Abstract
In spite of the clinical importance of prostate cancer (PCa) bone metastasis, the precise mechanisms for the directed migration of malignant cells remain unclear. In the present study, the expression of CXCR6 in human PCa and benign prostatic hyperplasia samples, and the expression of CXCL16 in human osseous tissues were determined by immunohistochemistry. It was found that the level of CXCR6 protein expression was elevated in human malignant prostate tumors, and CXCL16 was expressed positively by human osteocytes in vivo. The in vitro experiments further confirmed that the PCa cell lines PC3 and LNCap expressed CXCR6 at both the mRNA and protein levels, and exogenous CXCL16 has the potential to stimulate the invasion of PC3 and LNCap. To further elucidate the role of the CXCL16–CXCR6 axis in PCa progression, we compared the expression of CXCR6 and CXCR4 in human PCa tissues and the effects of CXCL16 and CXCL12 on the in vitro invasion of PC3 and LNCap cells. It was shown that CXCR6 and CXCR4 proteins were coexpressed and elevated in human PCa samples, and CXCL16 and CXCL12 promoted the invasion of PC3 and LNCap via their respective receptors. Furthermore, in contrast to CXCL12, which enhanced the activity of matrix metalloproteinase (MMP) 9 and MMP2 in PC3 and LNCap, CXCL16 ligation resulted in stronger MMP9 and MMP2 activity in LNCap but not in PC3. Our results suggest that besides CXCL12/CXCR4, CXCL16/CXCR6 might be another important factor involved in PCa bone metastasis. (Cancer Sci 2008; 99: 1362–1369)
Abbreviations:
- BPH
benign prostatic hyperplasia
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- MMP
matrix metalloproteinase
- PBS
phosphate‐buffered saline
- PCa
prostate cancer
- PCR
polymerase chain reaction
- SDS
sodium dodecyl sulfate
Prostate cancer is a common neoplasm and the second leading cause of cancer death in American men, and its morbidity has also increased in recent years in China.( 1 , 2 ) Despite advances in early diagnosis and therapeutics of PCa, metastasis to bone is one of the most severe complications and major causes of mortality of PCa.( 3 , 4 ) Many factors have been implicated in the process of metastasis, but the precise mechanisms for the directed migration and invasion of malignant cells into selective organs at both the cellular and molecular levels remain unclear.
Recent studies indicate that tumor cell migration and metastasis are not random processes; rather, chemokines and chemokine receptors may play important roles in determining the metastatic destination of tumor cells.( 5 , 6 ) For PCa, most investigations focused on the CXCL12–CXCR4 signaling pathway;( 7 , 8 , 9 ) however, little is known about the relationship between PCa specific metastasis and other chemokines or chemokine receptors.
CXCR6, initially described under the names Bonzo, STRL33 and TYMSTR, is a newly characterized chemokine receptor that until now was described to be expressed selectively by subsets of memory/effector T cells,( 10 ) NK cells,( 11 ) NK T cells,( 12 ) and plasma cells.( 13 ) CXCL16, the sole ligand of CXCR6, is a unique CXC chemokine that exists both in a transmembrane form and a soluble form.( 14 , 15 ) The interaction between CXCL16 and CXCR6 has been shown to mediate multiple biological activities, including selective trafficking of lymphocyte subsets, cell adhesion, cell survival, chronic inflammation, and antitumor immunity.( 16 , 17 , 18 , 19 , 20 ) In particular, human bone marrow plasma cells express CXCR6 selectively, and tissues known to be enriched with plasma cells as well as cultured human bone marrow stromal cells express CXCL16 constitutively,( 13 ) implying the importance of the CXCL16–CXCR6 axis in efficient recruitment to target tissues. Furthermore, it has been found that first‐trimester human cytotrophoblasts coexpress CXCL16 and CXCR6 as well as secreted CXCL16, which induces their invasion in an autocrine manner.( 18 ) The metastasis of circulating prostate carcinoma cells has similarities with not only the homing behavior of hematopoietic cells to the bone marrow, but also the invading process of first‐trimester human trophoblasts into the decidua. Thus, it is speculated that CXCL16–CXCR6 may be another chemokine ligand–receptor pair that mediates migration and invasion of prostate carcinoma cells to bone.
In the present study, the expression of CXCL16–CXCR6 in human PCa and BPH tissues and the expression of CXCL16 in human osseous tissues were determined by immunohistochemistry. The PCa cell lines PC3 and LNCap cells were used to investigate the effects of CXCL16 on the invasion of carcinoma cells in vitro. To further elucidate the role of the CXCL16–CXCR6 axis in PCa progression, we compared the expression of CXCR6 and CXCR4 in human PCa tissues and the effects of CXCL16 and CXCL12 on invasion of PC3 and LNCap cells. Finally, the gelatin zamography assay was conducted to test whether MMP9 and MMP2, which have been shown to be critical determinants for PCa invasion and metastasis,( 21 , 22 , 23 ) were the downstream effectors modulated by the CXCL16–CXCR6 signaling pathway. By elucidating the underlying biology of PCa progression, we hope to provide insights into better understanding and clinical management of this aggressive malignancy.
Materials and Methods
Human tissue collection. All procedures involving participants in the study were approved by the Zhongnan Hospital of Wuhan University Human Research Ethics Committee, and all subjects gave informed consent to collect tissue samples.
