Abstract
Adult T‐cell leukemia/lymphoma (ATL) is a highly aggressive disease with poor prognosis. CD30+ cells are frequently observed in lymph node cells and peripheral blood mononuclear cells of ATL patients. In order to elicit the role of CD30+ cells in ATL development, we investigated expression of the membrane type of CD30 (mCD30) and the soluble form of CD30 (sCD30) on ATL cells. Both mCD30 and sCD30 are expressed on various numbers of ATL cells in vivo as well as cell lines such as MT‐2, L540 and Karpas 299. The level of serum sCD30 in each clinical stage showed an elevated level in patients with acute type (mean ± standard error; 545.2 ± 18.6 U/mL) rather than with lymphoma type ATL (327.62 ± 94.85 U/mL). In four patients whose sera were stored and examined longitudinally, the levels decreased following the response to chemotherapy but not in patients with chemotherapy resistance. Thus, our results imply that sCD30 levels may be another useful marker for the activity and aggressiveness of ATL. (Cancer Sci 2005; 96: 810–815)
Abbreviations:
- ALCL
anaplastic large cell lymphoma
- ATL
adult T‐cell leukemia/lymphoma
- CR
complete remission
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FSC
forward scatter
- HD
Hodgkin's disease
- HLTV‐1
human T‐cell leukemia virus type 1
- H‐RS
Hodgkin and Reed‐Sternberg
- LDH
lactate dehydrogenase
- LN
lymph node
- mAb
monoclonal antibodies
- NHL
non‐Hodgkin's lymphoma
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- sIL‐2R
soluble interleukin‐2 receptor
- SSC
side scatter
- TNFR
tumor necrosis factor receptor
- TRAF
TNFR‐associated factor
Adult T‐cell leukemia/lymphoma (ATL) is a highly aggressive leukemia/lymphoma which was first proposed as a new disease entity in 1977.( 1 , 2 ) Subsequently, Shimoyama and the Lymphoma Study Group classified four clinical subtypes: acute type, lymphoma‐type, chronic type, and smoldering type.( 3 ) The long clinical latency and low incidence of ATL indicate that some genetic changes are involved in the malignant transformation and monoclonal expansion of human T‐cell leukemia virus type 1 (HTLV‐1)‐infected cells. Monoclonal proliferation of HTLV‐1‐infected cells is observed in some virus carriers, who are considered to be the high risk group for development of ATL.( 4 ) Constitutive activation of STAT proteins as well as the functional impairment and stabilization of p53 protein found in the peripheral blood mononuclear cells (PBMC) of ATL patients are supposed to be one base for ATL development.( 5 , 6 ) However, it remains to be studied what the additional factors are that allow the monoclonal proliferation of ATL cells.
CD30, a 120 kDa type I cell surface glycoprotein, is a member of the tumor necrosis factor receptor (TNFR) superfamily. The soluble form (sCD30) is produced by metalloprotease cleavage of the juxta‐membrane region releasing as an extracellular region of 85 kDa protein.( 7 , 8 ) CD30 is normally expressed by activated, but not by resting, B or T cells and also expressed on Hodgkin and Reed‐Sternberg (H‐RS) cells, anaplastic large cell lymphoma (ALCL) and ATL cells.( 9 ) CD30 regulates their proliferation, differentiation and apoptotic cell death, depending on the cell type and developmental stage.( 10 ) CD30+ cells release sCD30 in vitro and in vivo and it cannot be detected in the sera of healthy donors.( 8 , 11 )
CD30 ligand (CD30L, CD153) is a type II transmembrane protein and a member of the TNF ligand superfamily.( 12 ) It is expressed on resting B and activated T cells in addition to monocytes, granulocytes and natural killer cells.( 7 ) Its pleiotropic biologic activities were reported, including its proliferative effect and CD30L‐induced cell death.( 13 ) Recently, Su et al. also reported that Hodgkin's tumor cells inhibit proliferation and activation of T cells by the use of CD30, inducing the suppression of tumor surveillance.( 14 )
In ALCL and Hodgkin's disease (HD), elevated levels of sCD30 in the sera of patients are correlated with a poor prognosis, perhaps because a high level of sCD30 reflects a high tumor burden or because sCD30 blocks the biologic effects of CD30L.( 15 , 16 , 17 ) Moreover, sCD30 is detectable in the sera of ATL patients.( 11 ) In this study, we investigated the clinical significance of the membrane type of CD30 (mCD30) expression and serum sCD30 levels in ATL. Our results revealed the relationship between sCD30 levels and other serum markers of ATL.
