Abstract
We investigated the role of Nek2, a member of the serine‐threonine kinase family, in the tumorigenic growth of breast carcinoma. Increased expression of Nek2 was observed in all breast carcinoma cell lines examined (BT20, BT474, Hs578T, MCF7, MDA‐MB‐231, T47D, and ZR‐75‐1) by immunoblotting. By treatment with Nek2 short interfering RNA (siRNA), expression of Nek2 was clearly decreased in both estrogen receptor (ER)‐positive (MCF7) and ER‐negative (MDA‐MB‐231) breast carcinoma cell lines. Cell growth, colony formation in soft agar, and in vitro invasiveness of these cell lines were substantially suppressed by Nek2 siRNA treatment. In a xenograft nude mouse model with subcutaneous implantation of MCF7 or MDA‐MB‐231, subcutaneous injection of Nek2 siRNA around the tumor nodules resulted in a reduction of tumor size compared with those of control siRNA injection. Taken together, Nek2 appears to play a pivotal role in tumorigenic growth of breast carcinoma cells, and could be a useful therapeutic molecular target for the treatment of breast carcinoma both in ER‐positive and ER‐negative cases. (Cancer Sci 2009; 100: 111–116)
Breast carcinoma is one of the most common malignancies. There are a variety of treatments for breast carcinoma including molecular targeting therapy, which has made considerable advances. Trastuzumab, a humanized monoclonal antibody to human EGFR‐related (HER)‐2, is the first molecular targeting agent approved to be effective for breast carcinoma therapy.( 1 ) Other novel targeted treatments, including antiangiogenic compounds such as bevacizumab, sunitinib, and vatalanib, and bifunctional drugs such as lapatinib, HER‐2, and Epidermal Growth Factor Receptor (EGFR) inhibitor, are currently in clinical evaluation and, so far, show promising effects.( 2 ) Among all breast carcinomas, those that are positive for hormone receptor (estrogen receptor [ER] or progesterone receptor [PgR]) or ErbB2 currently account for 75–80 and 15–20% of cases, respectively. The remaining 10–15% of breast carcinomas are in a so‐called receptor‐negative or triple‐negative category, which is defined by the absence of the expression of these three proteins.( 3 ) Patients with this type of carcinoma are resistant to the existing targeted treatments so that finding medical treatments for these patients remains a challenge.
Nek2 is a member of the serine‐threonine kinase family Nek, which are structurally related to the essential mitotic regulator never in mitosis A (NIMA).( 4 ) Nek2 is involved in cell division and mitotic regulation by centrosome splitting.( 4 , 5 , 6 ) Interestingly, we found overexpression of Nek2 in cholangiocarcinoma in a tumor‐specific manner.( 7 ) Moreover, we found that Nek2 depletion in cholangiocarcinoma cell lines caused growth suppression and cell death, and that administration of short interfering RNA (siRNA) against Nek2 into tumor‐bearing mice induced substantial prolongation of survival.( 7 ) Although our observation is promising, it remains to be determined whether the critical role of Nek2 is specific for cholangiocarcinoma or not. In this context, increased expression of Nek2 has been reported in cell lines derived from breast, cervical, and prostate carcinomas, as well as lymphomas,( 8 , 9 ) yet the role of Nek2 in these carcinomas remains largely unclear.
To obtain more clues to the usefulness of Nek2 siRNA therapy, we investigated its effect in breast carcinoma. Here we show, for the first time, that Nek2 depletion by treatment with Nek2 siRNA results in the inhibition of cell proliferation and invasion of breast carcinoma cell lines including both ER‐positive and ER‐negative types. Moreover, in a xenograft nude mouse model, we show that subcutaneous injection of Nek2 siRNA around tumor nodules results in substantial suppression of tumor growth compared to control siRNA injection.
Materials and Methods
Cell culture. Cell lines derived from human breast carcinoma (BT20, BT474, Hs578T, MCF7, MDA‐MB‐231, MDA‐MB453, T47D, ZR75‐1), a cell line derived from human cholangiocarcinoma (HuCCT1), and human foreskin fibroblast (HFF) cells were maintained as described previously.( 10 , 11 , 12 ) HuCCT1 were provided by the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan) and Riken Cell Bank (Tsukuba, Japan).
