | 0.780 ± 0.694 | 1.001 ± 0.543 | 11/42 (26.2) | ||||
| Child–Pugh class | A | 0.919 ± 0.773 | 0.674 | 1.094 ± 0.560 | 0.878 | 21/92 (22.8) | 0.887 |
| B | 0.973 ± 0.709 | 1.078 ± 0.695 | 12/55 (21.8) | ||||
| Serum α‐FP (ng/mL) | <400 | 1.079 ± 0.890 | 0.176 | 1.214 ± 0.562 | 0.132 | 22/108 (20.4) | 0.315 |
| ≥400 | 0.890 ± 0.687 | 1.042 ± 0.625 | 11/39 (28.2) | ||||
| Tumor size (cm) | ≤2 | 0.903 ± 0.833 | 0.151 | 1.037 ± 0.658 | 0.117 | 2/31 (6.5) | 0.040 |
| 2–5 | 0.908 ± 0.577 | 0.997 ± 0.586 | 13/62 (21.0) | ||||
| 5–10 | 0.839 ± 0.631 | 1.144 ± 0.540 | 12/36 (33.3) | ||||
| >10 | 1.312 ± 1.178 | 1.376 ± 0.698 | 6/18 (33.3) | ||||
| Tumor number | 1 | 1.018 ± 0.713 | 0.070 | 1.135 ± 0.637 | 0.093 | 11/63 (17.5) | 0.583 |
| 2 | 0.612 ± 0.676 | 0.837 ± 0.522 | 6/27 (22.2) | ||||
| 3–4 | 0.907 ± 0.498 | 1.063 ± 0.409 | 6/22 (27.3) | ||||
| ≥5 | 1.071 ± 0.925 | 1.211 ± 0.697 | 10/35 (28.6) | ||||
| Tumor type | Well‐defined | 0.855 ± 0.638 | 0.118 | 1.034 ± 0.569 | 0.225 | 19/83 (22.9) | 0.884 |
| Poorly defined | 1.049 ± 0.862 | 1.158 ± 0.660 | 14/64 (21.9) | ||||
| Vascular invasion | Yes | 1.191 ± 0.677 | 0.072 | 1.026 ± 0.603 | 0.005 | 11/24 (45.8) | 0.005 |
| No | 0.891 ± 1.020 | 1.402 ± 0.569 | 22/123 (17.9) | ||||
| Lymph node invasion | Yes | 1.308 ± 0.612 | 0.218 | 1.737 ± 0.520 | 0.008 | 2/6 (33.3) | 0.514 |
| No | 0.924 ± 0.751 | 1.060 ± 0.601 | 31/141 (22.0) | ||||
| Extrahepatic metastasis | Yes | 0.943 ± 0.960 | 0.988 | 1.216 ± 0.670 | 0.519 | 2/9 (22.2) | 0.987 |
| No | 0.939 ± 0.736 | 1.079 ± 0.609 | 31/138 (23.7) | ||||
| Modified UICC stage | I | 1.063 ± 0.975 | 0.633 | 1.163 ± 0.738 | 0.215 | 1/15 (6.7) | 0.009 |
| II | 0.930 ± 0.579 | 1.047 ± 0.579 | 8/52 (15.4) | ||||
| III | 0.848 ± 0.621 | 0.993 ± 0.588 | 13/55 (23.6) | ||||
| IVa | 1.169 ± 1.285 | 1.341 ± 0.589 | 8/14 (57.1) | ||||
| IVb | 0.985 ± 0.865 | 1.324 ± 0.672 | 3/11 (27.3) | ||||
| AJCC/UICC stage | I | 0.966 ± 0.705 | 0.642 | 1.098 ± 0.626 | 0.044 | 9/54 (16.7) | 0.025 |
| II | 0.804 ± 0.608 | 0.895 ± 0.579 | 6/45 (13.3) | ||||
| IIIA | 1.058 ± 0.940 | 1.224 ± 0.575 | 14/35 (40.0) | ||||
| IIIC | 1.063 ± 0.236 | 1.625 ± 0.477 | 2/4 (50.0) | ||||
| IV | 0.943 ± 0.960 | 1.216 ± 0.670 | 2/9 (22.2) | ||||
| Lipiodol uptake | Compact | 0.938 ± 0.602 | 0.991 | 0.940 ± 0.670 | 0.186 | 4/25 (16.0) | 0.597 |
| Noncompact | 0.940 ± 0.070 | 1.118 ± 0.597 | . | 28/122 (23.0) | |||
HBV indicates hepatitis B virus; α‐FP, α‐fetoprotein; UICC, International Union Against Cancer; AJCC, American Joint Committee on Cancer.