Materials and Methods
Patients
Peripheral blood (PB) and lymph nodes (LN) were obtained from patients in the University Hospital, Kochi Medical School, Kochi University after informed consent according to the Helsinki Declaration was obtained from each patient. The clinical diagnosis was based on the presence of serum antibodies against HTLV‐1 antigens and monoclonal integration of HTLV‐1 provirus in PBMC or LN cells. The relevant characteristics of 10 patients (mean age ± standard deviation, 58.2 ± 12.7 years), seven with acute type and three with lymphoma‐type ATL, were investigated (Table 1). In addition, three acute type patients, one lymphoma‐type and one chronic type ATL patient, two HTLV‐1 carriers, two patients with B cell malignancy, including diffuse large B cell lymphoma of non‐Hodgkin's lymphoma (NHL) and multiple myeloma, and 21 healthy donors applied their serum to immunoassay for sCD30.
Table 1.
Clinical data of 10 patients with adult T‐cell leukemia/lymphoma
| Patient | Subtype | Age (years) | LDH* (IU/L) | Ca † (mg/dL) | WBC ‡ (/µL) | Abnormal (%) | sIL‐2R § (U/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Acute | 58 | 1403 | 11.6 | 13 200 | 26 | 216 000 |
| 2 | Acute | 54 | 3424 | 21.8 | 29 600 | 84 | 59 100 |
| 3 | Acute | 67 | 212 | 9.4 | 5800 | 34 | 36 100 |
| 4 | Acute | 36 | 383 | 20.4 | 11 900 | 20 | 5580 |
| 5 | Acute | 68 | 2202 | 23.2 | 141 300 | 52 | 175 000 |
| 6 | Acute | 43 | 1086 | 19.8 | 11 100 | 56 | 60 900 |
| 7 | Acute | 77 | 1754 | 17.2 | 19 500 | 59 | 35 000 |
| 8 | Lymphoma | 49 | 1710 | 10.6 | 6200 | 1 | 81 900 |
| 9 | Lymphoma | 65 | 971 | 11.6 | 4000 | 2 | 17 900 |
| 10 | Lymphoma | 65 | 486 | 19.8 | 5000 | 1 | 18 100 |
*The normal ranges of lactate dehydrogenase (LDH) are 110–220 IU/mL. † The normal ranges of Ca are 8–10.4 mg/dL. ‡The normal ranges of white blood cells (WBC) are 4000–8000/µL. §The normal ranges of sIL‐2R are 220–530 U/mL.
Cell culture
L540 and Karpas 299 were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). MT‐2 and Karpas 299 were suspended in standard RPMI‐1640 medium (Sigma, St Louis, Missouri) supplemented with 10% heat inactivated fetal bovine serum (FBS). L540 was suspended in the same medium supplemented with 20% FBS.
Flow cytometric analysis
Two‐color staining procedures were performed on freshly obtained PB or LN cells; red blood cells were destroyed using FACS‐brand lysing solution (Becton‐Dickinson, San Jose, CA, USA). Cryopreserved mononuclear cells were thawed in new calf serum (Life Technologies, Miami, FL, USA). Thawed PB and LN cells were treated with Dnase I (0.25 mg/mL), MgSO4 and heparin (125 U/mL); cells were washed prior to staining. Cell staining was performed using the following monoclonal antibodies (mAb): anti‐CD2 (T11) fluorescein isothiocyanate (FITC)/anti‐CD19 (B4) phycoerythrin (PE), anti‐CD3 FITC (CD3), anti‐CD8 PE (T8), anti‐CD4 FITC (T4), anti‐CD25 PE (CD25), anti‐CD14 FITC (CD14), anti‐CD5 PE (T1), anti‐CD30 FITC (CD30), anti‐CD22 PE (CD22), all obtained from Beckman Coulter (Miami, FL, USA); anti‐CD30 FITC was from Dako Cytomation (Glostrup, Denmark).