Preparation of siRNA. The sense and antisense strands of Nek2 siRNA obtained from Qiagen (Valencia, CA, USA) were as follows: Nek2 siRNA 19, 5′‐GGAGGGGAUCUGGCUAGUGdTdT‐3′ (sense), 5′‐CACUAGCCAGAUCCCCUCCdTdT‐3′ (antisense); Nek2 siRNA 27, 5′‐GGAAUGCCACAGACGAAGUdTdT‐3′ (sense), 5′‐ACUUCGUCUGUGGCAUUCCdTdT‐3′ (antisense); Nek2 siRNA 65, 5′‐GAGGGCGACAAUUAGGAGAdTdT‐3′ (sense), 5′‐UCUCCUAAUUGUCGCCCUCdTdT‐3′ (antisense); control siRNA, 5′‐UUCUCCGAACGUGUCACGUdTdT‐3′ (sense), 5′‐ACGUGACACGUUCGGAGAAdTdT‐3′ (antisense); and luciferase pGL2 siRNA, 5′‐CGUACGCGGAAUACUUCGAdTdT‐3′ (sense), 5′‐UCGAAGUACUCAGCGUACGdTdT‐3′ (antisense). Control siRNA and luciferase pGL2 siRNA were used as controls. In vitro transfection of siRNA was carried out using GenePORTER (Genlantis, San Diego, CA, USA).
Electrophoresis and immunoblotting. Sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunoblotting were carried out as described elsewhere.( 10 ) Mouse monoclonal anti‐Nek2 (Transduction Laboratories, Lexington, KY, USA) and mouse monoclonal anti‐α‐tubulin (Abnova Corporation, Taiwan) were used for the analysis.
Quantitative real‐time polymerase chain reaction. The procedure of quantitative real‐time polymerase chain reaction (PCR) using the 7300 Real Time PCR system (Applied Biosystems, Tokyo, Japan) was described previously.( 11 ) The TaqMan gene expression assay kit (Applied Biosystems) was used. 18S rRNA was used as an endogenous control.
Cell proliferation assay. Cell proliferation was determined using a colormined assay of cell viability that is based on the cleavage of tetrazolium salt by mitochondrial dehydrogenases (Tetra Color One; Seikagaku Corporation, Tokyo, Japan). Absorbance of the formazan dye was measured at 450 and 630 nm 1.5 h after adding the reagent. A dye‐exclusion test was carried out to assess cell viability after siRNA treatment.
Soft agar colony‐formation assay. To assess the anchorage‐independent growth, a soft agar colony assay was done. Twenty‐four hours after transfection with siRNA, 1 × 104 cells were mixed with 0.36% agar in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum and overlaid onto a 0.72% agarose layer in six‐well plates. After 3 weeks of incubation, colonies greater than 50 µm in diameter in randomly selected fields (×40 magnification) were counted.
In vitro cell‐invasion assay. Hs578T, MDA‐MB‐231, and MCF7 cells were assayed for their invasiveness using a modified Boyden chamber method.( 13 ) Briefly, 24 h after transfection with siRNA, 1 × 105 cells in 200 µL of serum‐free medium were added into the upper chambers, whereas the lower wells contained the conditioned medium from starvation culture as a chemoattractant. After 3 h of incubation, the non‐invading cells on the upper side of the chamber membranes were removed. The invading cells on the opposite side of the chamber membranes were fixed and counted.
Animal efficacy studies. Male nude mice, 8 weeks old and weighing 20–25 g (Crea Japan, Tokyo, Japan) were used in the present study. MCF7 or MDA‐MB‐231 cells (1 × 107) were inoculated subcutaneously into the femoral area of the mice. Ten mice were used for each type of carcinoma cell. After 4 weeks, the tumors generally reached 150–200 mm in diameter. After 1 week of tumor inoculation, we started siRNA administration. Twenty µmol/L Nek2 siRNA (siRNA 27 for MCF7 and siRNA 19 for MDA‐MB‐231) dissolved in 100 µL of cell matrix was administered directly around tumor nodules once a week for a total of 3 weeks. Phosphate‐buffered saline was used to dilute the siRNA. In the animal model experiment, luciferase pGL2 siRNA was used as a control. Growth was assessed based on tumor volume measured weekly.
Blood examination (white blood cell counts, aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) were carried out at 1 week after the final siRNA injection. Animal experiments were done in compliance with the guidelines of the Institute for Laboratory Animal Research, Nagoya University Graduate School of Medicine.