Human PCa tissues were obtained from 18 patients who underwent radical retropubic prostatectomy (eight samples) or prostate puncture (10 samples) at Zhongnan Hospital of Wuhan University from 2003 to 2006. The age, serum prostate‐specific antigen level, and prostate volume of PCa patients were 62.4 ± 4.42 years (55–70 years), 12.7 ± 5.08 ng/mL (5.8–23 ng/mL), and 47.4 ± 3.55 mL (40.4–52.5 mL), respectively. According to clinical stage, seven patients were T1 and 11 patients were T2. Tumors were graded using the Gleason grading system and examined to identify areas of PCa. In all of the tissues examined, there were nine samples classified as score 8–10, five samples as score 7, and four samples as score 2–6. BPH samples were collected from 10 patients who received transurethral resection of the prostate (five samples) or prostate puncture (five samples). Five patients who underwent bone surgery for non‐cancer reasons were recruited for the collection of bone tissues, including two cranial bone samples, two vertebrate samples, and one os longum sample. Placental tissues (five samples) were from elective terminations of first‐trimester pregnancies (gestational age 6–9 weeks) for non‐medical reasons.
Cell culture. PC3 and LNCap cells were obtained from the Cell Bank of Chinese Academy of Science (Shanghai, China). PCa cell lines were cultured in DMEM‐F12 containing 10% heat‐inactivated FBS (Gibco, Grand Island, NY, USA), 100 IU/mL penicillin, and 100 µg/mL streptomycin. The cells were recovered and passaged three times, then allowed to grow to confluence for the following experiments.
Human first‐trimester trophoblast VCT cells were used as a positive control in this study, and were isolated from first‐trimester placentas as described previously.( 24 ) The isolated VCT were seeded on dishes precoated with Matrigel (BD Biosciences, Bedford, MA, USA), and cultured in DMEM‐F12 supplied with 10% heat‐inactivated FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin, then incubated for 24 h before use.
Immunohistochemistry. The tissues were fixed routinely with formalin and embedded in paraffin. Antigen retrieval was carried out and 0.3% H2O2 in PBS was used to block endogenous peroxidase activity in the sections. After treatment with protein blocking solution containing 7% horse serum to block non‐specific binding, the sections were incubated overnight at 4°C with mouse antihuman CXCR4 (25 µg/mL), mouse antihuman CXCR6 (25 µg/mL), mouse antihuman CXCL12 (10 µg/mL), and goat antihuman CXCL16 (10 µg/mL) antibodies (all from R & D Systems, Abingdon, UK). A streptavidin–biotin detection reagent kit (Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) with 3,30‐diaminobenzidine tetrahydrochloride was used for signal detection and Harris hematoxylin was used as a counterstain. Mouse or goat isotype IgG (20 µg/mL) (Sino‐America, Shanghai, China) was used as a negative control and human first‐trimester villous tissues was used as a positive control. Immunostaining results were evaluated by two independent pathologists. The immunostaining intensity was observed and scored as no staining (score = 0), light yellow (score = 1), light brown (score = 2), or brown (score = 3). The percentage of positive cells was calculated as <5% (score = 0), 5–25% (score = 1), 26–50% (score = 2), 51–75% (score = 3), or >75% (4 score = ). Combining the immunostaining intensity score and the percentage of positive cells, we classified the scoring as follows: <2, negative expression; 2–3, weak expression; 4–5, moderate expression, and 6–7 as strong expression. Scoring was carried out blindly using a telepathology system without knowledge of overall Gleason score (e.g. tumor grade), tumor size, or clinical outcome.( 7 , 25 ) To facilitate statistical analysis of the protein expression between human PCa and BPH tissues, we assigned new values 1, 2, 3, and 4 to the negative, weak, moderate, and strong expression, respectively.
Reverse transcription–polymerase chain reaction. PC3 and LNCap cells were seeded at a density of 1 × 106 cells/mL in 50‐mL cell culture bottles. After 70–80% cell confluence was reached, total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and the total RNA from primary cultured human first‐trimester trophoblast cells was used as a positive control.( 26 ) Total RNA (2 µg) was used for first‐strand complementary DNA (cDNA) synthesis in a 20‐µL reaction volume with 200 U Moloney murine leukemia virus reverse transcriptase. Then cDNA (10 µL) was amplified by PCR in a final volume of 50 µL containing 2 mM dNTP, 0.8 µmol/L specific primers, 1.25 u Taq DNA polymerase, 1 mmol/L MgCl2 and 1× reaction buffer. In 5 min precycle at 95°C, the reaction was followed by 35 cycles of 1 min at 94°C, 30 s at 55°C, and 30 s at 72°C. When the final cycle was over, samples were kept at 72°C for 15 min to complete the synthesis. The primer pairs for cDNA amplification were as follows: 5′‐GAA CTT CCT ATG CAA GGC AGT CC‐3′ (forward) and 5′‐CCA TGA TGT GCT GAA ACT GGA AC‐3′ (reverse) for human CXCR4; 5′‐ATG GCA ATG TCT TTA ATC TCG ACA A‐3′ (forward) and 5′‐TGA AAG CTG GTC ATG GCA TAG TAT T‐3′ (reverse) for human CXCR6; and 5′‐GGG GAG CCA AAA GGG TCA TCA TCT‐3′ (forward) and 5′‐GAG GGG CCA TCC ACA GTC TTC T‐3′ (reverse) for human GAPDH.( 18 , 26 ) The expected fragment lengths of CXCR4, CXCR6, and GAPDH were 302, 295, and 235 bp, respectively. The PCR products (10 µL) were electrophoresed on 2% agarose gels and ethidium bromide‐stained bands were photographed and analyzed by gel‐imaging systems. The relative intensity of chemokine receptor = absorbance value of the receptor/absorbance of GAPDH. The experiments were carried out in triplicate and repeated three times.
Immunocytochemistry. At 70–80% cell confluence, PC3 and LNCap cells were digested with 0.25% trypsin (Bio Basic, Ontario, Canada) containing 0.1% ethylenediaminetetraacetic acid and seeded at a density of 2 × 105 cells/well in 24‐well plates preplaced with coverslips. The coverslips for LNCap were precoated with Matrigel. After 48 h of culture, PCa cell lines were fixed in 4% formalin for 20 min at room temperature, washed in PBS, and permeabilized for 15 min in 0.03% Triton X‐100‐PBS, then treated with 0.3% H2O2 to inactivate endogenous peroxidase activity. The procedure described above for immunohistochemistry was then followed. Primary cultured VCT were used as a positive control. The experiments were repeated three times.
