Abstract
Microvascular endothelial cells, which are recruited by tumors, have become an important target in cancer therapy. This study firstly examined the antitumor effect of angiogenesis inhibitor combined with ultrasound (US) irradiation for human cancer in vivo and evaluated its vascularity using color Doppler US in real time with a microbubble US contrast agent. A human uterine sarcoma cell line, FU‐MMT‐1, was used in vivo because this tumor is one of the most malignant neoplasms of the human solid tumors and it also has a poor response to any of the chemotherapeutic agents currently used, as well as to radiotherapy. In angiogenic inhibitors, TNP‐470 was selected to use in an in vivo study, because this agent showed a higher inhibitory effect in tube formation assay in vitro, than that of FR118487, or thalidomide. The FU‐MMT‐1 xenografts in nude mice were treated using US at a low‐intensity (2.0 w/cm2, 1MHZ) for 4 min three times per week each after the subcutaneous injection of TNP‐470 (30 mg/kg), an angiogenesis inhibitor, and this treatment was continued for 8 weeks. Either treatment of US alone or TNP‐470 alone showed a suppression of tumor growth, in comparison to the non‐treatment group (control), and a significantly enhanced effect was obtained using the combined treatment. A reduction in the intratumoral vascularity, which was evaluated using both color Doppler and immunohistochemistry, was significantly demonstrated using the combined treatment, in comparison to each treatment alone, and the control. No side‐effect was observed in any mice in the combined treatment group. These results suggest that the antitumor effect of TNP‐470 for uterine sarcoma was accelerated by US irradiation in vivo and this combination might be a potentially effective for new cancer therapy. (Cancer Sci 2007; 98: 929–935)
Angiogenesis, the growth of new capillary blood vessels from pre‐existing vasculature, is a crucial process for tumor progression and metastasis.( 1 ) The microvascular endothelial cells (EC), which are recruited by tumors, have thus become an important second target in cancer therapy.( 2 ) Angiogenesis inhibitors have thus been developed to target vascular EC and block tumor angiogenesis. Anti‐angiogenic therapy alone has been shown to be able to suppress the growth of established tumors and a recent clinical trial showed successful results for advanced rectal cancer.( 3 ) The addition of antiangiogenic agents to chemotherapy,( 4 , 5 ) radiation,( 6 , 7 ) or molecular‐targeting agents has thus been suggested to potentially increase clinical efficacy. Gorski et al. showed that radiotherapy and antibodies against VEGF had a synergistic effect against primary tumors.( 6 ) According to an analysis of a bibliographic database, MEDLINE, however, no previous study has ever examined the combination of angiogenesis inhibitors and ultrasound (US) irradiation in the field of cancer research.
O‐(chloroacetyl‐carbamoyl)fumagillol (TNP‐470) is a low‐molecular‐weight synthetic analog of fumagillin, a natural compound secreted by the fungus Aspergillus fumigatus fresenius.( 8 ) TNP‐470 blocks endothelial cell cycle progression in the late G1 phase by activating p53 through a mechanism leading to cyclin‐dependent kinase inhibitor p21CIP/WAF expression.( 9 ) The cellular target of TNP‐470 was found to be methionine aminopeptidase‐2 (MetAP‐2), an intracellular enzyme necessary for the process of protein myristolation.( 10 ) The antitumor effect of TNP‐470 has been shown in various tumors of human malignancies both in vitro and in vivo.( 11 , 12 , 13 ) Our previous studies have shown that TNP‐470 inhibited the VEGF production and proliferation of a uterine sarcoma cell line, FU‐MMT‐1, in vitro ( 13 ) which had been established from a patient with uterine carcinosarcoma.( 14 ) The combination of TNP‐470 and cytotoxic agents( 4 , 5 ) or radiation( 15 ) has showed a successful outcome in vivo.
US has been shown to enhance the antitumor effect of a chemotherapeutic agent in vitro and in vivo.( 16 , 17 , 18 , 19 ) Transiently increased permeability of the cell membrane is one of the mechanisms of the US‐enhanced chemotherapy.( 20 ) Sonoporation, and resealing of the cell membrane by acoustic pressure are considered to be a primary reason for an increased intracytoplasmic concentration of the administered agent.( 21 ) Ablation of adult T‐cell leukemia cells and lysis of HL‐60 cells by low‐intensity US is enhanced in the presence of a photosensitizing drug, indicating that the photosensitive drug potentiates the cytotoxicity of US.( 21 , 22 ) The potentiation of some anticancer agents occurs when the agent may become more potent against the tumor cells when used in conjunction with US. The absorption of ultrasound energy by the agent and the production of free radicals seem to be the likely mechanisms of this increase.( 23 , 24 ) In order to assess the accelerated (synergistic) drug effect of angiogenesis inhibitor using US energy, this study examined for the first time the therapeutic effect of an angiogenesis inhibitor combined with US irradiation for human cancer in vivo. In addition, the effect of antiangiogenesis in this combined treatment was assessed non‐invasively using color Doppler US in real‐time with a microbubble contrast agent.
Materials and Methods
Cell line and nude mice. A human uterine sarcoma cell line, FU‐MMT‐1, previously established by us from a patient with uterine carcinosarcoma, was used in this study because this tumor is one of the most malignant neoplasms of the human solid tumors and it also has a poor response to any of the chemotherapeutic agents currently used, as well as to radiotherapy. FU‐MMT‐1 shows highly progressive activity both in vitro and in vivo. This cell line is chiefly composed of rhabdomyosarcoma cells and the immunophenotype, tumorigenicity, and cytogenetic characteristics have been reported previously.( 14 ) Female BALB/cA Jcl‐nu athymic nude mice were obtained from Clea (Tokyo, Japan). Five to 6‐week‐old mice weighing 20 g were used in the experiments. All animals were kept in isolation rooms at a controlled temperature and they were caged in groups of five of fewer and had free access to standard animal chow and water according to the Instructions of the Institute of Experimental Animal Science, Fukuoka University Medical School.