Values in the columns are represented as means ± standard deviations.
Values in the column are represented as ratio (%).
P‐values for each clinical variable relative to loge(VEGF/platelet) calculated using Student's t‐test or ANOVA test.
P‐values for each clinical variable relative to ΔVEGF > 0.5 calculated using χ2 test or Fisher's exact test.
Change in serum VEGF/platelets level after TACE and association with HCC characteristics. We arbitrarily defined a cutoff of ΔVEGF > 0.5; i.e. a 50% increase in serum VEGF/platelets level from baseline, and we assessed the association between ΔVEGF > 0.5 and various clinical variables (Table 3). Of the 147 enrolled patients, 33 (22.4%) showed a ΔVEGF > 0.5. There were significant correlations between ΔVEGF > 0.5 and tumor size (P = 0.04), vascular invasion (P = 0.005), and modified UICC (P = 0.009) and AJCC/UICC stage (P = 0.025).
Changes in serum VEGF/platelets level after TACE (DVEGF) and tumor responsiveness.
One month follow‐up. All patients underwent regular follow‐up 1 month after TACE and surveillance for tumor responsiveness by CT scan at least every 2–3 months (Table 4). The median duration of follow‐up was 23.2 months (range, 1.5–25.5 months). At a median 1.1 months (range, 0.6–2.1 months) after TACE, ΔVEGF > 0.5 was not significantly associated with either intrahepatic tumor status or nodal invasion. However, ΔVEGF > 0.5 was observed in 20.1% (28/139) of patients with stable extrahepatic metastases and in 62.5% (5/8) of those with new extrahepatic metastases, indicating a significant correlation between ΔVEGF > 0.5 and newly developed extrahepatic metastases (P = 0.005).
Table 4.
Effect of ΔVEGF (vascular endothelial growth factor) >0.5 on hepatocellular carcinoma (HCC) status after transcatheter arterial chemoembolization (TACE)
| Characteristics | 1 month (n = 147) | 6 months (n = 126) | 12 months (n = 95) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔVEGF > 0.5 | % | P‐value* | ΔVEGF > 0.5 | % | P‐value* | ΔVEGF > 0.5 | % | P‐value* | |
| Intrahepatic tumor status | 0.178 | 0.246 | 0.920 | ||||||
| Improved | 9/59 | 15.3 | 10/60 | 13.4 | 8/41 | 19.5 | |||
| Stable | 8/34 | 23.5 | 12/43 | 27.9 | 5/24 | 20.8 | |||
| Aggravated | 16/54 | 29.6 | 3/23 | 13.4 | 5/30 | 16.7 | |||
| Lymph node invasion | 0.099 | 0.396 | 0.518 | ||||||
| Stable | 30/141 | 21.3 | 23/120 | 19.2 | 17/92 | 18.5 | |||
| New † | 3/6 | 50.0 | 2/6 | 33.3 | 1/3 | 33.3 | |||
| Extrahepatic metastasis | 0.005 | 0.003 | 0.627 | ||||||
| Stable | 28/139 | 20.1 | 19/115 | 16.5 | 17/87 | 19.5 | |||
| New ‡ | 5/8 | 62.5 | 6/11 | 54.5 | 1/8 | 12.5 | |||
HCC indicates hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
P‐values for each clinical variable relative to ΔVEGF > 0.5 calculated using χ
2 test or Fisher's exact test.
Development of nodal invasion in patients without prior enlarged nodes.