Matrigel invasion assay. The invasion of PCa cells across Matrigel was evaluated objectively in an invasion chamber based on the previous procedure.( 27 ) Briefly, the cell culture inserts (8‐µm pore size, 6.5‐mm diameter; Corning, Corning, NY, USA) coated with 10 µL pure Matrigel were placed in a 24‐well plate. The isolated PC3 or LNCap cells (2 × 105 in 200 µL serum‐free DMEM‐F12) were plated in the upper chamber. The lower chambers were filled with 800 µL serum‐free DMEM‐F12 supplied with recombinant human CXCL16 at final concentrations of 0, 50, 100, and 200 ng/mL, CXCL12 (200 ng/mL), or a combination of CXCL12 (200 ng/mL) with CXCL16 (200 ng/mL). Before being stimulated with CXCL12 (200 ng/mL) or CXCL16 (200 ng/mL), cells in different chambers were preincubated with neutralizing antibodies to CXCL12 (50 µg/mL), CXCL16 (30 µg/mL), or CXCR4 (20 µg/mL). The cells were then incubated at 37°C for 48 h. The inserts were removed, washed in PBS, and the non‐invading cells together with the Matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 min at room temperature, and stained with hematoxylin. The results were observed under a light microscope (Olympus, Tokyo, Japan) and cells that had migrated to the lower surface were counted. Each experiment was carried out in triplicate, and repeated four times.
Gelatin zymography. The culture medium in the upper chamber in the invasion assay was harvested and the proteolytic activity of both MMP9 and MMP2 was measured by the technique of gelatin zymography as described by Chinni et al., with slight modification.( 28 ) Briefly, collected culture supernatants containing equal amounts of total protein were mixed with SDS loading buffer, loaded into each lane, and electrophoresed on 10% SDS‐polyacrylamide gels copolymerized with 0.2% gelatin. After electrophoresis, the gel was rinsed in 2.5% Triton‐X 100 for 1 h to remove SDS. The gel was then incubated for 18 h at 37°C in 50 mmol/L Tris‐HCl (pH 7.5), 200 mmol/L NaCl, 10 mmol/L CaCl2, and stained with 2.5% Coomassie blue R250 (Sigma Chemical, St Louis, MO, USA) dissolved in 40% (v/v) methanol and 10% acetic acid. The gels were then rinsed sequentially in three different decolorant solutions (A, 30% methanol, 10% acetic acid; B, 20% methanol, 10% acetic acid; C, 10% methanol, 5% acetic acid). The gel was photographed and assayed using the HPIAS‐1000 high‐intensity image analysis system (Championimage, Wuhan, China). The gelatinolytic activity was visualized as a clear white band against a dark background of stained gelatin. The proteolytic activity of MMP was equal to the intensity of MMP9 or MMP2 of the treatment group over that of the control. The experiments were carried out in triplicate, and repeated four times.
Statistics. Differences in CXCR4 or CXCR6 potein expression between PCa and BPH were evaluated statistically using Pearson's χ2‐test. The in vitro experiments are shown as the mean ± SE. Differences in the mRNA levels of chemokine receptors were evaluated using Student's t‐test, and the invasion assay and zymography data were assessed using the post hoc Dunnett's t‐test and Tambane's T2 test, when appropriate. The differences were accepted as significant at P < 0.05.
Results
Protein expression of CXCL16–CXCR6 in human PCa and BPH in vivo. To verify our hypothesis that CXCL16–CXCR6 is involved in PCa metastasis, we first determined the protein expression of CXCR6 in human PCa (18 samples) and BPH (10 samples) obtained from Zhongnan Hospital of Wuhan University from 2003 to 2006. The results revealed moderate to strong brown‐colored staining for CXCR6 in the cytoplasm and membrane of human native PCa cells (Fig. 1a). The expression of CXCR6 protein was also observed in the hyperplasic epithelia, but the staining for CXCR6 was weaker in human BPH (Fig. 1g). Quantitative analysis of CXCR6 expression in human PCa and BPH tissues was then carried out by scoring protein expression as negative (score = 1), weak (score = 2), moderate (score = 3), or strong (score = 4). Differences in CXCR6 protein expression were evaluated statistically using Pearson χ2‐test. As shown in Figure 2, the protein level of CXCR6 in malignant prostate epithelia was significantly higher than in hyperplasic epithelia (Fig. 2).
Figure 1.
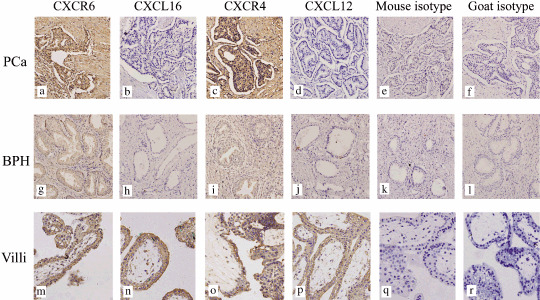
The expression of CXCL16–CXCR6 and CXCL12–CXCR4 in human prostate cancer (PCa) and benign prostatic hyperplasia (BPH). (a,c) Moderate to strong, brown‐colored staining for CXCR6 and CXCR4 was recognized in the cytoplasm and cytomembrane of the human native PCa cells. (g,i) The hyperplasic epithelia of BPH was also shown to express CXCR6 and CXCR4 protein, but the staining was weak. (b,d,h,j) No obvious CXCL16 or CXCL12 protein expression was detected in either malignant epithelia or hyperplasic cells. (m,n,o,p) Specific staining for CXCR6, CXCL16, CXCR4, and CXCL12 was clearly present in the positive control (villous tissue). (e,f,k,l,q,r) No background staining was observed in the isotype control experiments. Magnification, ×200.