In vitro tube formation assay. Experiments on tube formation were conducted in triplicate in 24‐multiwell dishes using an Angiogenesis kit (Kurabo, Osaka, Japan), according to the manufacturer's instructions. Briefly, human umbilical vein endothelial cells (HUVEC) cocultured with human fibroblasts were cultivated with TNP‐470 (1 µg/mL), FR118487 (1 µg/mL), and thalidomide (1 µg/mL) in the medium containing 10 ng/mL of vascular endothelial growth factor (VEGF). An angiogenesis inhibitor, FR118487 was synthesized by chemical modification of the fermentation products of a fungus, Scolecobasidium arenarium (F‐2015), at Fujisawa Pharmaceutical Co., Ltd (Tsukuba, Japan). The medium was changed every 3 days. After 10 days, the dishes were washed with phosphate‐buffered saline (PBS) and fixed with 70% ethanol at 4°C. After the fixed cells were rinsed three times with PBS, the cells were then incubated with mouse antihuman CD31 (Kurabo, Osaka, Japan) in PBS containing 1% bovine serum albumin (BSA) for 60 min. After washing with 1% BSA–PBS three times, the cells were incubated with goat antimouse IgG AlkP conjugate (Kurabo, Osaka, Japan). Metal‐enhanced 3,3′‐diamino‐benzidine‐tetrahydrochloride (DAB) was the substrate, the reaction yielding a dark reddish‐brown insoluble end‐product. Finally, the cells were washed with PBS five times, and viewed using an Olympus microscope. The area and tube length were measured using the Kurabo angiogenesis image analyzer (Kurabo, Osaka, Japan) in five different fields per each well, and then were statistically analyzed.
Chemicals. TNP‐470 was kindly donated by Takeda Chemical Industries (Osaka, Japan). Its structure and characteristics have been described previously.( 11 ) TNP‐470 was suspended in a vehicle of 0.5% ethanol plus 5% gum arabic in saline. FR118487 was kindly donated by Fujisawa Pharmaceutical Co., Ltd (Tsukuba, Japan). The inhibitory effect of this drug on angiogenesis in the rabbit cornea has previously been described.( 25 )
Injection of TNP‐470 and ultrasound irradiation. The mice were injected subcutaneously with 2 × 105 FU‐MMT‐1 cells in 0.2 mL DMEM in the right auxiliary region of the flank. Mice bearing the resultant tumors measuring 5–10 mm in diameter on the 14th day were randomly separated into four groups as follows: i) US irradiation alone (n = 8); ii) TNP‐470 injection alone (n = 8); iii) combination of TNP‐470 and US irradiation (n = 8), and iv) non‐treatment as the control (n = 12: injection of 0.5% ethanol plus 5% gum arabic in saline) and these therapies were continued for 8 weeks. TNP‐470 was injected subcutaneously at a dose of 30 mg/kg three times per week for each mouse in groups of TNP‐470 alone and the combined treatment. The mice were anesthetized with ether and US (continuous wave, at 1 MHZ frequency, and 2.0 w/cm2 intensity), was irradiated through a probe onto subcutaneous tumors for 4 min three times per week using Sonitron 1000 (Rich‐mar, Inola, OK) for each mouse in groups of US alone and the combined treatment. Tumor growth was monitored by measuring the weekly volume twice, calculated as V = a × b2/2 (a = length; b = width). An autopsy was done on all mice soon after finishing these therapies or when they died during the course of therapies and the tumors were then harvested, and the size and the weights of these tumors were measured. Mean, SD, median, and SE of the tumor size during the courses and those of the tumor weight after the therapies in each group were calculated.
Evaluation of tumor vascularity with contrasted color Doppler US. The intratumoral vascularity in xenografts was examined after finishing each treatment using color Doppler US (SSD‐4000, Aloka Ltd, Tokyo, Japan) with a 7.5‐MHz curved array transducer (UST‐987‐7.5, Aloka Ltd) using a microbubble ultrasound contrast agent, Optison® (Molecular Biosystems Inc., San Diego, CA, USA) at our animal center in Fukuoka University. The US examinations were standardized using a medium wall filter, pulsed repetition frequency of 1000 Hz, moderate‐to‐long persistence, and a slow and steady movement of the transducer to achieve the highest sensitivity without apparent background noise. The intratumoral blood flow was enhanced after an i.v. bolus injection of Optison® (0.6 mL/kg). Optison® is approved for use in echocardiography by the USA Food and Drug Administration (FDA) and consists of a suspension of perfluoropropane‐filled albumin microspheres with a concentration of 6.3 × 108 bubbles/mL. After the examination, the previously stored images were retrieved and displayed on the monitor. The area in the longitudinal image on the US monitor of each tumor was automatically calculated using the manual‐trace measurement of the length of tumor circumference, then, the number of colored vessels within the tumor was counted. The average sonographic vascular density (ASVD) was calculated as the number of colored‐vessels within a longitudinal tumor section divided by the area. The ASVD in each treatment group was calculated (±SD) and statistically compared. Moreover, the areas of highest neovascularization were then identified on their stored images. The tumor vessel counts over an area of 19.625‐mm2 (a field of a circle with 5 mm in diameter) per field in each of these three areas within the tumor were then counted as the highest sonographic vascular density (HSVD). The averages of the HSVD in the three 19.625‐mm2 fields of each tumor were calculated in each treatment group (±SD) and statistically compared.