The staining of nucleated cells was determined by gating, based on forward scatter and side scatter (FSC/SSC) properties. Cell debris and red blood cells were excluded by FSC/SSC gating. A minimum of 50 000 events were measured using FACSCalibur apparatus (Becton‐Dickinson). Two‐color staining was analyzed using the CellQuest software package (Version 3.1; Becton‐Dickinson). The percentage of positive cells was calculated by subtracting isotype‐control histograms from the mCD30 histograms using the enhanced normalization subtraction protocol from the CellQuest software package. Mean fluorescence intensities were calculated for mCD30. Normalization was performed by calibrating the flow cytometer using Calibrate beads (Becton‐Dickinson). These assays were performed by Special Reference Laboratories (Tokyo, Japan).
Expression of mCD30 was studied by flow cytometry (FACSCalibur, Becton‐Dickinson) on cell lines incubated with an FITC‐cojugated anti‐CD30 mAb (Ber‐H83; BD Biosciences, San Jose, CA, USA) in combination with PE‐conjugated mAb against T‐cell associated antigen CD4 (RPA‐T4; eBioscience, San Diego, CA, USA).
Immunoassays for sCD30
The levels of sCD30 were determined in sera stored at −80°C by a sandwich enzyme‐linked immunosorbent assay (human sCD30 ELISA; Bender MedSystems, Vienna, Austria), following the manufacturer's instructions. Each sample was measured in duplicate by a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA, USA) and the results were reported as the average absorbance values for each set of two samples. The limit of detection for sCD30 was determined to be 0.5 U/mL.
Statistical analysis
When comparing two groups, Student's t‐test was used. All statistical analyses were carried out using spss software (Version 11.03; spss, Tokyo, Japan) and the results were considered to be significant when the P‐value was < 0.05, and highly significant when the P‐value was < 0.01.
Results
Analysis of cell lines
CD30 expression was examined in L540, Karpas 299 and MT‐2 cell lines. L540 is a T‐cell‐like HD‐derived cell line, in this instance taken from the bone marrow of a 20‐year‐old woman in the clinical stage IVB of histologically‐proven nodular sclerosing type HD.( 18 ) Karpas 299 is a CD30+ ALCL cell line, in this instance established from blast cells in PB of a 25‐year‐old man with a diagnosis of CD30+ high‐grade NHL.( 19 ) MT‐2 is an ATL cell line, established by transformation of cord blood T cells by in vitro transmission of HTLV‐1.( 20 ) All cell lines expressed moderate levels of mCD30 on the cell surface (Fig. 1a) and sCD30 in culture supernatant (Fig. 1b).
Figure 1.
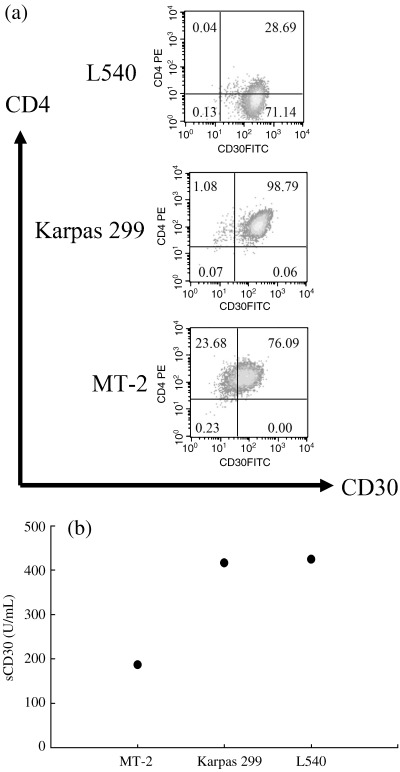
CD30 expressing in cell lines. Flow cytometric analysis with anti‐CD4 and anti‐CD30 antibodies and enzyme‐linked immunosorbent assay of culture supernatant were performed using CD30 expressing cell lines. All cell lines express both mCD30 (a) and sCD30 (b). L‐540, Hodgkin and Reed‐Sternberg cell line; Karpas 299, anaplastic large cell lymphoma cell line; MT‐2, human T‐cell leukemia virus type 1‐infected cell line.