Figure 2.
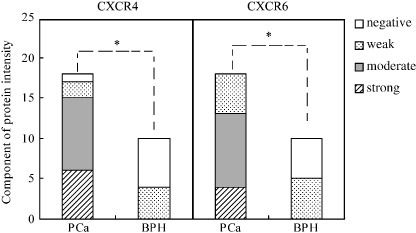
Quantitative evaluation of the protein expression of CXCR6 and CXCR4 in prostate cancer (PCa) and benign prostatic hyperplasia (BPH). Immunostaining intensity was scored as either absent (1), weak (2), moderate (3), or strong (4). Statistically significant differences in CXCR6 and CXCR4 protein expression were noted between human PCa and BPH tissues. *P < 0.01.
Previous studies have found that CXCR4 protein expression is increased in localized and metastastic PCa.( 7 ) Therefore, in the present study also determined the expression of CXCR4 and compared the protein level of CXCR6 with that of CXCR4. As shown in 1, 2, human primary PCa cells showed specific staining for CXCR4 and CXCR6 protein, and no significant difference in protein levels between CXCR6 and CXCR4 was detected in PCa tissues, but the protein expression of both CXCR6 and CXCR4 in BPH samples was at a relatively lower level than in PCa tissues. It could be concluded that CXCR6 and CXCR4 proteins are coexpressed and elevated in human PCa tissues.
In addition, the protein expression of CXCL16 (ligand for CXCR6) and CXCL12 (ligand for CXCR4) in human PCa and BPH sections was determined. The results in Figure 1 clearly show that no specific staining for CXCL16 or CXCL12 proteins was detected in either malignant or hyperplasic epithelia.
Protein expression of CXCL16 in human bone tissues in vivo. Bone is the preferred organ site for metastasis of PCa, and PCa cells adhere preferentially to human bone marrow endothelial cells compared with other types of vascular endothelial cells.( 3 , 4 , 29 ) Thus, the expression of CXCL16, the only ligand for CXCR6 in human bone tissues, was detected by immunohistochemistry. Moderate staining for CXCL16 was observed clearly in the cytoplasm and membrane of osteocytes (Fig. 3a–c). In addition, we also observed positive CXCL12 expression in human osteocytes (Fig. 3e–g). Thus, CXCL16 and CXCL12 could be coexpressed by human bone tissues.
Figure 3.
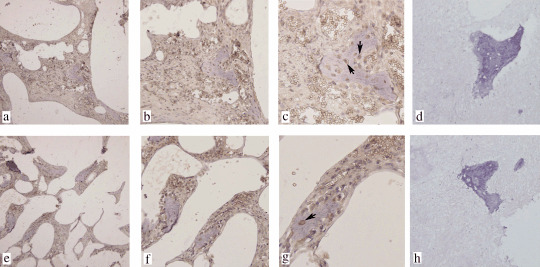
The expression of CXCL16 and CXCL12 in human bone tissues. (a–c) Specific brown‐colored staining for CXCL16 was recognized in the cytoplasm and cytomembrane of human osteocytes in bone tissue. (e–g) Furthermore, CXCL12 protein was also expressed by human osteocytes with positive staining in the cytoplasm and cytomembrane. (d) Goat and (h) mouse isotype controls. No background staining was observed in the control experiments. (c,g) Osteocytes stained positive for CXCL16 or CXCL12 are marked with arrowheads. (a,d,e,h) Magnification, ×100; (b,f) magnification, ×200; (c,g) magnification, ×400.
Semiquantitative analysis of mRNA for CXCR6 in human PCa cell lines. To validate the level of gene expression of CXCR6 in the PCa cell lines, we detected the gene expression intensities for both PC3 and LNCap by reverse transcription‐PCR and compared the relative mRNA level between CXCR4 and CXCR6 in these cells. Total RNA from human first‐trimester trophoblast cells was used as a positive control. Figure 4 shows that both PC3 and LNCap cells expressed CXCR6 mRNA and there was no detectable difference in the mRNA intensities between CXCR4 and CXCR6 in PC3, and the same phenomenon was also observed in LNCap. Furthermore, no significant difference in the mRNA level of CXCR6 was detected between PC3 and LNCap cells.
Figure 4.
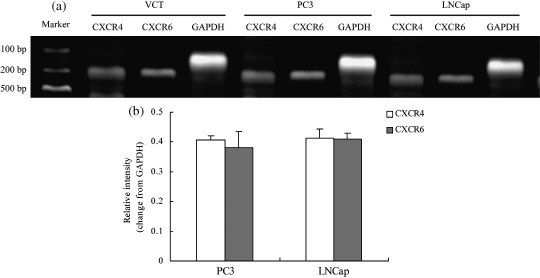
The expression of CXCR6 mRNA in the human prostate cancer (PCa) cell lines PC3 and LNCap. The 302‐bp polymerase chain reaction (PCR) product specific for CXCR4, the 295‐bp PCR product specific for CXCR6, and the 235‐bp PCR product specific for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were separated on 2% agarose gels and detected using gel imaging systems. (a) VCT human first‐trimester trophoblast cells were used as a positive control. At the RNA level, both PC3 and LNCap cells expressed CXCR6. No significant difference in the mRNA level of CXCR6 was detected between PC3 and LNCap cells. Moreover, there was no detectable difference in the mRNA intensities between CXCR4 and CXCR6 in PC3 cells. (b) The same results were observed in LNCap cells. The results were highly reproducible in three independent experiments. Error bars depict the standard error of the mean.