Evaluation of tumor vascularity with immunohistochemistry. The paraffin sections from each tumor were reacted with each primary antibody for 1 h at room temperature in our pathology laboratory. The attached antibodies were visualized using the labeled streptavidin‐biotin (LSAB) method (Zymed, San Francisco, CA, USA). The monoclonal antibody used was anti‐CD34 (an endothelial marker: 1:50; Dako) for endothelial cells in the tumor vessels. The negative controls consisted of an omission of the primary antibody. Microvessel density (MVD) was measured in all tumors treated in this therapy. Intratumoral microvessels were highlighted using anti‐CD34 immunostaining in formalin‐fixed, paraffin‐embedded sections in each tumor. The MVD quantitation in the highest vascularization (so‐called hot spots) was examined in each tumor in the same manner as in our previous study,( 26 ) which was named as the highest immunohistochemical MVD (HIMVD). The average of the HIMVD in these three groups and that in the controls was then calculated. Moreover, to assess the average vascularization in entire tumor sections, 10 areas were randomly chosen in each tumor then the tumor vessels were counted, and then the obtained density was considered to be the average immunohistochemical MVD (AIMVD).
Statistical analysis. These in vivo data were expressed as the mean ± SD. The Mann–Whitney U‐test (non‐parametric) was used to compare tumor growth, or tumor weight between three treatment groups and the control. The unpaired t‐test (parametric) was used to compare tumor vessel density between three treatment groups and the control. These statistical analyses were done using the software package StatView 5.0 (SAS Institute, Inc., Cary, NC, USA) for Macintosh. The results were judged to be statistically significant if the P‐value of each respective test statistic was less than 0.0001.
Results
In vitro tube formation assay. To investigate the antiangiogenic effect of angiogenesis inhibitors, a tube formation assay was performed using HUVEC cocultured with human fibroblasts. When HUVEC were cultured in the presence of VEGF, efficient tube formation was observed (positive control). In a quantitative analysis of both tube area and length, TNP‐470 significantly inhibited the tube formation in vitro, in comparison to the other two antiangiogenic agents, thalidomide, and FR118487 in this study (P < 0.01; Fig. 1a–f). We therefore finally selected TNP‐470 to use in our following in vivo study as a representative agent.
Figure 1.
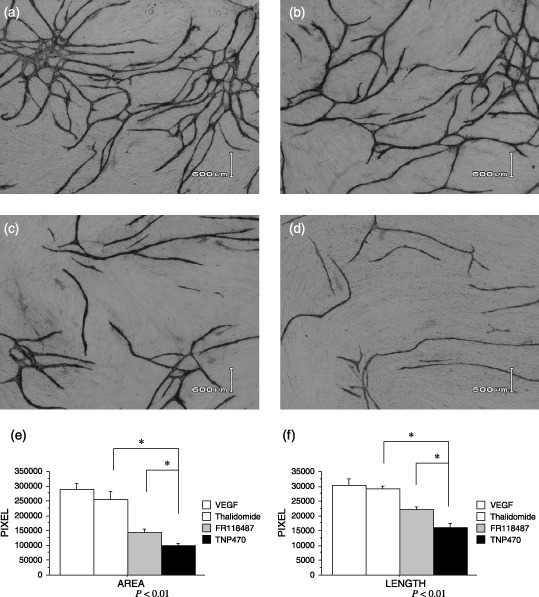
(a–f) Effects on tube formation in vitro. (a) When human umbilical vein endothelial cells (HUVEC) were cultured in the presence of VEGF (10 ng/mL), efficient tube formation was observed. (d) TNP‐470 (1 µg/mL) significantly inhibited the tube formation in vitro, in comparison to the other two antiangiogenic agents, (b) thalidomide (1 µg/mL) and (c) FR118487 (1 µg/mL), in quantitative analysis of both (e) tube area and (f) length using an image analyzer.
Suppression of tumor growth. The weekly changes in the mean tumor volume in xenografts during the courses of therapies are shown in Fig. 2. Either treatment of US alone or TNP‐470 alone inhibited the growth of the FU‐MMT‐1 xenografts, in comparison to that of the control (Mann–Whitney‐U‐test: P = 0.151, and P = 0.006). Furthermore, a significantly enhanced effect to suppress the tumor growth was obtained using the combined treatment (vs US alone: P < 0.0001; and vs TNP‐470 alone: P < 0.0001; Fig. 1). No significant difference in the mean tumor volume was observed between the treatments using US alone and TNP‐470 alone (P = 0.176).
Figure 2.
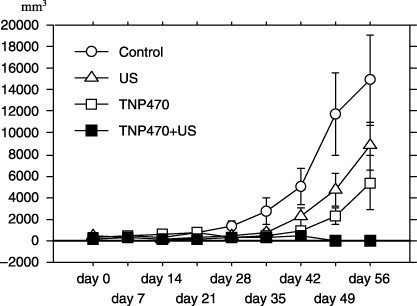
Effects on growth of the FU‐MMT‐1 xenografts. Each curve represents the mean and SD of the tumor (mm3) volume in each group. Either treatment with ultrasound alone or TNP‐470 alone significantly inhibited the growth of the FU‐MMT‐1 xenografts, in comparison to that of the non‐treatment group (control; Mann–Whitney‐U, P = 0.151, and P = 0.006). Moreover, a significantly enhanced effect was obtained using the combined treatment, in comparison to that of each therapy alone (Mann–Whitney‐U, vs US alone: P < 0.0001, and vs TNP‐470 alone: P < 0.0001).