Statistical analysis. All data are presented as the mean ± SE. The statistical differences were analyzed by Student's t‐test and repeated measures of ANOVA. The results were considered significant when P < 0.05.
Results
Overexpression of Nek2 in breast cancers. We first examined the expression of Nek2 in eight breast carcinoma cell lines (BT20, BT474, Hs578T, MCF7, MDA‐MB‐231, MDA‐MB‐453, T47D, and ZR‐75‐1) by immunoblotting (Fig. 1). Nek2 has three spliced variants, Nek2A, Nek2B, and Nek2A‐T. Nek2A encodes a protein of 445 amino acids (48 kDa), whereas Nek2B encodes a protein of 384 amino acids (44 kDa). The different molecular weight is due to a different length of their non‐catalytic C‐terminus. Nek2A is degraded upon mitotic entry, whereas Nek2B remains stable.( 4 , 5 , 6 ) Upregulation of Nek2A and Nek2B was observed in all eight breast carcinoma cell lines examined, whereas it was hardly detectable in HFF, a cell line derived from normal human fibroblasts. It should be noted that these eight cell lines included both ER‐negative and ER‐positive human breast carcinoma cell lines. HuCCT1, a cholangiocarcinoma cell line in which high expression of Nek2 was reported in our previous study,( 7 ) was used as a positive control.
Figure 1.
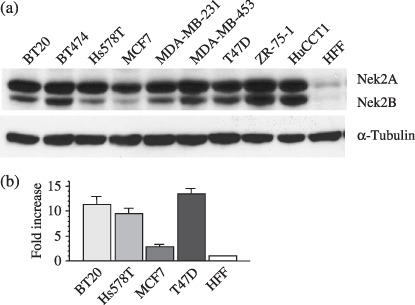
The expression of Nek2 in breast carcinoma cell lines. (a) The expression of Nek2 in eight breast carcinoma cell lines, BT20, BT474, Hs578T, MCF7, MDA‐MB‐231, MDA‐MB‐453, T47D, and ZR‐75‐1, by immunoblotting. HuCCT1 (cholangiocarcinoma cell line) was used as a positive control and the HFF (human fibroblast cell line) was used as a negative control. α‐Tubulin was used as an internal control. (b) The expression of Nek2 in four breast carcinoma cell lines was measured by quantitative real‐time polymerase chain reaction. Graphs show the relative expression levels of Nek2 in BT20, Hs578T, MCF7, and T47D, as compared with that of HFF (negative control).
To confirm the increased expression of Nek2 in breast carcinoma cell lines, we next carried out quantitative real‐time PCR. As shown in Figure 1b, Nek2 expression was activated, at least in part, at the transcriptional level in a tumor‐specific manner.
Effect of Nek2 siRNA treatment on cell growth. ‘RNA interference has emerged as a natural and highly efficient mechanism for gene silencing. We have designed approximately 100 siRNA candidates based on their sequence, the presence of UU at their 5′ end, and their GC content. Among them, we selected three types of siRNA (Nek2 siRNA 65, 27, and 19) based on their secondary structure. These siRNA showed clear suppression of Nek2 protein expression and cellular growth in cholangiocarcinoma cell lines in our previous study.( 7 ) In this study, these siRNA were tested for their ability to suppress Nek2 expression in two types of breast carcinoma cell lines. Although MCF‐7 was chosen as a representative ER‐positive cell line, MDA‐MB‐231and Hs578T were examined as ER‐negative cell lines. In all of these cell lines, MCF‐7, MDA‐MB‐231, and Hs578T treatment with Nek2‐specific siRNA substantially suppressed Nek2 expression (Fig. 2a). In siRNA‐treated MCF7, relative amounts of Nek2A were decreased to 48% by siRNA 19 and 23% by Nek2 siRNA 27, respectively, compared with those of control siRNA‐treated cells. Similarly, relative amounts of Nek2B in MCF7 were decreased to 39% by siRNA 19 and 31% by siRNA 27, respectively. The reduction rates of Nek2B signals were almost similar to those of Nek2A. In contrast, transfection with either luciferase siRNA or control siRNA did not alter Nek2 expression significantly.
Figure 2.