Protein expression of CXCR6 in human PCa cell lines. After identifying CXCR6 transcripts in the PCa cell lines, we further analyzed the CXCR6 protein expression in PC3 and LNCap cells by immunocytochemistry. The results shown in Figure 5 demonstrate that CXCR6 and CXCR4 protein were coexpressed by PC3 and LNCap cells. Specific moderate to strong brown‐colored staining for CXCR6 in the cytoplasm and cytomembrane of both PC3 and LNCap was observed clearly.
Figure 5.
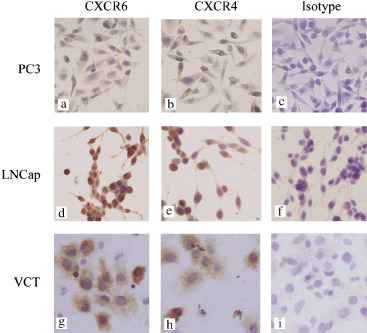
CXCR6 and CXCR4 proteins were coexpressed in both PC3 and LNCap cells in vitro. Specific brown‐colored staining for CXCR6 was recognized in the cytoplasm and cytomembrane of (a) PC3 and (d) LNCap cells. (a,b,d,e) The figures clearly show that the chemokine receptors, CXCR6 and CXCR4 protein are coexpressed in PC3 and LNCap cells. (g,h) Specific staining for CXCR6 and CXCR4 is clearly present in the VCT trophoblast cells, which were used as a positive control. (c,f,i) No background staining was observed in the isotype control experiments. Magnification, ×200.
CXCL16 induces the invasion of PCa cell lines in vitro. The Matrigel invasion assay was used to investigate whether exogenous CXCL16 has the potential to stimulate the invasion of PC3 and LNCap cells in vitro. Figure 6a shows that the different concentrations of CXCL16 substantially increased the invasion of PC3 cells in a dose‐responsive manner. The number of cells that migrated to the lower surface increased significantly in each CXCL16 treatment group (50, 100, 200 ng/mL; P < 0.01) compared to the vehicle controls, and reached a peak at a CXCL16 concentration of 200 ng/mL. Only at the concentration of 200 ng/mL CXCL16 could this chemokine remarkably promote the invasion of LNCap cells (compared to the control group; P < 0.01). CXCL16 at 200 ng/mL induced a 3.2‐fold and 2‐fold increase in PC3 and LNCap invasiveness, respectively. Therefore, to keep consistent in the experiments, the following studies were all carried out using 200 ng/mL CXCL16.
Figure 6.
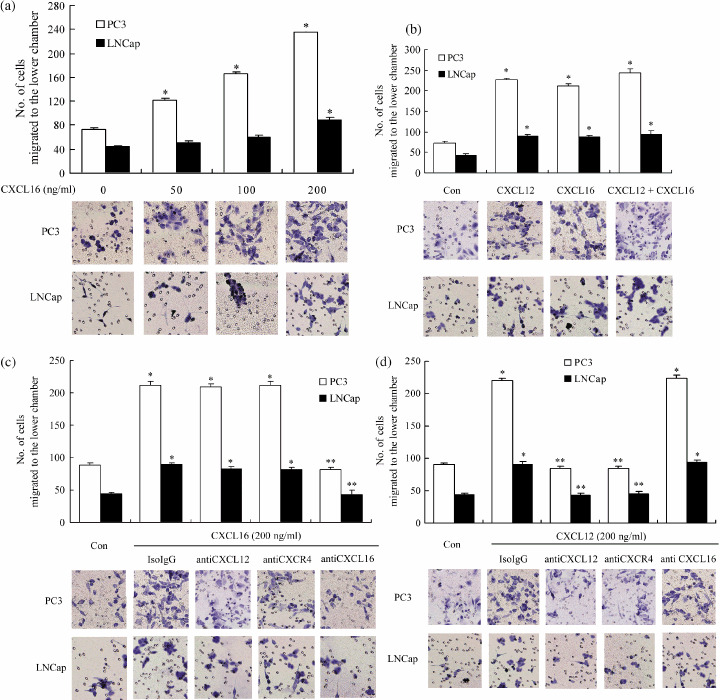
CXCL16 promoted the invasion of human PCa cell lines independent of the CXCL12–CXCR4 axis. A Matrigel invasion assay was carried out to assess the invasive ability of PC3 and LNCap cells stimulated by CXCL16 and CXCL12. The number of cells that migrated to the lower chambers was counted to represent the invasiveness of PCa cell lines and the images are representative of the experiments. (a) CXCL16 promoted the invasion of PC3 in a dose‐dependent manner and the invasion of LNCap cells was increased significantly when the concentration of CXCL16 was raised to 200 ng/mL. (b) Combined addition of CXCL16 (200 ng/mL) and CXCL12 (200 ng/mL) had a similar stimulatory effect on the invasive ability of PC3 and LNCap cells compared with either CXCL16 (200 ng/mL) or CXCL12 (200 ng/mL) treatment alone. (c) Neutralizing antibodies to CXCL12 (50 µg/mL) and CXCR4 (20 µg/mL) were unable to change the increased invasion induced by CXCL16, and (d) addition of neutralizing antibody to CXCL16 (30 µg/mL) could not change the stimulatory effect of CXCL12. (c,d) Similar results were observed in both PC3 and LNCap cells. *P < 0.01 compared to the vehicle control. **P < 0.01 compared to the CXCL6 alone or CXCL12 alone treatment groups. The results were highly reproducible in four independent experiments. Error bars depict the standard error of the mean. Magnification, ×200.