The mean weight after treatment of either US alone (10.98 ± 2.27 g, range: 4.8–17.2 g) or TNP‐470 alone (8.94 ± 3.07 g, range: 0.0–21.2 g) was smaller than that of the control (Mann–Whitney‐U‐test vs US alone: P = 0.0042; vs TNP‐470 alone: P = 0.025). The enhanced effect of the weight reduction in xenografts was shown by the combined treatment (0.75 ± 0.52 g, range: 0.0–4.8 g), in comparison to either US alone (P = 0.027) or TNP‐470 alone (P = 0.050). The mean weight of the xenografts measured after the combined treatment was significantly lower than that of the control (25.04 ± 3.85 g, range: 7.6–30.8 g; P = 0.0007; Fig. 3). After these treatments, disappearance of the tumor (tumor free) was seen in five of eight (62.5%) mice in the combined treatment, and two of eight (25.0%) in TNP‐470 alone (χ2 test; P = 0.13), whereas no such disappearance was seen after US alone, or in the control (vs combined treatment: P = 0.007, and P = 0.001). Dissemination, or direct invasion into the abdominal cavity by the tumor was only seen in the control (33.3%; four of 12 mice).
Figure 3.
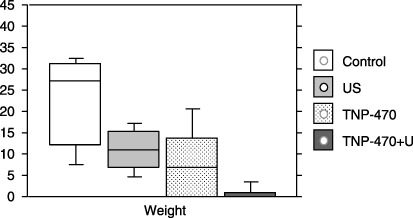
The mean (SD) tumor weights (g) in the FU‐MMT‐1 xenografts resected after treatment. The mean tumor weight after treatment of either ultrasound (US) alone (10.98 ± 2.27 g, range: 4.8–17.2 g) or TNP‐470 alone (8.94 ± 3.07 g, range: 3.1–21.2 g) was smaller than that of the control (25.04 ± 3.85 g, range: 7.6–30.8 g; Mann–Whitney‐U‐test, vs US alone: P = 0.0042; vs TNP‐470 alone; P = 0.025). The enhanced effect of the weight reduction in xenografts was shown by the combined treatment (0.75 ± 0.52 g, range; 0.0–4.8 g), in comparison to either US alone (P = 0.027) or TNP‐470 alone (P = 0.050). The mean tumor weight of the xenografts measured after the combined treatment was significantly lower than that of the control (P = 0.0007).
Evaluation of the antiangiogenic effect using contrast color Doppler ultrasound. The effects of antiangiogenesis for FU‐MMT‐1 xenografts by these therapies were evaluated using contrast Doppler US in real‐time after the 8 weeks of treatment (Fig. 4a–d). In the evaluation with ASVD, the mean of the group of combination treatment was significantly lower than that of the control (unpaired t‐test: P = 0.04), whereas no significant difference was observed between either the treatment of US alone or TNP‐470 alone and the control (P = 0.39, P = 0.41). In the evaluation with HSVD, the mean of the group of either TNP alone or combination treatment was thus significantly lower than that of the control (unpaired t‐test: P = 0.002, P = 0.000004), whereas no significant difference was observed between the treatment of US alone and the control (P = 0.16). The reduction in the vascular density (HSVD) was significantly enhanced by the combined treatment, in comparison to that of the US treatment alone (P = 0.0003), or the TNP‐470 alone (P = 0.00009; Table 1). The intratumoral vascularity could not be fully evaluated using either non‐contrasted color Doppler or power Doppler ultrasound because the intensity of the signals of the colored‐blood flow in these approaches were apparently lower than that of contrasted color Doppler ultrasound.
Figure 4.
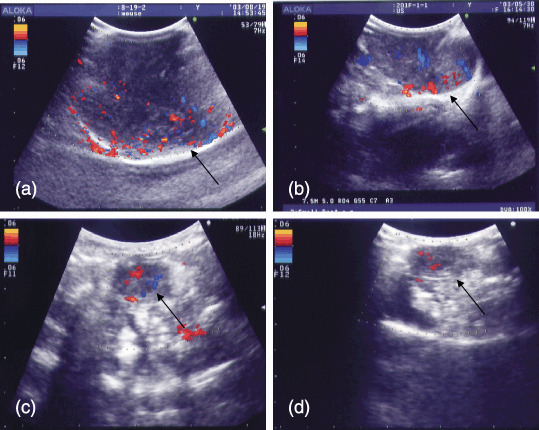
(a–d) Visualization of the intratumoral blood flow using non‐invasive contrasted color Doppler ultrasound (US) in FU‐MMT‐1 human uterine sarcoma growing subcutaneous on the backs of BALB/cA Jcl‐nu mice. Representative Doppler ultrasound pictures are taken at maximal signal intensity after injecting the US contrast agent (10 s after injection). The tumor vessel density in (c) the group of TNP‐470 treatment alone was lower than that of (a) the control (unpaired t‐test, P = 0.0007), whereas no significant difference was observed between (b) the treatment of US alone and the control (unpaired t‐test, P = 0.11). The reduction of tumor vessel density was significantly enhanced by (d) the combined treatment, in comparison to that of the US treatment alone (P = 0.00002), or the TNP‐470 alone (unpaired t‐test, P = 0.00009).
Table 1.