mCD30 expression in fresh ATL cells
Table 2 shows the results of flow cytometric analysis including PB from five patients with acute type, LN cells from four patients and spleen cells from one patient (Patient 2). The results confirmed the cell surface phenotype of ATL cells that were reported previously,( 21 ) that is CD2+CD3+CD4+CD8−CD25+, except for the LN cells of patient 10. The results showed a low percentage of CD4+ cells (24.8%) with a low percentage of CD8+ cells (35.0%). Considering the results of chromosomal analysis and Southern blot analysis (data not shown), the LN cells of patient 10 are considered to be composed of two clones with surface phenotypes of CD4+CD8+ and CD4−CD8−. As shown in Table 2, significant percentages of cells were shown to be positive for mCD30. As the majority of cells analyzed showed T cell surface phenotype, the cells expressing mCD30 were considered to be ATL cells.
Table 2.
Results of flow cytometric analysis in 10 samples in nine patients with adult T‐cell leukemia/lymphoma
| Patients | Subtype | CD2 (%) | CD3 (%) | CD4 (%) | CD5 (%) | CD8 (%) | CD25 (%) | CD30 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Acute (PB) | 93.1 | 89.3 | 90.1 | 93.1 | 4.5 | 88.7 | 74.1 |
| 2 | Acute (PB) | 98.3 | 79.3 | 85.1 | 70.6 | 6.4 | 45.3 | 7.2 |
| 2 | Acute (spleen) | 100.0 | 96.0 | 99.3 | 57.3 | 1.6 | 94.7 | 27.2 |
| 3 | Acute (PB) | 84.3 | 88.7 | 96.0 | 89.8 | 6.0 | 68.4 | 27.2 |
| 4 | Acute (LN) | 94.7 | 20.2 | 52.5 | 12.9 | 24.0 | 28.4 | 10.1 |
| 5 | Acute (PB) | 93.9 | 69.7 | 84.8 | 14.5 | 3.3 | 66.5 | 33.3 |
| 6 | Acute (PB) | 92.3 | 60.6 | 89.3 | 76.6 | 5.5 | 82.9 | 16.9 |
| 8 | Lymphoma (LN) | 100.0 | 100.0 | 99.8 | 86.9 | 40.2 | 98.0 | 11.4 |
| 9 | Lymphoma (LN) | 95.0 | 91.9 | 93.8 | 91.7 | 90.0 | 84.7 | 19.6 |
| 10 | Lymphoma (LN) | 90.3 | 91.1 | 24.8 | 98.7 | 35.0 | 86.6 | 45.4 |
LN, lymph node; PB, peripheral blood.
Relationship between sCD30 levels and clinical state of ATL
Next, we measured the serum levels of sCD30 using available sera from five acute type and two lymphoma‐type ATL patients (Table 1), in addition to three acute type patients, one lymphoma‐type and one chronic type ATL patient, two HTLV‐1 carriers, two B cell malignancy patients and 21 healthy donors (Fig. 2). Among them, serum levels of sCD30 on admission and after chemotherapy relapse and clinical complete remission (CR) were connected by a solid line in three patients with acute type ATL and one patient with lymphoma‐type ATL. The highest serum sCD30 levels were observed in patients with acute type ATL. The levels in acute ATL patients before treatment and after relapse of patient 7 (mean ± standard error; 545.2 ± 18.6 U/mL) were significantly higher than those of healthy donors (38.1 ± 2.0 U/mL, P < 0.01) and four post‐treatment patients with CR, except patient 5 (68.63 ± 9.98 U/mL, P < 0.01). CR is established when measurable diseases including abnormal cells in the PB have disappeared completely.( 22 ) In patients with lymphoma‐type ATL, serum levels (327.62 ± 94.85 U/mL) were also lower at the point of clinical CR (80.92 U/mL). Furthermore, levels in the pretreatment patients with B cell malignancy, as well as asymptomatic HTLV‐1 carriers, were much lower than in pretreatment ATL patients. Although serum sCD30 levels in post‐treatment patients were higher than that of healthy donors, this was probably due to minimum residual disease and HTLV‐1‐infected non‐tumor cells in patients. Thus, the serum level of sCD30 is associated with the development of ATL.