Development of de novo distant metastases regardless of the presence or progression of prior metastases.
Six month follow‐up. We obtained follow‐up data from 126 of the 147 enrolled patients, at a median 6.1 months (range, 4.8–7.9 months) after TACE (Table 4). Four of the 21 patients who were not included in the 6‐month evaluation had died, and an objective response could not be determined in the others. In agreement with the results observed at 1 month, ΔVEGF > 0.5 at 6 months was not significantly correlated with either intrahepatic tumor status or nodal invasion. However, ΔVEGF > 0.5 was observed in 16.5% (19/115) of patients with stable extrahepatic metastases and in 54.5% (6/11) of those with new extrahepatic metastases, indicating a significant correlation between ΔVEGF > 0.5 and newly developed extrahepatic metastases at 6 months (P = 0.003).
One year follow‐up. Of the 126 patients evaluated 6 months after TACE, 95 were evaluated at a median 12.1 months (range, 10.7–14.6 months) after TACE (Table 4). At this follow‐up, ΔVEGF > 0.5 was not significantly correlated with the development of new extrahepatic metastases.
Changes in serum VEGF/platelets level and survival. We assessed PFS and OS using the Kaplan–Meier method (Fig. 5a,b). Through 4.7 months after TACE, PFS was significantly higher in patients with ΔVEGF ≤ 0.5 than in those with ΔVEGF > 0.5 (P < 0.001). OS was also higher in patients with ΔVEGF ≤ 0.5 than in those with ΔVEGF > 0.5 through the entire follow‐up period, but this difference was not statistically significant (P = 0.054).
Figure 5.
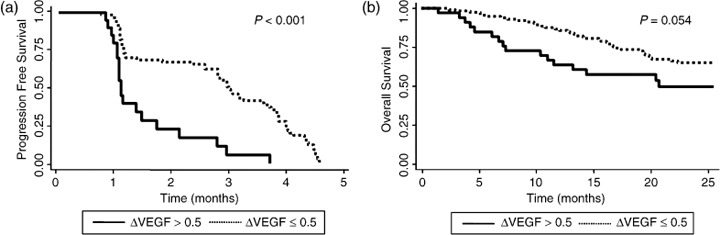
Relation of progression free survival (PFS) and overall survival (OS) with ΔVEGF (vascular endothelial growth factor)*. Kaplan–Meier survival plots dichotomized by ΔVEGF ≤ 0.5 or >0.5. (a) Through 4.7 months after transcatheter arterial chemoembolization (TACE), PFS was significantly higher in patients with ΔVEGF ≤ 0.5 than in those with ΔVEGF > 0.5; (b) OS was higher in patients with ΔVEGF ≤ 0.5 than in those with ΔVEGF > 0.5 through the entire follow‐up period, but this difference was not statistically significant. P‐values were calculated using the log‐rank test.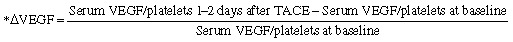
In univariate analysis, tumor number and ΔVEGF had a statistically significant influence on PFS through 4.7 months after TACE (Table 5). In multivariate analysis, both ΔVEGF and tumor type were the variables significantly related PFS through 4.7 months after TACE. ΔVEGF > 0.5 increased the risk of progression by approximately 3‐fold (hazard ratio, 3.111; 95% confidence interval, 1.680–5.762; P < 0.001; Table 5).
Table 5.