The sonographic and immunohistochemical vascular density in FU‐MMT‐1 xenografts after 8 weeks’ treatments
| Control | US alone | TNP‐470 alone | TNP‐470 + US | |
|---|---|---|---|---|
| 1) Average sonographic vascular density (per 100 mm2) | 8.6 ± 2.1 | 7.7 ± 2.0 | 7.6 ± 2.3 | 5.5 ± 1.3 |
| 2) Highest sonographic vascular density (per 19.63 mm2) | 14.6 ± 1.8 | 12.9 ± 2.7 | 11.1 ± 1.4 | 3.6 ± 1.1 |
| 3) Average immunohistochemical microvessel density† | 49.8 ± 10.2 | 25.9 ± 12.1 | 25.1 ± 14.1 | 9.4 ± 3.2 |
| 4) Highest immunohistochemical microvessel density† | 61.3 ± 10.7 | 60.8 ± 14.7 | 50.8 ± 13.7 | 11.3 ± 2.2 |
Per high‐power field (0.74 mm2). 1) Control versus US: P = 0.39, or TNP‐470: P = 0.41, or TNP‐470 + US: P = 0.04. TNP‐470 versus TNP‐470 + US: P = 0.19. 2) Control versus US: P = 0.16, or TNP‐470: P = 0.002, or TNP‐470 + US: P = 0.000004. TNP‐470 + US versus US alone: P = 0.0003, or TNP‐470: P = 0.00009. 3) Control versus US: P = 0.0008, or TNP‐470: P = 0.002, or TNP‐470 + US: P = 0.0001. TNP‐470 + US versus US alone: P = 0.05, or P = 0.04. 4) Control versus US: P = 0.94, or TNP‐470: P = 0.13, or TNP‐470 + US: P = 0.000002. TNP‐470 + US versus US alone: P = 0.0001, or TNP‐470 alone: P = 0.001. Bald: P<0.05 (versus control).
MVD. The mean of AIMVD in each treatment group was significantly lower than that of the control (unpaired t‐test, US: P = 0.0008; TNP‐470: P = 0.002; combination: P = 0001). The reduction of AIMVD was significantly enhanced by the combined treatment, in comparison to that of the TNP‐470 treatment alone (P = 0.04). In contrast, no difference in the mean of HIMVD was observed between the group of either US treatment alone or TNP‐470 treatment alone and the control (P = 0.94, P = 0.13). However, the reduction of HIMVD was significantly enhanced by the combined treatment, in comparison to that of the US treatment alone (P = 0.0001), or the TNP‐470 alone (P = 0.001; Table 1; Fig. 5a–d). The destruction of the tumor vessels and areas of coagulative necrosis were apparently seen in tumors of either US therapy alone or combination treatment, in comparison to either the control or TNP‐470 treatment alone group.
Figure 5.
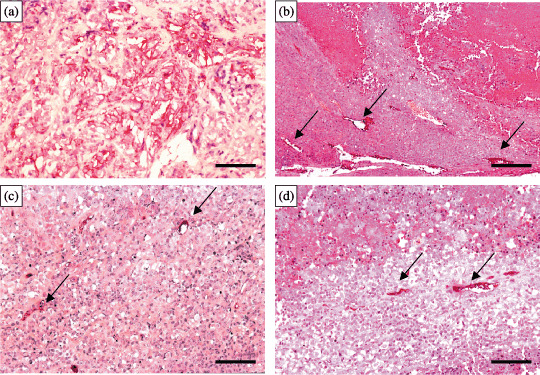
(a–d) Intratumoral microvessels immunohistochemically stained (red colored) by anti‐CD34. The tumor vessel densities in both groups of (b) ultrasound (US) alone and (c) TNP‐470 treatment alone were significantly lower than that of (a) the control. The reduction of tumor vessel density was significantly enhanced by (d) the combined treatment, in comparison to that of (b) the US treatment alone, or (c) the TNP‐470 alone. The destruction of tumor vessels (→) and areas of coagulative necrosis (upper areas in both b and d) are apparently shown in either US treatment alone or after combination treatment. Scale bars: 200 µm.
Toxicity. No side‐effect such as a loss of body weight, or neurotoxicity was significantly observed in any mice in this study.
Discussion
TNP‐470 blocks endothelial cell cycle progression in the late G1 phase to activate p53 in endothelial cells, thus leading to an increase in cyclin‐dependent kinase inhibitor p21CIP/WAF expression and subsequent growth arrest.( 10 ) The molecular target of TNP‐470 has been found to be MetAP‐2, an intracellular enzyme necessary for the process of protein myristolation, thus preventing membrane proteins from translocating to the cell surface.( 9 ) Moreover, TNP‐470 suppresses the production of bFGF, or VEGF in vitro.( 5 , 13 ) TNP‐470 has been shown to act for human malignancies as a single agent in a wide variety of preclinical models( 11 , 12 , 13 ) and some clinical trials.( 27 , 28 ) Recent studies have showed a synergistic effect of radiation and angiogenesis inhibitors.( 6 , 7 ) The combination of angiostatin, an antiangiogenic agent, and radiotherapy has been shown to work best when angiostatin was given simultaneously with radiotherapy in vivo.( 6 ) The successful outcome from the combination of TNP‐470 with cytotoxic therapies( 5 ) or radiotherapy( 15 ) has been also shown in vivo.