Figure 2.
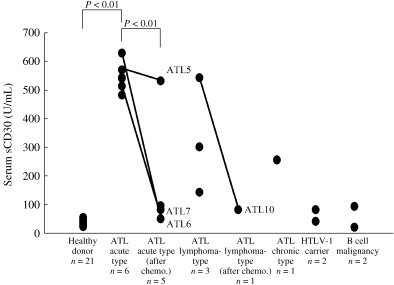
sCD30 levels in sera of adult T‐cell leukemia/lymphoma (ATL) patients. Serum levels of sCD30 were plotted in patients with ATL and B cell malignancy, human T‐cell leukemia virus type 1 carriers and healthy donors. The pre‐ and post‐treatment sCD30 concentrations in patients with ATL were correlated with the clinical stage of disease. In patients 5 and 6, serum sCD30 levels of pre‐ and post‐treated states are connected by a solid line. The terms were 93 days in patient 5 and 100 days in patient 6. In patients 7 and 10, the levels after and before relapse are also lined. The terms were 134 days in patient 7 and 189 days in patient 10.
Clinical disease progression
To demonstrate whether the serum level of sCD30 can be useful as a clinical marker of disease progression, we studied the clinical features of three patients with acute type and one patient with lymphoma‐type ATL in terms of the chronological changes in their serum sCD30 levels, as well as other markers that indicate the tumor burden, lactate dehydrogenase (LDH) and soluble interleukin‐2 receptor (sIL‐2R), along with the number of ATL cells (Fig. 3). The patients were treated with three kinds of regimens, CHOP, CHOP‐V‐MMV and THP‐COP (cyclophosphamide, adriamycin, vincristine, prednisolone, etoposide, ranimustine, mitoxantrone, vindesine, pinorubicin and granulocyte colony‐stimulating factor).( 22 ) In the case of patient 5, who showed resistance to chemotherapy and died of ATL, serum sCD30 levels remained high although serum levels of LDH and sIL‐2R and the number of ATL cells in PB decreased after chemotherapy (Fig. 3a). On the other hand, in patient 6, who showed a good response to chemotherapy and achieved complete remission, serum levels of sCD30, LDH and sIL‐2R decreased and normalized in response to chemotherapy (Fig. 3b). Furthermore, the results shown in Fig. 3c describe the levels of these markers before and after relapse in patient 7, who died of ATL. Gradual elevation of serum levels of sCD30 was observed before disease relapse was clinically evident. The high serum levels of sCD30 remained after relapse, whereas serum levels of LDH and sIL‐2R and the number of ATL cells showed prompt decrease after chemotherapy. In addition, with regard to patient 10, who relapsed and died of ATL, serum levels of sCD30 as well as sIL‐2R increased according to the clinical relapse (Fig. 3d). Considering that patients 5, 7 and 10 showed resistance to all modalities of chemotherapy and that patient 6, with complete remission, kept a low level of serum sCD30, it may be suggested that maintenance of high serum sCD30 levels even after therapy is associated with resistance to chemotherapy.
Figure 3.