It has been confirmed that CXCL12 promotes the invasion of PCa cell lines in vitro.( 8 ) In present study, we compared the invasive effects induced by CXCL16 and CXCL12. Both PC3 and LNCaP cells migrated and invaded toward CXCL16 or CXCL12; however, treatment with a combination of CXCL16 and CXCL12 appeared to have a stimulatory effect on invasion, similar to treatment with CXCL16 or CXCL12 alone (Fig. 6b). The unavailability of neutralizing antibody to CXCR6 prevented us from confirming our results through blocking CXCR6, but as CXCR6 is the only receptor for CXCL16,( 14 , 15 ) the following experiments were conducted with CXCL16 neutralizing antibody. It was found that addition of neutralizing antibodies of CXCL16 failed to abolish the stimulatory effect of CXCL12, and meanwhile, CXCL16‐dependent invasion was blocked by treatment with CXCL16 neutralizing antibody, but not by anti‐CXCR4 or CXCL12 neutralizing antibody (Fig. 6c,d). Similar results were observed in PC3 and LNCap cells, which suggests that CXCL16 and CXCL12 stimulated the invasion of PCa cell lines in an independent extracellular way.
Effects of the CXCL16–CXCR6 axis on the proteolytic activity of MMP9 and MMP2 in PC3 and LNCap cells. The MMP are a large family of proteolytic enzymes thought to play an important role in cancer invasion and metastasis, due to their ability to degrade the extracellular matrix and basement membrane.( 30 ) Among various proteinases, MMP9 and MMP2 have been found to be highly associated with prostate cancer metastasis.( 21 , 22 , 23 ) Therefore, the conditioned medium from the upper chamber in the invasion assay was collected and the gelatinolytic activity of MMP9 and MMP2 was analyzed by gelatin zymography. As is shown in Figure 7, in contrast to CXCL12, which enhanced the activity of MMP9 and MMP2 in both PC3 and LNCap cells, CXCL16 ligation resulted in stronger MMP9 and MMP2 activity in LNCap cells but not in PC3 cells. Although both CXCL16 and CXCL12 promoted the activity of MMP9 and MMP2 in LNCap cells, no remarkable synergized effect was observed in the CXCL16 and CXCL12 combination group, compared to the CXCL16 alone or CXCL12 alone groups. The addition of neutralizing antibodies to CXCL12 and CXCR4 did not diminish the elevated activity of MMP9 and MMP2 in LNCap cells stimulated by CXCL16, and neutralizing antibody to CXCL16 failed to abolish the increased MMP activity in these cells induced by CXCL12. Furthermore, neither CXCL16 nor CXCL16 neutralizing antibodies affected the stimulatory effect of CXCL12 on the activity of MMP9 and MMP2 in PC3 cells.
Figure 7.
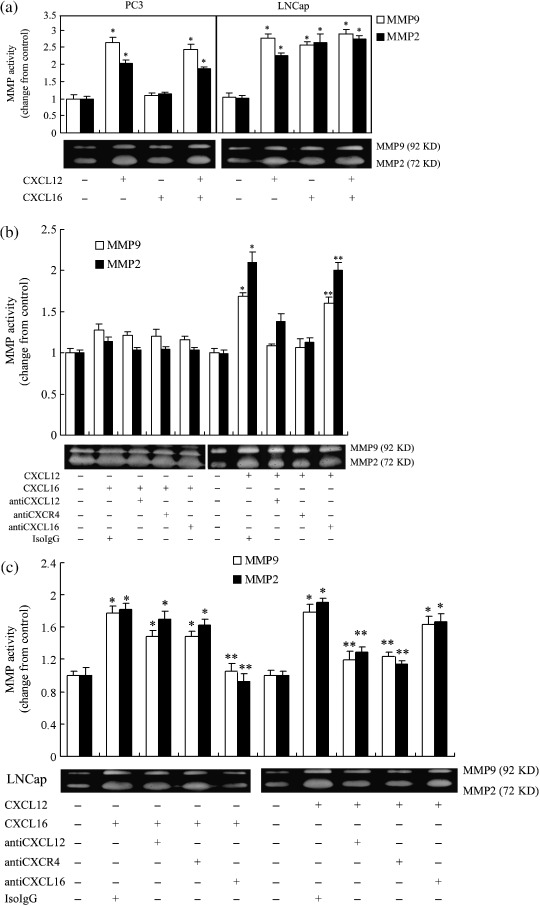
Effects of the CXCL16–CXCR6 and CXCL12–CXCR4 axis on the activity of matrix metalloproteinase (MMP) 9 and MMP2 by prostate cancer cell lines. The supernatant from the upper well in the invasion assay was collected, and the activities of MMP9 (92 kDa) and MMP2 (72 kDa) were measured by gelatin zymography. Gelatinolytic activity was visualized as a clear white band against a dark background of stained gelatin. The photographs were from a representative experiment. (a) In contrast to CXCL12, which enhanced the activity of MMP9 and MMP2 in both the PC3 and LNCap cell lines, CXCL16 ligation resulted in stronger MMP9 and MMP2 activity in LNCap cells but not in PC3 cells. (b) CXCL12 increased the activity of MMP9 and MMP2 in PC3 cells independent of the CXCL16–CXCR6 axis. (c) The addition of neutralizing antibodies to CXCL12 and CXCR4 was unable to diminish the elevated activity of MMP9 and MMP2 in LNCap cells stimulated by CXCL16, and neutralizing antibody to CXCL16 failed to abolish the increased MMP activity in these cells induced by CXCL12. *P < 0.01 compared to the vehicle control. **P < 0.01 compared to the CXCL6 alone or CXCL12 alone treatment groups. The results were highly reproducible in four independent experiments. Error bars depict the standard error of the mean.
Discussion
Most patients with PCa will die eventually from the disease. In spite of the clinical importance of tumor metastasis, much remains to be learned about the metastatic process. Here, we report for the first time that in addition to the CXCL12–CXCR4 signaling pathway, CXCL16–CXCR6, a less‐well‐characterized chemokine–receptor axis, might be another attractive candidate for mediating PCa selective metastasis to bone.