Weight loss has been frequently observed in animals receiving TNP‐470, and the dose‐limiting neurotoxicity associated with TNP‐470 has been reported in clinical trials.( 28 ) In the present study, the dose of TNP‐470 was thus kept low (30 mg/kg/day, three times/week), so that no remarkable side‐effects were observed in any mice treated using TNP‐470 with or without US irradiation. To reduce the toxicity of TNP‐470, however, a recent study tried using water‐soluble synthetic polymers, N‐(2‐hydroxypropyl) methacrylamide (HPMA) copolymer, as a specific carrier of TNP‐470, so that this HPMA copolymer‐TNP‐470 substantially enhanced and prolonged the activity of TNP‐470 in vivo and did not cause any weight loss or neurotoxic effects in mice.( 29 ) Further approaches or new ideas may be required to reduce the toxicity of antiangiogenic agents.
US, which is routinely used for diagnostic imaging applications, is now being adopted in various therapeutic applications. The effect of US in activating antitumor drugs or agents has been studied both in vitro and in vivo.( 16 , 17 , 19 ) Our previous study showed that leukemia cells could be selectively eliminated using low‐intensity US in the presence of photosensitive drugs.( 22 ) The mechanism behind the augmentation of the activity of anticancer drugs and other agents by US remains to be fully elucidated; however, several mechanisms have been suggested: i) increased permeability: the increased intracellular concentration of drugs after ultrasonic irradiation suggests an increased permeability (also called sonoporation), or the opening of pores in the cells.( 20 ) Sonoporation is the term used for the phenomenon by which US may transiently alter the structure of the cellular membrane, thus inducing an enhanced uptake of low‐ and high‐molecular‐weight molecules into the cell. There have been a large number of studies in which a synergistic effect between US and different drugs has been sought. Regarding the mechanisms of action in an in vitro environment, acoustic cavitation and streaming may be predominant.( 30 ) Our previous study showed that a direct observation of the cells using electron microscopy confirmed the presence of pore‐like disruptions in the cell membrane after the combined treatment;( 21 ) ii) increased sensitivity of the cells to the agent: US alone can cause lethal or sublethal cellular damage.( 31 ) Interestingly, our current study also showed that US alone could inhibit the growth of a FU‐MMT‐1 xenograft established from a uterine sarcoma,( 14 ) which is one of the most aggressive tumors among the human malignancies. Moreover, histopathologically, the destruction of tumor vessels and area of coagulative necrosis were apparently seen in tumors treated with either US therapy alone or combination treatment, compared to those treated with TNP‐470 therapy alone, thus suggesting direct cellular damage by US irradiation. One commonly observed vascular effect, usually characterized by an increased tumor blood flow during the initial period of therapeutic US irradiation and eventual destruction of the vasculature, renders the tumor mass more hypoxic.( 31 ) Our current study supported the vascular effect of US in vivo because the mean AIMD in US treatment alone was significantly lower than that of control, and destruction of tumor vessels was apparently seen in the US treatment group. A recent study showed that malignant cells were found to be sensitive to therapeutic US treatment, thus resulting in a transient decrease in cell proliferation.( 32 ) In a suspension of carcinoma cells exposed to 1 MHz ultrasound, cell killing was induced, accompanied by DNA strand breaks. This might be mainly attributable to free radical formation and the pyrolyitic processes.( 24 ) Cells damaged sublethally by US are thus suggested to be more biologically susceptible to the antitumor agents; iii) potentiation of the agent: it is also suggested that anticancer agents became more potent against tumor cells when they are used in conjunction with US.( 16 , 17 , 19 ) The absorption of US energy by the agent and the production of free radicals have been cited as the likely mechanisms of this increase.( 33 ) Inertial cavitation is required in this process, primarily in the production of free radicals.( 18 ) We thus irradiated with low‐intensity US within a few minutes after the subcutaneous injection of TNP‐470 to accelerate the drug potentiation because the mean plasma half‐life of TNP‐470 has been reported to be short (2–6 min in humans by intravenous injection).( 28 ) As a result, this combined treatment could significantly inhibit the growth of FU‐MMT‐1 in vivo, in comparison to either TNP‐470 used alone or US alone, thus suggesting an accelerated (booster, or synergistic) effect of US for TNP‐470. We suppose that the possible mechanism of our combination therapy using a low‐intensity US might be chiefly the first mechanism (sonoporation) described above.
As TNP‐470 has been shown to possess antiproliferation effects for not only endothelial cells but also for tumor cells, the uptake of this agent might thus be enhanced in both cells by sonoporation. In addition, direct cellular damage including a vascular effect by US irradiation (the second mechanism) might be added because of the evidence of wide areas of coagulative necrosis in irradiated tissue specimens. Feril et al. recently reported monocytic leukemia cells (U937) were killed by combining hyperthermia sensitive drug, 2,2′‐azobis (2‐amidinopropane) dihydrochloride (AAPH) and exposure to non‐thermal 1 MHz US for 1 min at an intensity of 2.0 W/cm2.( 34 ) Apoptosis measured using flow cytometry and free radical investigation using electron paramagnetic resonance (EPR) spin trapping showed that US‐induced cell lysis and apoptosis were enhanced in the presence of AAPH, regardless of the temperature at the time of sonication. Although free radicals were increased in the combined treatment, this increase did not correlate well with cell killing.( 34 ) The mechanism of enhancement pointed to the increased uptake of the agent during sonication rather than potentiation by AAPH. However, direct measurement of free radicals in the present experiment was technically impossible and further analysis will be needed to affirm these suppositions.