Cox survival analysis of predictors for progression free survival (PFS) through 4.7 months after transcatheter arterial chemoembolization (TACE)
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value* | HR | 95% CI | P‐value* | |
| Age, ≥60 years | 0.996 | 0.973–1.021 | 0.763 | NE | ||
| Sex, male | 1.237 | 0.733–2.088 | 0.426 | NE | ||
| Etiology, HBV | 0.980 | 0.606–1.587 | 0.936 | NE | ||
| Child–Pugh class, B | 1.106 | 0.711–1.719 | 0.655 | NE | ||
| Serum α‐FP, ≥400 ng/mL | 1.350 | 0.813–2.242 | 0.246 | NE | ||
| Tumor size, 2–5 cm | 0.980 | 0.506–1.898 | 0.953 | 1.550 | 0.790–3.043 | 0.203 |
| Tumor size, 5–10 cm | 1.167 | 0.578–2.355 | 0.666 | 1.564 | 0.738–3.318 | 0.243 |
| Tumor size, >10 cm | 1.914 | 0.812–4.513 | 0.138 | 1.632 | 0.533–4.998 | 0.391 |
| Tumor number, 2 | 1.261 | 0.686–2.318 | 0.455 | 1.139 | 0.594–2.184 | 0.694 |
| Tumor number, 3–4 | 1.006 | 0.561–1.806 | 0.983 | 1.085 | 0.574–2.052 | 0.801 |
| Tumor number, ≥5 | 2.074 | 1.170–3.679 | 0.013 | 1.849 | 0.946–3.614 | 0.072 |
| Tumor type, poorly defined | 1.526 | 0.976–2.387 | 0.064 | 1.672 | 1.033–2.705 | 0.036 |
| Vascular invasion, present | 1.279 | 0.740–2.208 | 0.379 | NE | ||
| Lymph node invasion, present | 2.177 | 0.773–6.130 | 0.141 | 1.250 | 0.422–3.700 | 0.687 |
| Extrahepatic metastasis, present | 1.655 | 0.791–3.463 | 0.181 | 1.294 | 0.478–3.506 | 0.612 |
| ΔVEGF, >5 | 3.059 | 1.731–5.405 | <0.001 | 3.111 | 1.680–5.762 | <0.001 |
| Lipiodol uptake, non‐compact | 1.786 | 0.853–3.737 | 0.124 | 1.709 | 0.823–3.546 | 0.150 |
PFS indicates progression free survival; TACE, transcatheter arterial chemoembolization; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; α‐FP, α‐fetoprotein; NE, not evaluated.
P‐values generated from the Cox proportional hazards model.
Discussion
Most patients with unresectable or inoperable HCC are treated by various palliative therapies, including TACE,( 2 ) which is thought to lead to tumor necrosis through the promotion of a hypoxic condition. Ischemic injury after TACE has been found to induce the up‐regulation of circulating VEGF in patients with HCC, reaching a peak value 1–7 days after TACE.( 19 , 20 , 21 , 22 ) We also found that serum VEGF concentrations increased transiently 1–2 days after TACE, but then declined to baseline levels, 1 month after TACE, suggesting that the effect of TACE on serum VEGF concentration nearly disappeared 1 month after TACE. Instead, platelet counts decreased temporarily 1–2 days after TACE, although they increased gradually up to 1 month of TACE, which might be due to TACE‐related coagulopathy with thrombocytopenia( 30 ) and myelosuppression induced by chemotherapeutics during TACE. In addition, this study showed that the number of prior TACE sessions had no effect on serum VEGF level, either at baseline or 1–2 days after TACE.
Platelets are the major physiological transporters of VEGF in blood, and serum VEGF concentrations highly correlated with platelet counts.( 24 ) Moreover, the platelet load of VEGF in patients with malignancy correlated quantitatively with tumor expression of VEGF.( 31 ) We observed a strong positive correlation between serum VEGF concentration and platelet count. Therefore, we used loge(serum VEGF/platelet count) as a standardized measure of circulating VEGF and as an indirect estimate of tumor VEGF expression.
Previous studies have shown a significant correlation between pre‐TACE level of circulating VEGF or VEGF up‐regulation after TACE and HCC characteristics, including tumor size, vascular invasion and metastasis.( 12 , 19 , 21 ) In the present study, serum VEGF level before and after TACE also correlated positively with tumor size and tumor number, and/or vascular invasion, although not with nodal and remote metastases. However, loge (VEGF/platelets) values at baseline were not associated with any clinical parameters, although loge(VEGF/platelets) values 1–2 days after TACE were significantly correlated with vascular or nodal invasion and tumor stage. It might be probably because the scales of loge(VEGF/platelets) values at baseline were too small for their correlations with clinical variables to reach the statistical significance, compared with serum VEGF levels themselves, but instead, loge(VEGF/platelets) values 1–2 days after TACE all increased by TACE‐related hypoxia enough for them to reach the statistical significance.