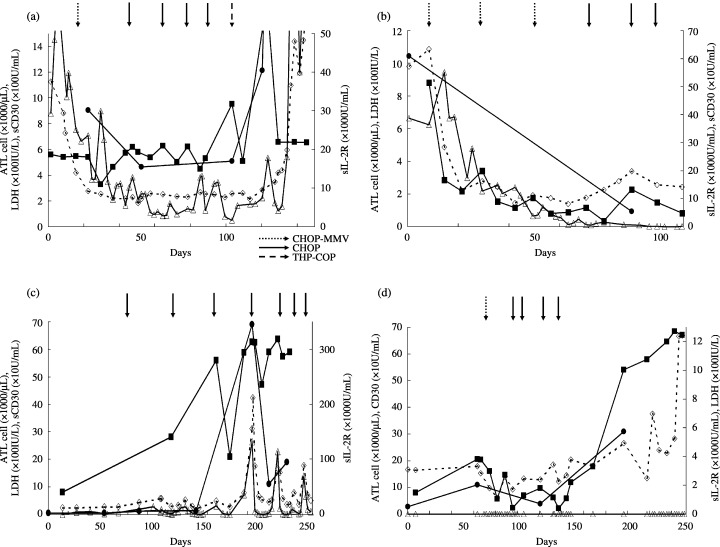
The association between serum sCD30 levels and clinical disease progression. Longitudinal monitoring of serum sCD30 concentrations was performed in three patients with acute type and one patient with lymphoma‐type adult T‐cell leukemia/lymphoma (ATL). Serum sCD30(▪),sIL‐2R(•) and lactate dehydrogenase(◊) and the number of ATL cells(▵) in peripheral blood of ATL patients were examined. Arrows indicate the chemotherapy regimens, CHOP‐MMV, CHOP and THP‐COP, respectively. (a) Patient 5 with therapy resistance showed high levels of sCD30 even after chemotherapy. (b) In patient 6, who responded well to therapy, sCD30 decreased following effective chemotherapy. (c) In patient 7, the level of sCD30 elevated before a relapse. After relapse, patient 7, who was resistant to therapy, showed high levels of sCD30. (d) In patient 10, who relapsed with lymphoma‐type ATL, sCD30 increased according to the clinical relapse.
Discussion
We found the level of serum sCD30 correlated with the progression and activity of disease in this study (Fig. 2). As most ATL patients either fail to reach a complete remission or subsequently relapse, sCD30 is thought to be useful for prediction of ATL development and monitoring the patients as a marker of the responsiveness to therapy.
Serum levels of sCD30 in patient 6 positively correlated with other serum markers of disease aggressiveness, such as sIL‐2R and LDH,( 23 , 24 ) and the number of ATL cells in the PB (Fig. 3b). However, in patient 5, who showed therapy resistance, sCD30 levels remained high and correlated with the progression and activity of disease, but not with levels of LDH and the number of ATL cells in the PB (Fig. 3a). The results suggested that sCD30 levels may be useful as a marker of disease aggressiveness in ATL as well as serum levels of sIL‐2R and LDH, or more useful than other serum markers, and may help to assess the status of ATL patients and predict the relapse and/or progression of ATL. From the relationship between elevation of serum sCD30 levels and clinical stage, it may be possible to identify patients with a very high or very low risk of treatment failure with standard therapy.( 16 , 17 )
Whereas recombinant CD30L enhanced proliferation of ATL cell lines,( 13 ) it was also reported that sCD30 binds to CD30L with high affinity, decreases the availability of CD30L on lymphocytes and blocks the apoptosis of CD30+ tumors.( 15 , 25 ) Because sCD30 levels became higher with the increasing number of ATL cells (Fig. 3b,c), sCD30 might protect ATL cells from apoptosis as shown in our cases. In addition, it has been reported that mCD30 might play an important role in the suppression of the immune system including antitumor surveillance.( 14 ) Thus, it could be possible that sCD30 bound to CD30L, prevented CD30–CD30L interaction and inhibited the proliferation of activated T cells and the cell death of CD30+ ATL cells.