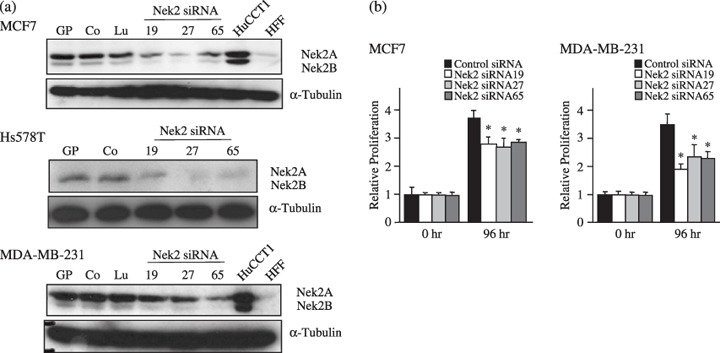
The effect of Nek2 short interfering RNA (siRNA) treatment on cell growth. (a) At 48 h after transfection with Nek2 siRNA (Nek2 siRNA 19, Nek2 siRNA 27, and Nek2 siRNA 65), relative levels of Nek2 in MCF7 (a representative estrogen receptor [ER]‐positive cell line), MDA‐MB‐231, and Hs578T (representative ER‐negative cell lines) were examined by immunoblotting. Control siRNA (Co) and luciferase pGL2 siRNA (Lu) were used as controls. GP cells were treated only with Gene PORTER. α‐Tubilin was used as a control. (b) Effect of Nek2 siRNA treatment on the growth of breast carcinoma cells. Graphs show the relative proliferation levels in MCF7 and MDA‐MB‐231 48 h after transfection with Nek2 siRNA (Nek2 siRNA 19, Nek2 siRNA 27, and Nek2 siRNA 65). *Statistically significant (P < 0.05 vs control siRNA by Student's test).
We next examined the effect of siRNA treatment on the growth of breast carcinoma cells. As shown in Figure 2b, growth of MCF7 was substantially suppressed by treatment with Nek2 siRNA, compared with that of control siRNA‐treated cells. The proliferation in MDA‐MB‐231 was also suppressed by treatment with Nek2 siRNA to levels similar to those of MCF7.
Effect of Nek2 siRNA treatment on anchorage‐independent growth and in vitro invasiveness of breast carcinoma cells. Anchorage‐independent growth is a growth property unique for transformed cells that can be assayed by soft agar colony formation. We next examined the effect of Nek2 siRNA treatment on soft agar colony formation of MCF7 (ER positive) and Hs578T (ER negative). Because MDA‐MB‐231cells form tiny colonies in our hands, we examined Hs578T as an ER‐negative cell line. Both in ER‐positive (MCF7) and ER‐negative (Hs578T) cell lines, we found an approximately fourfold decrease in the number of colonies formed after Nek2 siRNA treatment compared with control siRNA treatment (Fig. 3). In addition, the size of the colonies was also reduced in Nek2 siRNA‐transfected Hs578T cells.
Figure 3.
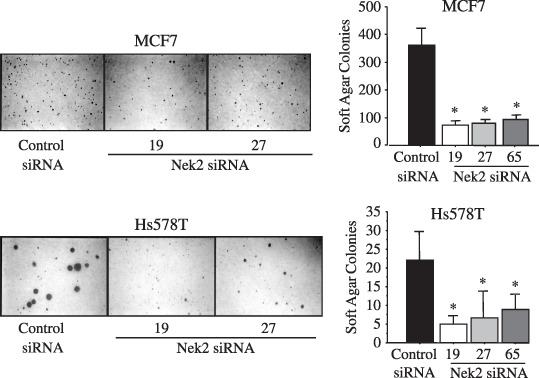
The effect of Nek2 short interfering RNA (siRNA) treatment on the anchorage‐independent growth of breast carcinoma cells, which was assayed by soft agar colony formation. Left, photographs of colony formation; right, the mean numbers of colonies counted by soft agar assay for each of the control or siNek2 treatments. *P < 0.05 vs control siRNA by Student's t‐test.