A recent study reported a correlation between TACE‐induced changes of angiogenic factors and overall survival.( 32 ) Our prospective study also found that a >50% increase in serum VEGF concentration relative to baseline (ΔVEGF) was related to the advent of extrahepatic metastases up to 6 months after TACE (Table 4), although the best predictive cut‐off level should be defined through further research with a larger size cohort. Moreover, ΔVEGF may have the advantage of standardization, since absolute level of circulating VEGF varies widely. However, ΔVEGF > 0.5 did not had a pivotal role in de novo development of extrahepatic metastases at 1‐year assessment points, which might be indubitably because, of the 147 enrolled patients, 52 patients who mostly had aggressive tumors had been dead, or were lost to follow‐up at that time, and more predominantly, the repetition of subsequent TACE sessions could confound the effect of ΔVEGF > 0.5 on distant tumor spread. Previous studies suggested that basal level of circulating VEGF might be related to current distant metastases.( 9 , 22 ) Further, we have shown clearly that the temporary overproduction of VEGF caused by a single session of TACE was related with future distant metastases, primarily in the lungs and bones. Therefore, this study suggested a rationale of additional treatment of antiangiogenesis to TACE in some cases of HCC. The findings presented here are the first to show that transiently increased serum VEGF level following a single session of TACE had a strong influence on PFS as an independent prognostic factor, even for a short period of time (Fig. 5a; Table 5). After multivariate analysis, both ΔVEGF and tumor type were statistically significant predictors for PFS through 4.7 months (Table 5). Although ΔVEGF did not lead to a significant difference in survival rate (P = 0.054; Fig. 5b), a larger size of patients seems likely to lead to a positive relationship. In fact, it may be difficult to determine if ΔVEGF > 0.5 is significantly associated with OS, because the mortality of HCC patients is subject to many other factors, including underlying liver function and cirrhosis complications. Nevertheless, ΔVEGF > 0.5 may be a prognostic indicator, especially relevant to distant metastasis. We showed that although serum VEGF/platelets level at baseline showed no significant positive correlation with various parameters, serum VEGF/platelets level 1–2 days after TACE was significantly higher in patients with vascular or nodal invasion, especially in men (Table 3). These findings suggest that HCC and/or surrounding liver reacting more sensitively to TACE‐induced hypoxia may have a tendency to invade more aggressively.
Our study could suggest that measuring the difference between pre‐ and post‐TACE concentrations of serum VEGF may be useful in selecting HCC patients with a predicted high risk of tumor progression after TACE, especially relevant to remote metastasis. These patients may benefit from strategies that inhibit VEGF signaling concurrently with TACE to prevent tumor spread. Recently, molecularly targeted agents that act directly on the VEGF signaling pathway have been approved for clinical use, with bevacizumab, a monoclonal antibody targeting VEGF( 33 ) and sorafenib, a multikinase inhibitor targeting VEGFR‐2/–3,( 14 , 34 ) being approved for patients with HCC. Better clinical outcomes may be accomplished by using anti‐VEGF agents in the adjuvant setting after TACE,( 14 ) especially in patients with advanced HCC or those with significant incremental increases of serum VEGF concentrations.
In conclusion, our results provide evidence that the transient up‐regulation of VEGF expression after a single session of TACE was associated with extrahepatic metastases and PFS in HCC patients. This study may provide a rationale for use of VEGF antagonists in a select group of advanced HCC patients undergoing TACE.
Financial support: This work was supported by the National Cancer Center, Korea (Grant #0810260‐1).