In the current investigation, the protein expression of CXCL16–CXCR6 in human PCa and BPH samples was determined by immunohistochemistry. The data demonstrated moderate to strong CXCR6 protein expression in the primary PCa cells with brown‐colored staining in the cytoplasm and membrane, and the levels of CXCR6 protein expression in the malignant epithelia were significantly higher than those in the hyperplasic epithelia. We were not able to demonstrate CXCL16 at the protein level in either PCa or BPH tissues, but we found that the CXCL16 protein was expressed by human osteocytes and endothelial cells in bone sections. CXCL16 has been confirmed to serve as a powerful chemoattractant for directed migration of CXCR6‐positive lymphocytes.( 12 , 13 , 16 ) Thus, it is reasonable to speculate that the carcinoma cells move along a chemical gradient toward some sites having a higher local concentration of CXCL16 produced by osseous tissues, which might be one of the mechanisms for selective trafficking of PCa to bone. Moreover, CXCL16 is a novel membrane‐anchored chemokine, and has been shown to potentially function not only as a chemokine but also as an adhesion molecule.( 13 , 14 , 15 , 17 ) Therefore, membrane‐anchored CXCL16 may also contribute to tissue localization of metastatic PCa cells in bone through its direct binding to CXCR6.
To further explore the biological and functional significance of CXCR6 expression in PCa cells, we conducted several in vitro experiments because of the difficulties involved in carrying out in vivo migration and invasion experiments. The results showed that both of the PCa cell lines, PC3 and LNCap, expressed CXCR6 at the mRNA and protein levels and exogenous CXCL16 had the potential to stimulate the invasion of these cells in vitro, which further confirmed our speculation that CXCL16 and its CXCR6 receptor help to mediate the bone‐specific metastasis of prostate carcinomas. The invasive capacity of PC3 cells was increased remarkably at each concentration of CXCL16 (50, 100, 200 ng/mL). However, in contrast to the PC3 cells, only at 200 ng/mL CXCL16 could this chemokine significantly promote the invasion of LNCap cells. Moreover, CXCL16 ligation resulted in stronger MMP9 and MMP2 activity in LNCap cells but not in PC3 cells. PC3 cells were originally isolated from a vertebral metastasis of a human PCa patient and belong to a metastatic phenotype of PCa cell lines, whereas LNCaP cells, a locally invasive phenotype of PCa cell lines, were harvested from the lymph node of a patient with disseminated bony and lymph node involvement. Thus, malignant cells of different types might present different sensitivities to CXCL16 stimulation and activate different signaling pathways and molecules.
The CXCL12–CXCR4 axis has been suggested to regulate the migration and invasion of PC3 and LNCap cells and might play a pivotal role in PCa metastasis.( 7 , 8 ) Therefore, in the present study we also compared the expression of CXCR6 and CXCR4 in human PCa samples as well as the effect of the CXCL16–CXCR6 and CXCL12–CXCR4 axis on PCa cell invasion. It was shown that the CXCR6 and CXCR4 proteins were coexpressed and elevated in human PCa tissues, and CXCL16 and CXCL12 were capable of inducing the invasion of PCa cell lines in vitro. Moreover, we found that CXCL16 and CXCL12 were coexpressed by human osteocytes and endothelial cells in bone tissues. Thus, metastasis of carcinoma cells to bone might be mediated via multiple chemokine–receptor pairs rather than a singular molecule. However, CXCL16 and CXCL12 seemed to promote the invasion of PCa cell lines via their respective receptors, independently of each other. Furthermore, in contrast to CXCL12, which enhanced the activity of MMP9 and MMP2 at similar levels in both PC3 and LNCap cells, CXCL16 showed different effects on PC3 and LNCap cells. In LNCap cells, although it remains unknown whether there are similar intracellular signal transduction pathways or junctions, MMP9 and MMP2 appeared to be the common downstream effectors for CXCL12–CXCR4 and CXCL16–CXCR6. However, in PC3 cells, CXCL16–CXCR6 and CXCL12–CXCR4 might facilitate the invasion through activating different signaling pathways and molecules.
Some reports have indicated that several chemokine ligands and receptors correlate with PCa metastatic behavior,( 31 , 32 , 33 , 34 ) for example, CXCR1, the receptor for interleukin‐8, and CCR2, the receptor for CCL2, correlate with PCa progression.( 33 , 34 ) In the present investigation, we found that, besides CXCL12–CXCR4, CXCL16–CXCR6 also mediated the invasion of PCa cells. Thus, there might be a complicated chemokine and chemokine receptor network through which malignant cells migrate and invade into specific target organs, and we are still a long way from elucidating the action of chemokines on tumor metastasis.
It is very difficult for us to obtain PCa metastatic bone samples and we are unable to examine CXCL16 and CXCL12 in such specimens. However, our study has demonstrated for the first time that CXCR6 protein is expressed and elevated in human native PCa cells, and CXCL16, the only ligand for CXCR6, is expressed by human osseous tissue. Furthermore, CXCL16 induced the migration and invasion of PC3 and LNCaP cells in vitro. In addition, the expression pattern of CXCL16–CXCR6 in human PCa and bone specimens is similar to that of CXCL12–CXCR4, which has been shown to participate in PCa bone metastasis. Therefore, although the mechanisms of exactly how CXCL16–CXCR6 participates and directs the invasive patterns of PCa cells deserve further investigation, our research suggests a role for CXCL16–CXCR6 in the metastasis of prostate carcinoma.
Acknowledgments
This work is partially supported by the Hubei Science and Technology Project (no. 2007AA301B42‐2) to Weidong Hu.