Destruction or penetration of the basement membrane is thought to be an essential step in successful metastasis by malignant tumor cells. Because Nek2 controls microtubule organization, overexpressed Nek2 might affect tumor invasion. We therefore, studied the effect of Nek2 siRNA treatment on the in vitro invasiveness of breast carcinoma cells using a modified Boyden chamber method. Basement membrane matrix was reconstituted on to a filter in the Boyden chamber and the ability of cancer cells to invade through the coated filter was assayed. The cells (MCF7, Hs578T, and MDA‐MB‐231) were transfected with Nek2 siRNA, and subsequently incubated for 24 h. As shown in Figure 4, all of the examined cell lines, showed a reduction in their in vitro invasiveness by treatment with Nek2 siRNA. These results suggest the important role of Nek2 in the invasiveness as well as the anchorage‐independent growth of the breast carcinoma cells.
Figure 4.
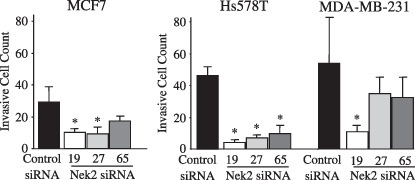
The effect of Nek2 short interfering RNA (siRNA) treatment on in vitro invasiveness of breast carcinoma cells, which was assayed using the modified Boyden chamber method. After 3 h incubation, cells that penetrated the membrane and appeared at the bottom side of the filter were fixed and counted.
Effect of Nek2 siRNA treatment on the growth of breast carcinoma in a xenograft nude mouse model. We next examined the effect of Nek2 siRNA treatment on the growth of breast carcinoma tumor in the xenograft nude mouse model. Mice were subcutaneously inoculated with either MCF7 or MDA‐MB‐231. One week following the tumor inoculation, we started siRNA injection. Twenty µM of siRNA (Nek2 siRNA 27 for MCF7 and Nek2 siRNA 19 for MDA‐MB‐231) or control siRNA in the cell matrix was subcutaneously injected around the tumor nodules of mice once a week for 3 weeks. The tumor sizes after inoculation of MCF7 and MDA‐MIB‐231 were measured once a week until 49 days. As shown in Figure 5, mean tumor volumes in the Nek2 siRNA‐treated mice were significantly lower than those of the control siRNA‐treated mice. It should be noted that, as far as we examined, these mice did not lose bodyweight and tolerated the siRNA treatment well. The average bodyweights of mice were 18.3 versus 18.6 g (Nek2 siRNA vs luciferase pGL2 siRNA) before siRNA treatment, and 23.0 versus 22.9 g at the time of final siRNA injection and 22.2 g versus 22.3 g after 4 weeks of observation. We observed no sign of toxicity, such as a decrease or increase in white blood cell counts and abnormal liver function by blood test, in siRNA‐treated mice (Fig. 5c).
Figure 5.
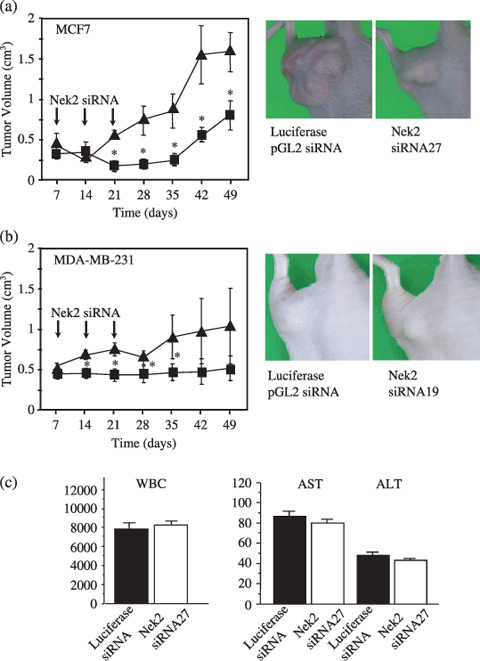
The effect of Nek2 short interfering RNA (siRNA) treatment on the growth of subcutaneously implanted breast carcinoma cells. (a) Left, tumor volume of the MCF7 xenograft. One week after tumor inoculation, siRNA injection was started. Nude mice bearing MCF7 tumors were treated with Nek2 siRNA 27 (20 µmol/L) once a week for 3 weeks as indicated by arrows. The volume of MCF7 tumors was measured weekly ( , control siRNA;
, control siRNA;  , Nek2 siRNA). Luciferase pGL2 siRNA was used as control siRNA. Right, photos of the MCF7 xenograft at 4 weeks after the final siRNA injection. (b) The same experiments as for MDA‐MB‐231 were carried out with Nek2 siRNA 19 (20 µmol/L). *P < 0.05 versus control siRNA by Student's t‐test. (c) Left, white blood cell (WBC) counts in MCF7‐inoculated mice treated with either control siRNA or Nek2 siRNA27 at 4 weeks after the final siRNA injection. Right: liver function (AST, aspartate aminotransferase; ALT, alanine aminotransferase) in MCF7‐inoculated mice treated with either control siRNA or Nek2 siRNA27 at 4 weeks after the final siRNA injection.