References
- 1. Bruix J, Sherman M, Llovet JM et al . Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona‐2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–30. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–36. [DOI] [PubMed] [Google Scholar]
- 3. Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti‐angiogenesis in hepatocellular carcinoma. Cancer Treat Rev 2006. [DOI] [PubMed]
- 4. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long‐term results of treatment and prognostic factors. Ann Surg 1999; 229: 216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg 1998; 228: 479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semela D, Dufour JF. Vascular endothelial growth factor signaling. In: Dufour JF, Calvien PA, eds. Signaling Pathways in Liver Diseases. Heidelberg: Springer‐Verlag Berlin, 2005; 91–104. [Google Scholar]
- 7. Miura H, Miyazaki T, Kuroda M et al . Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol 1997; 27: 854–61. [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 1998; 28: 68–77. [DOI] [PubMed] [Google Scholar]
- 9. Jinno K, Tanimizu M, Hyodo I et al . Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol 1998; 33: 376–82. [DOI] [PubMed] [Google Scholar]
- 10. Poon RT, Ng IO, Lau C et al . Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: A prospective study. Ann Surg 2001; 233: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chao Y, Li CP, Chau GY et al . Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol 2003; 10: 355–62. [DOI] [PubMed] [Google Scholar]
- 12. Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Report 2004; 11: 1077–84. [PubMed] [Google Scholar]
- 13. Kong SY, Park JW, Lee JA et al . Association between vascular endothelial growth factor gene polymorphisms and survival in hepatocellular carcinoma patients. Hepatology 2007; 46: 446–55. [DOI] [PubMed] [Google Scholar]
- 14. Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer 2008; 112: 250–9. [DOI] [PubMed] [Google Scholar]
- 15. Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol 2005; 23: 8093–108. [DOI] [PubMed] [Google Scholar]
- 16. Park KW, Park JW, Choi JI et al . Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus‐endemic area. J Gastroenterol Hepatol 2007; 23: 467–73. [DOI] [PubMed] [Google Scholar]
- 17. Von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 2001; 48: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 19. Xiong ZP, Yang SR, Liang ZY et al . Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2004; 3: 386–90. [PubMed] [Google Scholar]
- 20. Suzuki H, Mori M, Kawaguchi C, Adachi M, Miura S, Ishii H. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol 1999; 14: 1087–90. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004; 10: 2878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Influence of transarterial chemoembolization on angiogenesis and expression of vascular endothelial growth factor and basic fibroblast growth factor in rat with Walker‐256 transplanted hepatoma: an experimental study. World J Gastroenterol 2003; 9: 2445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park JW. [Practice guideline for diagnosis and treatment of hepatocellular carcinoma]. Korean J Hepatol 2004; 10: 88–98. [PubMed] [Google Scholar]
- 24. Verheul HM, Hoekman K, Luykx‐de Bakker S et al . Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res 1997; 3: 2187–90. [PubMed] [Google Scholar]
- 25. Park JW, Park KW, Cho SH et al . Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV‐related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol 2005; 100: 2194–200. [DOI] [PubMed] [Google Scholar]
- 26. Ueno S, Tanabe G, Nuruki K et al . Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002; 24: 395–403. [DOI] [PubMed] [Google Scholar]
- 27. AJCC . AJCC Cancer Staging Manual, 6th edn. New York: Springer, 2005. [Google Scholar]
- 28. Takayasu K, Arii S, Matsuo N et al . Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 2000; 175: 699–704. [DOI] [PubMed] [Google Scholar]
- 29. Kanematsu T, Furuta T, Takenaka K et al . A 5‐year experience of lipiodolization: Selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology 1989; 10: 98–102. [DOI] [PubMed] [Google Scholar]
- 30. Chung JW, Park JH, Han JK et al . Hepatic tumors: Predisposing factors for complications of transcatheter oily chemoembolization. Radiology 1996; 198: 33–40. [DOI] [PubMed] [Google Scholar]
- 31. Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res 2003; 63: 3121–6. [PubMed] [Google Scholar]
- 32. Sergio A, Cristofori C, Cardin R et al . Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol 2008; 103: 914–21. [DOI] [PubMed] [Google Scholar]
- 33. Zhu AX, Blaszkowsky LS, Ryan DP et al . Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24: 1898–903. [DOI] [PubMed] [Google Scholar]
- 34. Abou‐Alfa GK, Schwartz L, Ricci S et al . Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24: 4293–300. [DOI] [PubMed] [Google Scholar]