References
- 1. Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med 1993; 118: 793–803. [DOI] [PubMed] [Google Scholar]
- 2. Zhang W, Xiang YB, Liu ZW et al . Trends analysis of common urologic neoplasm incidence of elderly people in Shanghai, 1973–99. Ai Zheng 2004; 23: 555–8. [PubMed] [Google Scholar]
- 3. Bubendorf L, Schopfer A, Wagner U et al . Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum Pathol 2000; 31: 578–83. [DOI] [PubMed] [Google Scholar]
- 4. Jacobs SC. Spread of prostatic cancer to bone. Urology 1983; 21: 337–44. [DOI] [PubMed] [Google Scholar]
- 5. Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine–chemokine receptor network to treat cancer. Cancer 2007; 109: 2392–404. [DOI] [PubMed] [Google Scholar]
- 6. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4: 540–50. [DOI] [PubMed] [Google Scholar]
- 7. Sun YX, Wang J, Shelburne CE et al . Expression of CXCR4 and CXCL12 (SDF‐1) in human prostate cancers (PCa) in vivo . J Cell Biochem 2003; 89: 462–73. [DOI] [PubMed] [Google Scholar]
- 8. Singh S, Singh UP, Grizzle WE, Lillard Jr JW. CXCL12–CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Laboratory Invest 2004; 84: 1666–76. [DOI] [PubMed] [Google Scholar]
- 9. Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross‐talk between paracrine‐acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res 2007; 67: 4244–53. [DOI] [PubMed] [Google Scholar]
- 10. Kim CH, Kunkel EJ, Boisvert J et al . Bonzo/CXCR6 expression defines type 1‐polarized T‐cell subsets with extralymphoid tissue homing potential. J Clin Invest 2001; 107: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol 2000; 165: 3284–92. [DOI] [PubMed] [Google Scholar]
- 12. Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24+ V beta 11+ NKT cell subsets with distinct cytokine‐producing capacity. Blood 2002; 100: 11–16. [DOI] [PubMed] [Google Scholar]
- 13. Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol 2003; 170: 1136–40. [DOI] [PubMed] [Google Scholar]
- 14. Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV‐coreceptor Bonzo. Nat Immunol 2000; 1: 298–304. [DOI] [PubMed] [Google Scholar]
- 15. Wilbanks A, Zondlo SC, Murphy K et al . Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol 2001; 166: 5145–54. [DOI] [PubMed] [Google Scholar]
- 16. Hara T, Katakai T, Lee JH et al . A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion. Int Immunol 2006; 18: 301–11. [DOI] [PubMed] [Google Scholar]
- 17. Shimaoka T, Nakayama T, Fukumoto N et al . Cell surface anchored SR‐PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6‐expressing cells. J Leukoc Biol 2004; 75: 267–74. [DOI] [PubMed] [Google Scholar]
- 18. Huang Y, Zhu XY, Du MR, Wu X, Wang MY, Li DJ. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first‐trimester human trophoblast cells in an autocrine manner. Hum Reprod 2006; 21: 1083–91. [DOI] [PubMed] [Google Scholar]
- 19. Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N. Pathogenic role of the CXCL16–CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum 2005; 52: 3004–14. [DOI] [PubMed] [Google Scholar]
- 20. Hojo S, Koizumi K, Tsuneyama K et al . High‐level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor‐infiltrating lymphocytes in colorectal cancer. Cancer Res 2007; 67: 4725–31. [DOI] [PubMed] [Google Scholar]
- 21. Lokeshwar BL. MMP inhibition in prostate cancer. Ann NY Acad Sci 1999; 878: 271–89. [DOI] [PubMed] [Google Scholar]
- 22. Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res 1998; 58: 1395–9. [PubMed] [Google Scholar]
- 23. Nemeth JA, Yousif R, Herzog M et al . Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst 2002; 94: 17–25. [DOI] [PubMed] [Google Scholar]
- 24. Zhou WH, Du MR, Dong L et al . Cyclosporin A increases expression of matrix metalloproteinase 9 and 2 and invasiveness in vitro of the first‐trimester human trophoblast cells via the mitogen‐activated protein kinase pathway. Hum Reprod 2007; 22: 2743–50. [DOI] [PubMed] [Google Scholar]
- 25. Perrone EE, Theoharis C, Mucci NR et al . Tissue microarray assessment of prostate cancer tumor proliferation in African‐American and white men. J Natl Cancer Inst 2000; 92: 937–9. [DOI] [PubMed] [Google Scholar]
- 26. Wu X, Li DJ, Yuan MM, Zhu Y, Wang MY. The expression of CXCR4/CXCL12 in first‐trimester human trophoblast cells. Biol Reprod 2004; 70: 1877–85. [DOI] [PubMed] [Google Scholar]
- 27. Chuan YC, Pang ST, Cedazo‐Minguez A, Norstedt G, Pousette A, Flores‐Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem 2006; 281: 2938–48. [DOI] [PubMed] [Google Scholar]
- 28. Chinni SR, Sivalogan S, Dong Z et al . CXCL12/CXCR4 signaling activates Akt‐1 and MMP‐9 expression in prostate cancer cells: the role of bone microenvironment‐associated CXCL12. Prostate 2006; 66: 32–48. [DOI] [PubMed] [Google Scholar]
- 29. Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst 1998; 90: 118–23. [DOI] [PubMed] [Google Scholar]
- 30. Curran S, Murray GI. Matrix metalloproteinases. Molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 2000; 36: 1621–30. [DOI] [PubMed] [Google Scholar]
- 31. Heresi GA, Wang JC, Taichman R et al . Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: report of a case, review of the literature, and analysis of chemokine receptor expression. Urol Oncol 2005; 23: 261–7. [DOI] [PubMed] [Google Scholar]
- 32. Shen H, Schuster R, Lu B, Waltz SE, Lentsch AB. Critical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growth. Prostate 2006; 66: 1721–8. [DOI] [PubMed] [Google Scholar]
- 33. Araki S, Omori Y, Lyn D et al . Interleukin‐8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res 2007; 67: 6854–62. [DOI] [PubMed] [Google Scholar]
- 34. Lu Y, Cai Z, Xiao G et al . CCR2 expression correlates with prostate cancer progression. J Cell Biochem 2007; 101: 676–85. [DOI] [PubMed] [Google Scholar]