, Nek2 siRNA). Luciferase pGL2 siRNA was used as control siRNA. Right, photos of the MCF7 xenograft at 4 weeks after the final siRNA injection. (b) The same experiments as for MDA‐MB‐231 were carried out with Nek2 siRNA 19 (20 µmol/L). *P < 0.05 versus control siRNA by Student's t‐test. (c) Left, white blood cell (WBC) counts in MCF7‐inoculated mice treated with either control siRNA or Nek2 siRNA27 at 4 weeks after the final siRNA injection. Right: liver function (AST, aspartate aminotransferase; ALT, alanine aminotransferase) in MCF7‐inoculated mice treated with either control siRNA or Nek2 siRNA27 at 4 weeks after the final siRNA injection.
Discussion
Breast carcinoma is one of the leading causes of morbidity and mortality in women. In cases of early‐stage breast carcinoma, surgical and radiochemotherapeutic treatments show effective results. In contrast, those of an advanced or aggressive carcinoma are far more difficult to control by these treatments, so a new therapeutic modality needs to be developed. Recently, we reported that siRNA against Nek2, a cell cycle‐related kinase, could suppress the growth of cholangiocarcinoma.( 7 ) Although the effect of Nek2 siRNA in cholangiocarcinoma was quite promising, it remained unclear whether the effect of the siRNA was limited to cholangiocarcinoma or not. We therefore studied the role of Nek2 in breast carcinoma as another model for molecular targeting therapy. All of the breast carcinoma cell lines we examined had high levels of Nek2 expression to levels similar to those of the cholangiocarcinoma. Furthermore, we showed for the first time that siRNA against Nek2 could substantially suppress the proliferation, anchorage‐independent growth, in vitro invasiveness, and in vivo tumor formation of breast carcinoma cell lines.
In recent years, endocrine therapy for breast carcinoma has progressed with drugs such as ER modulators and aromatase inhibitors. In many cases, ER plays a crucial role in the development and proliferation of breast carcinoma. Many reports have shown that the expression of ER in breast carcinoma affects sensitivity to drug therapy, including hormonal therapy,( 14 ) chemotherapy,( 13 , 15 ) and molecular targeting therapy.( 16 , 17 ) ER has also been used as a predictive marker for endocrine therapy and clinicopathological factors.( 18 ) However, approximately 20–25% of breast carcinoma is ER negative, indicating that their growth is independent of estrogen. For these ER‐negative carcinomas, a therapy targeting ER may be ineffective and another modality that is not affected by the expression of ER is required. In this regard, our data showed promising results in which Nek2 siRNA had an inhibitory effect on cellular proliferation and invasion for both ER‐positive and ER‐negative breast carcinoma. Our results also suggest that increased expression of Nek2 and ER expression in breast carcinoma are mutually independent.
Although the precise mechanism by which Nek2 siRNA causes suppression of tumor growth remains to be clarified, our results strongly suggest that Nek2 is required for the tumorigenesis of breast carcinoma cells. Nek2 siRNA could suppress anchorage‐independent growth, suggesting that breast carcinoma cells have a specific dependence on Nek2 for their tumorigenic growth, which was proposed by Weinstein et al. as ‘oncogene addiction’.( 19 ) Oncogene addiction is an unexplained dependency of cancer cells on a particular cellular pathway for maintenance of the malignant phenotype, cell survival, or proliferation.( 19 ) In breast carcinoma, Her‐2/neu,( 20 ) Myc,( 21 ) and Wnt( 22 ) have been proposed as candidate genes for oncogene addiction. In the present study, we demonstrated that the Nek2 pathway is necessary to maintain the tumorigenic growth of breast carcinoma cells, suggesting Nek2 to be another candidate gene for oncogene addiction. It should be noted here that Nek2 depletion suppresses not only the anchorage‐independent growth but also the invasiveness of the breast carcinoma. Although the downstream effectors of Nek2 should be identified, our results suggest that Nek2 has multiple downstream signaling pathways that control not only cell growth but also invasiveness.