Mori N et al. reported that constitutive activation of NF‐κB occurs through a Tax‐independent mechanism in primary ATL cells.( 26 ) CD30 contains TNFR‐associated factor (TRAF) domains in its cytoplasmic portion, therefore it is possible that they interact with TRAF‐1, ‐2, ‐3 and ‐5, through which CD30 mediates NF‐κB activation in ATL cells.( 9 ) This may also explain how CD30 activation suppresses the apoptotic potential of chemotherapeutic agents and contributes to resistance.
MT‐2 harbors about 10 copies of HTLV‐1 provirus and constitutively expresses viral genes. It cannot be a model of in vivo transformed ATL cells, which usually harbor a single copy of HTLV‐1 provirus that is heavily methylated or deleted. However, our results indicate that such virus‐producing cell lines may be useful for the characterization of CD30 expressing CD4+ cells in PBMC and LN cells from ATL patients. As MT‐2 expresses both mCD30 and sCD30, further investigation of MT‐2 would be useful to elicit how CD30+ cells interact with CD30L+ cells and why a certain number of CD30+ ATL cells are protected from activated T cells through high levels of sCD30 expression in vivo.
In conclusion, we identified a certain number of CD4+CD30+ cells among ATL cells in vivo and their sCD30 production correlated with the aggressiveness of ATL. Thus, sCD30 expression may be associated with the proliferation and survival of ATL cells. Further study is required to elicit the characteristics of CD4+CD30+ATL cells, probable regulatory T cells,( 27 ) and they may be a possible target of new ATL therapy.
Acknowledgments
We thank A. Ichikawa and E. Oohara for the preparation of sera from patients and I. Miyoshi and K. Yamaguchi for their critical reading. This work was supported by the Fund for Academic Research from Kochi University, and in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Takatsuki K, Uchiyama T, Sagawa K, Yodoi J. Adult T‐cell leukemia in Japan. In: Seno S, Takaku F, Irino S, eds. Topics in Hematology, Proceedings of the 16th International Congress of Hematology. Amsterdam, The Netherlands: Excerpta Medica, 1977; 73–7. [Google Scholar]
- 2. Uchiyama T, Yodoi J, Segawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. [PubMed] [Google Scholar]
- 3. Shimoyama M, Members of The Lymphoma Study Group ( 1984– 87). Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukemia‐lymphoma. Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 4. Takemoto S, Matsuoka M, Yamaguchi K, Takatsuki K. A novel diagnostic method of adult T‐cell leukemia: Monoclonal integration of human T‐cell lymphotropic virus type I provirus DNA detected by inverse polymerase chain reaction. Blood 1994; 84: 3080–5. [PubMed] [Google Scholar]
- 5. Takemoto S, Mulloy JC, Cereseto A et al. Proliferation of adult T‐cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA 1997; 94: 13897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takemoto S, Trovato R, Cereseto A et al. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14ARF‐MDM2 loop in ex vivo and cultured adult T‐cell leukemia/lymphoma cells. Blood 2000; 95: 3939–44. [PubMed] [Google Scholar]
- 7. Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol 1998; 10: 457–70. [DOI] [PubMed] [Google Scholar]
- 8. Josimovic‐Alasevic O, Durkop H, Schwarting R, Backe E, Stein H, Diamantstein T. Ki‐1 (CD30) antigen is released by Ki‐1‐positive tumor cells in vitro and in vivo I. Partial characterization of soluble Ki‐1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme‐linked immunosorbent assay. Eur J Immunol 1989; 19: 157–62. [DOI] [PubMed] [Google Scholar]
- 9. Takeshita M, Akamatsu M, Ohshima K et al. CD30 (Ki‐1) expression in adult T‐cell leukaemia/lymphoma is associated with distinctive immunohistological and clinical characteristics. Histopathology 1995; 26: 539–46. [DOI] [PubMed] [Google Scholar]