US has been used as a modality for diagnostic imaging in various clinical fields without producing any significant adverse effects. The neovascularization of tumors could be displayed using color Doppler US in various human solid tumors. The changes in intratumoral vascularity in xenotransplanted tumors treated using antiangiogenesis have been demonstrated using color Doppler US in real‐time.( 35 , 36 ) In our recent clinical report, using color Doppler US with a microbubble contrast agent was shown to enhance the vascularity of solid tumors, even in small lesions.( 37 ) In the present study, the outcome of antiangiogenic treatment thus could be more efficiently evaluated using contrast color Doppler US than non‐contrast Doppler. Our histopathological results supported the findings of sonographic vascular density and, immunohistochemically, both HIMVD and AIMVD in the combined treatment group were significantly lower than those in the TNP‐470 treatment alone group. The discrepancy in the results between the HIMVD and AIMVD in US treatment alone as well as in TNP‐470 treatment alone might be the result of the alternative angiogenesis (increased vascularity) focally seen near the hypoxic areas. In the present study, although a difference in the results of vascular density between color Doppler sonography and immunohistochemical examination was partially observed, another recent study has also showed the usefulness of contrast color Doppler to assess angiogenesis without an immunohistochemical examination in vivo.( 35 ) Moreover, another study additionally reported that the color Doppler vascularity index is a better indicator of tumor behavior than the immunohistochemical microvessel density in colon cancer patients.( 38 ) As a result, the combined treatment of TNP‐470 and US irradiation was found to be more effective in suppressing angiogenesis for uterine sarcoma xenografts than either therapy alone. The mechanisms of this antiangiogenic therapy using low‐intensity US for intratumoral microvessels as well as feeding vessels into the tumors therefore need to be more precisely elucidated.
The xenotransplanted model used in this study is originated from a human uterine carcinosarcoma,( 14 ) which is one of the highest angiogenic tumors in all human solid malignancies. Our previous studies apparently showed higher expressions of VEGF‐A and angiopoietin‐2 genes as well as a higher frequency of lymphovascular invasion and a high‐MVD of these tumors, in comparison to those of the other human uterine carcinomas.( 26 , 39 ) As a result, the success of the antiangiogenic combination therapy used in the present study might therefore be associated with the high angiogenic activities (vascular‐rich) of this tumor. Further trials of this combination therapy for other tumor models with either moderate or low levels of angiogenic activity might therefore be needed.
In conclusion, our results support the positive interaction between TNP‐470 and US energy regarding the inhibition of growth, and angiogenesis of human uterine sarcoma. As a result, US irradiation is thus suggested to have enhanced the effect in antiangiogenesis therapy on this tumor, and this combination might be potentially useful for a new cancer therapy.
Acknowledgments
This research was partly supported by a grant from the Ministry of Education, Science and Culture, Japan (#11671164). A part of the content in this study was orally announced by the first author as the ‘Effect of antiangiogenesis drug therapy combined with ultrasound irradiation for uterine cancer evaluated with contrasted ultrasonography’ at the 14th World Congress on Ultrasound in Obstetrics and Gynecology at Stockholm, Sweden, September 4, 2004.
References
- 1. Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 3. Willett CG, Boucher Y, Di Tomaso E et al. Direct evidence that the VEGF‐specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Madden TL, Tran HT et al. Safety and pharmacokinetic effects of TNP‐470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non‐small‐cell lung cancer. J Clin Oncol 2002; 20: 4440–7. [DOI] [PubMed] [Google Scholar]
- 5. Inoue K, Chikazawa M, Fukata S, Yoshikawa C, Shuin T. Docetaxel enhances the therapeutic effect of the angiogenesis inhibitor TNP‐470 (AGM‐1470) in metastatic human transitional cell carcinoma. Clin Cancer Res, 2003; 9: 886–99. [PubMed] [Google Scholar]
- 6. Gorski DH, Mauceri HJ, Salloum RM et al. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res 1998; 58: 5686–9. [PubMed] [Google Scholar]
- 7. Mauceri HJ, Hanna NN, Beckett MA et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 1998; 394: 287–91. [DOI] [PubMed] [Google Scholar]
- 8. Ingber D, Fujita T, Kishimoto S et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature (Lond), 1990; 348: 555–7. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Griffith EC, Sage J, Jacks T, Liu JO. Cell cycle inhibition by the anti‐angiogenic agent TNP‐470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci USA 2000; 97: 6427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti‐angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP‐2. Proc Natl Acad Sci USA 1997; 94: 6099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yanase T, Tamura M, Fujita K, Kodama S, Tanaka K. Inhibitory effect of angiogenesis inhibitor TNP‐470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res 1993; 53: 2566–70. [PubMed] [Google Scholar]