In summary, this is the first report demonstrating that Nek2 plays a critical role in carcinogenesis, tumor invasion, and tumorigenic growth of breast carcinoma, and that inhibition of Nek2 expression with siRNA causes suppression of cancer growth and invasion in both ER‐positive and ER‐negative cells. Our data, especially the in vivo results, provide a strong rationale for further investigation of Nek2 inhibitors as a new breast carcinoma therapy.
Competing interests statement
The authors declare that they have no competing financial interests.
Acknowledgments
The authors would like to thank members and staff of the Hamaguchi Laboratory for their technical assistance and helpful discussions. This work was supported by a Grant‐in‐Aid for the Center of Excellence Research from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1. Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti‐HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–31. [PubMed] [Google Scholar]
- 2. Longo R, Torino F, Gasparini G. Targeted therapy of breast cancer. Curr Pharm Des 2007; 13: 497–517. [DOI] [PubMed] [Google Scholar]
- 3. Cleator S, Heller W, Coombes RC. Triple‐negative breast cancer: therapeutic options. Lancet Oncol 2007; 8: 235–44. [DOI] [PubMed] [Google Scholar]
- 4. Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J 1998; 17: 470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 2002; 21: 6184–94. [DOI] [PubMed] [Google Scholar]
- 6. Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ, Muschel RJ. Inhibition of centrosome separation after DNA damage: a role for Nek2. Radiat Res 2004; 162: 128–35. [DOI] [PubMed] [Google Scholar]
- 7. Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res 2007; 67: 9637–42. [DOI] [PubMed] [Google Scholar]
- 8. Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res 2004; 64: 7370–6. [DOI] [PubMed] [Google Scholar]
- 9. De Vos S, Hofmann WK, Grogan TM et al . Gene expression profile of serial samples of transformed B‐cell lymphomas. Lab Invest 2003; 83: 271–85. [DOI] [PubMed] [Google Scholar]
- 10. Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v‐src causes tyrosine phosphorylation and inactivation of the N‐cadherin–catenin cell adhesion system. Embo J 1993; 12: 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanimura Y, Kokuryo T, Tsunoda N et al . Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett 2005; 219: 205–13. [DOI] [PubMed] [Google Scholar]
- 12. Mon NN, Hasegawa H, Thant AA et al . A role for focal adhesion kinase signaling in tumor necrosis factor‐alpha‐dependent matrix metalloproteinase‐9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res 2006; 66: 6778–84. [DOI] [PubMed] [Google Scholar]
- 13. Toi M, Nakamura S, Kuroi K et al . Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat 2008; 110 (3): 531–9. [DOI] [PubMed] [Google Scholar]
- 14. Esslimani‐Sahla M, Simony‐Lafontaine J, Kramar A et al . Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res 2004; 10: 5769–76. [DOI] [PubMed] [Google Scholar]
- 15. Aas T, Geisler S, Eide GE et al . Predictive value of tumour cell proliferation in locally advanced breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 2003; 39: 438–46. [DOI] [PubMed] [Google Scholar]
- 16. Conforti R, Boulet T, Tomasic G et al . Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracycline‐based chemotherapy: a biomarker study from two randomized trials. Ann Oncol 2007; 18: 1477–83. [DOI] [PubMed] [Google Scholar]
- 17. Shao W, Brown M. Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res 2004; 6: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida N, Omoto Y, Inoue A et al . Prediction of prognosis of estrogen receptor‐positive breast cancer with combination of selected estrogen‐regulated genes. Cancer Sci 2004; 95: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinstein IB. Cancer: Addiction to Oncogenes – the Achilles heal of cancer. Science 2002; 297 (5578): 63–4. [DOI] [PubMed] [Google Scholar]
- 20. Moody SE, Perez D, Pan TC et al . The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 2005; 8: 197–209. [DOI] [PubMed] [Google Scholar]
- 21. D’Cruz CM, Gunther EJ, Boxer RB et al . c‐MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med 2001; 7: 235–9. [DOI] [PubMed] [Google Scholar]
- 22. Gunther EJ, Moody SE, Belka GK et al . Impact of p53 loss on reversal and recurrence of conditional Wnt‐induced tumorigenesis. Genes Dev 2003; 17: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]