- 10. Amakawa R, Hakem A, Kundig TM et al. Impaired negative selection of T‐cells in Hodgkin's disease antigen CD30‐deficient mice. Cell 1996; 84: 551–62. [DOI] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Pohl C, Berenbeck C et al. Detection of a soluble form of the CD30 antigen in sera of patients with lymphoma, adult T‐cell leukemia and infectious mononucleosis. Int J Cancer 1990; 45: 869–74. [DOI] [PubMed] [Google Scholar]
- 12. Smith CA, Gruss HJ, Davis T et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 1993; 73: 1349–60. [DOI] [PubMed] [Google Scholar]
- 13. Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30‐expressing cells and lymphoma cell lines. Blood 1994; 83: 2045–56. [PubMed] [Google Scholar]
- 14. Su CC, Chiu HH, Chang CC, Chen JC, Hsu SM. CD30 is involved in inhibition of T‐cell proliferation by Hodgkin's Reed‐Sternberg cells. Cancer Res 2004; 64: 2148–52. [DOI] [PubMed] [Google Scholar]
- 15. Younes A, Con Soli U, Snell V et al. CD30 ligand in lymphoma patients with CD30+ tumors. J Clin Oncol 1997; 15: 3355–62. [DOI] [PubMed] [Google Scholar]
- 16. Nadali G, Tavecchia L, Zanolin E et al. Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin's disease at high risk of unfavorable outcome. Blood 1998; 91: 3011–6. [PubMed] [Google Scholar]
- 17. Zinzani PL, Poleri S, Bendandi M et al. Clinical implications of serum levels of soluble CD30 in 70 adults anaplastic large‐cell lymphoma patients. J Clin Oncol 1998; 16: 1532–7. [DOI] [PubMed] [Google Scholar]
- 18. Diehi V, Kirchner H, Fonatsch C, Stein H, Gerdes F, Boie C. Hodgkin's disease: Establishment and characterization of four in vitro cell lines. J Cancer Res Clin Oncol 1981; 101: 111–24. [DOI] [PubMed] [Google Scholar]
- 19. Fischer P, Nacheva E, Mason DY et al. A Ki‐1 (CD30)‐positive human cell line (Karpas 299) established from a high‐grade non‐Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T‐cell receptor β‐chain gene. Blood 1988; 72: 234–40. [PubMed] [Google Scholar]
- 20. Miyoshi I, Taguchi H, Kubonishi S et al. Type C virus‐producing cell lines derived from adult T‐cell leukemia. Gann Monogr Cancer Res 1982; 28: 219–28. [Google Scholar]
- 21. Hattori T, Uchiyama T, Toibana T, Takatsuki K, Uchino H. Surface phenotype of Japanese adult T‐cell leukemia cells characterized by monoclonal antibodies. Blood 1981; 58: 645–7. [PubMed] [Google Scholar]
- 22. Taguchi H, Kinoshita KI, Takatsuki K et al. An intensive chemotherapy of adult T‐cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, ranimustine, and mitoxantrone with granulocyte colony‐stimulating factor support. J Acquir Immun Defic Syndr Hum Retrovirol 1996; 12: 182–6. [DOI] [PubMed] [Google Scholar]
- 23. Yasuda N, Lai PK, Ip SH et al. Soluble interleukin 2 receptors in sera of Japanese patients with adult T‐cell leukemia mark activity of disease. Blood 1988; 71: 1021–6. [PubMed] [Google Scholar]
- 24. Yamaguchi K, Nishimura H, Kawano F et al. A proposal for smoldering adult T‐cell leukemia – Diversity in clinical pictures of adult T‐cell leukemia. Jpn J Clin Oncol 1983; 13: 189–99. [PubMed] [Google Scholar]
- 25. Hargreaves PG, Al‐Shamkhani A. Soluble CD30 binds to CD153 with high affinity and blocks transmembrane signaling by CD30. Eur J Immunol 2002; 32: 163–73. [DOI] [PubMed] [Google Scholar]
- 26. Mori N, Fujii M, Ikeda S et al. Constitutive activation of NF‐κB in primary adult T‐cell leukemia cells. Blood 1999; 93: 2360–8. [PubMed] [Google Scholar]
- 27. Dai Z, Li Q, Wang Y et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30‐dependent mechanism. J Clin Invest 2004; 113: 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