- 12. Yoshida T, Kaneko Y, Tsukamoto A, Han K, Ichinose M, Kimura S. Suppression of hepatoma growth and angiogenesis by a fumagillin derivative TNP 470. Cancer Res 1998; 58: 3751–6. [PubMed] [Google Scholar]
- 13. Miura S, Emoto M, Matsuo Y, Kawarabayashi T, Saku K. Carcinosarcoma‐induced endothelial cells tube formation through KDR/Flk‐1 is blocked by TNP‐470. Cancer Lett 2004; 203: 45–50. [DOI] [PubMed] [Google Scholar]
- 14. Emoto M, Iwasaki H, Kikuchi M et al. Two cell lines established from mixed Müllerian tumors of the uterus: Morphological, immunocytochemical, and cytogenetic analyses. Cancer 1992; 69: 1759–68. [DOI] [PubMed] [Google Scholar]
- 15. Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP‐470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res 2000; 6: 971–8. [PubMed] [Google Scholar]
- 16. Saad AH, Hahn GM. Ultrasound enhanced drug toxicity on Chinese hamster ovary cells in vitro . Cancer Res 1989; 49: 5931–4. [PubMed] [Google Scholar]
- 17. Loverock P, Ter Haar G, Ormerod MG, Imrie PR. The effect of ultrasound on the cytotoxicity of adriamycin. Br J Radiol 1990; 63: 542–6. [DOI] [PubMed] [Google Scholar]
- 18. Worthington AE, Thompson J, Rauth AM, Hunt JW. Mechanism of ultrasound enhanced porphyrin cyototoxicity. Part I. A search for free radical effects. Ultrasound Med Biol 1993; 19: 123–5. [DOI] [PubMed] [Google Scholar]
- 19. Tomizawa M, Ebara M, Saisho H, Sakiyama S, Tagawa M. Irradiation with ultrasound of low output intensity increased chemosensitivity of subcutaneous solid tumors to an anti‐cancer agent. Cancer Lett 2001; 173: 31–5. [DOI] [PubMed] [Google Scholar]
- 20. Brayman AA, Coppage ML, Vaidya S, Miller MW. Transient poration and cell surface receptor removal from human lymphocytes in vitro by 1 MHz ultrasound. Ultrasound Med Biol 1999; 25: 999–1008. [DOI] [PubMed] [Google Scholar]
- 21. Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura, K. Induction of cell‐membrane porosity by ultrasound. Lancet 1999; 353: 1409. [DOI] [PubMed] [Google Scholar]
- 22. Tachibana K, Uchida T, Hisano S, Morioka E. Eliminating adult T‐cell leukaemia cells with ultrasound. Lancet 1997; 349: 325. [DOI] [PubMed] [Google Scholar]
- 23. Tata D, Hahn G, Dunn F. Ultrasonic absorption frequency dependence of two widely used anti‐cancer drugs: doxorubicin and daunorubicin. Ultrasonics 1993; 31: 447–50. [DOI] [PubMed] [Google Scholar]
- 24. Misik V, Riesz P. Free radical intermediates in sonodynamic therapy. Ann NY Acad Sci 2000; 899: 335–48. [DOI] [PubMed] [Google Scholar]
- 25. Otsuka T, Ohakawa T, Shibata T et al. A new potent angiogenesis inhibitor, FR118487. J Microbiol Biotechnol 1991; 1: 163–8. [Google Scholar]
- 26. Emoto M, Iwasaki H, Ishiguro M et al. Angiogenesis in carcinosarcomas of the uterus: differences in the microvessel density and expression of vascular endothelial growth factor between the epithelial and mesenchymal elements. Hum Pathol 1999; 30: 1232–41. [DOI] [PubMed] [Google Scholar]
- 27. Kudelka AP, Verschraegen CF, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP‐470. N Eng J Med 1998; 338: 991–2. [DOI] [PubMed] [Google Scholar]
- 28. Bhargava P, Marshall JL, Rizvi N et al. A phase I and pharmacokinetic study of TNP‐470 administered weekly to patients with advanced cancer. Clin Cancer Res 1999; 5: 1989–95. [PubMed] [Google Scholar]
- 29. Satchi‐Fainaro R, Puder M, Davies JW et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP‐470. Nat Med 2004; 10: 255–61. [DOI] [PubMed] [Google Scholar]
- 30. Ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol 2007; 93: 111–29. [DOI] [PubMed] [Google Scholar]
- 31. Longo FW, Longo WE, Tomashefsky P, Lattimer JK, Rivin BD, Tannenbaum M. Interaction of ultrasound with neoplastic tissue. Local effect on subcutaneously implanted Furth‐Columbia rat Wilms’ tumor. Urology 1975; 6: 631–4. [DOI] [PubMed] [Google Scholar]
- 32. Yang R, Reilly CR, Rescorla FJ et al. High‐intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg 1991; 126: 1002–9. [DOI] [PubMed] [Google Scholar]
- 33. Nicolai H, Steinbach P, Knuechel‐Clarke R et al. Proliferation of tumor spheroids after shock‐wave treatment. J Cancer Res Clin Oncol 1994; 120: 438–41. [DOI] [PubMed] [Google Scholar]
- 34. Feril LB Jr, Kondo T, Zhao QL et al. Enhancement of ultrasound‐induced apoptosis and cell lysis by echo‐contrast agents. Ultrasound Med Biol 2003; 29: 331–7. [DOI] [PubMed] [Google Scholar]
- 35. Gee MS, Saunders HM, Lee JC et al. Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Res 2001; 61: 2974–82. [PubMed] [Google Scholar]
- 36. Abdollahi A, Lipson KE, Sckell A et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res 2003; 63: 8890–8. [PubMed] [Google Scholar]
- 37. Emoto M, Fujimitsu R, Iwasaki H, Kawarabayashi T. Diagnostic challenges in patients with tumors: case 3. A normal‐sized ovarian cancer detected by color Doppler ultrasound using a microbubble contrast agent. J Clin Oncol 2003; 21: 3703–5. [DOI] [PubMed] [Google Scholar]
- 38. Chen C‐N, Cheng Y‐M, Liang J‐T et al. Color Doppler vascularity index can predict distant metastasis and survival in colon cancer patients. Cancer Res 2000; 60: 2892–7. [PubMed] [Google Scholar]
- 39. Emoto M, Charnock‐Jones DS, Licence D et al. Localisation of the VEGF and angiopoietin genes in uterine carcinosarcoma. Gynecol Oncol 2004; 95: 474–82. [DOI] [PubMed] [Google Scholar]


