Abstract
The usual primary endpoint in clinical trials for first‐line chemotherapy in advanced non‐small cell lung cancer is overall survival. Second‐line chemotherapy can also prolong overall survival. Non‐smoking history has been associated with a treatment effect for epidermal growth factor receptor‐tyrosine kinase inhibitor (EGFR‐TKI) versus placebo for overall survival. We performed a retrospective analysis to identify prognostic factors for progression‐free survival and overall survival in patients with advanced non‐small cell lung cancer treated with first‐line carboplatin/paclitaxel, and to examine the effect of second‐line therapy on progression‐free survival and overall survival. Ninety‐eight patients (median age 61 years, 35 female, 74 adenocarcinoma, 68 smokers, 56 performance status 0) fulfilled our criteria, of which 75 patients (78%) received more than second‐line therapy (docetaxel [54%] gefitinib [48%] erlotinib [4%]). For overall survival, smoking history and histology were significant prognostic factors. The 2‐year overall survival rates were as follows: smokers, 17%; non‐smokers, 52%, P < 0.0001; adenocarcinoma, 40%; other 15%, P = 0.0017. Multivariate analysis in patients who received second‐line therapy showed treatment with EGFR‐TKI was an independent predictor of overall survival. Smoking history and adenocarcinoma histology were prognostic factors for an improved outcome with carboplatin/paclitaxel in patients with non‐small cell lung cancer. Our study results suggest that the use of EGFR‐TKI after first‐line treatment may be associated with an improvement in overall survival. (Cancer Sci 2007; 98: 226–230)
Lung cancer is the malignant tumor with the highest mortality rates in the world.( 1 ) Approximately 80% of all lung cancer cases are non‐small cell lung cancer (NSCLC) and patients with postoperative recurrence or advanced NSCLC may be treated with systemic chemotherapy. Platinum‐based chemotherapy is widely used as first‐line treatment. Various combination regimens are available – the Four‐Arm Cooperative Study (FACS) conducted in Japan between October 2000 and June 2002 did not demonstrate any superiority of three experimental platinum‐based regimens (cisplatin/gemcitabine, cisplatin/vinorelbine and carboplatin/paclitaxel) compared with the reference arm of cisplatin/irinotecan.( 2 , 3 ) However, due to its good tolerability, ease of use and experience in Western countries, carboplatin/paclitaxel is currently the standard first‐line chemotherapy for NSCLC in Japan.
Docetaxel has been widely used as second‐line therapy for NSCLC in Japan. However, since its approval in July 2002, the use of gefitinib (IRESSA), an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR‐TKI), has been increasing each year. Erlotinib, another EGFR‐TKI, which is approved in a number of Western markets has also been used in clinical registration trials in some Japanese medical institutions. Gefitinib was the first molecular targeted agent to be approved for the treatment of NSCLC in Japan. Two international cooperative Phase II studies (IRESSA Dose Evaluation in Advanced Lung Cancer Trial: IDEAL1 and 2) demonstrated efficacy (response rates, 12.0–18.9%) and favorable tolerability of gefitinib in the treatment of NSCLC after failure of platinum‐based chemotherapy.( 4 , 5 ) Furthermore, the results of subset analyses of IDEAL1 indicated that the patient characteristics of Japanese nationality, female gender and adenocarcinoma histology were associated with longer overall survival (OS).( 4 )
In a placebo‐controlled Phase III study (BR21) erlotinib significantly prolonged OS compared with placebo in patients with previously treated NSCLC.( 6 ) A similar Phase III study (IRESSA Survival Evaluation in Lung Cancer [ISEL]) of gefitinib in refractory, advanced NSCLC showed an improvement in survival compared with placebo in the overall study population, which did not reach statistical significance.( 7 ) However, in a subset analysis, statistically significantly longer survival was demonstrated in patients of Asian origin and in patients who had never smoked.( 7 ) With the availability of new second‐line anticancer agents such as gefitinib and erlotinib, it is necessary to consider more fully the influence of second‐line treatment on evaluation of OS following standard first‐line treatment. Since the opening of our department in October 2002, carboplatin/paclitaxel has been used as the standard first‐line therapy for NSCLC, while the use of gefitinib as second‐line therapy is increasing each year. In this study we performed retrospective analyses of data from patients who had received carboplatin/paclitaxel, in order to identify prognostic variables affecting OS and progession‐free survival (PFS), and also to determine the contribution of second‐line and subsequent treatment to prolongation of OS.
Patients and Methods
Patients. This retrospective study recruited patients with NSCLC who had received chemotherapy at the Thoracic Oncology Division, Shizuoka Cancer Center, Japan, between October 2002 and September 2005. Patients met all of the following criteria:( 1 ) clinical stage IIIB or IV;( 2 ) patients were administered carboplatin area under the curve (AUC) 6 + paclitaxel 200 mg/m2 as first‐line chemotherapy; and( 3 ) performance status (PS) 0 or 1.
Target patients were identified in our electronically controlled clinical database and the following information extracted from their data:( 1 ) patient demographics at the start of first‐line chemotherapy (age, gender, smoking history, histology, stage);( 2 ) objective tumor response;( 3 ) time to disease progression;( 4 ) OS; and second‐line and subsequent chemotherapy regimens.( 5 ) The tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) using existing images and graded as complete response, partial response, stable disease, progressive disease or not evaluable.
Treatment. Patients received carboplatin and paclitaxel as first‐line chemotherapy. Patients received paclitaxel 200 mg/m2 as a 3‐h intravenous infusion, followed by carboplatin AUC 6 (Calvert's setting) as a 1‐h infusion on Day 1. Courses of treatment were repeated every 3 or 4 weeks for 4–6 cycles, until disease progression or severe toxicity. When a patient developed National Cancer Institute Common Toxicity Criteria (NCI‐CTC) grade 3 non‐hematological toxicity (except nausea and anorexia) after the start of treatment, the dose was reduced to carboplatin AUC 5 + paclitaxel 150 mg/m2.
Statistical analysis. Kaplan‐Meier plots were prepared for OS and PFS and median values were calculated. OS was measured from the first day of first‐line treatment to the day of death or the day last seen alive (cut‐off). PFS was measured from the first day of first‐line treatment to the earliest observation of documented progressive disease, or the day of death if the patient died before observation of progressive disease. Univariate and multivariate analyses were performed for OS and PFS stratified by baseline factors. To identify factors influencing PFS and OS, multivariate analysis was performed with covariates including disease stage (IIIB versus IV), histology (adenocarcinoma versus other), smoking history (non‐smoker versus smoker), gender (female versus male) and PS (0 versus 1). Multivariate analysis was performed by the stepwise regression method using a Cox proportional hazards model. To evaluate potential interaction between clinical variables such as smoking history or histology and EGFR‐TKI treatment, patients who received second‐line therapy were included in subsequent exploratory Cox analysis in which non‐smokers and adnocarcinoma patients were divided by EGFR‐TKI treatment, with smokers and nonadnocarcinoma patients set as references, respectively. Statistical analyses for this study were conducted using the Stat View software statistical tool.
Results
Patient characteristics. In total, 98 patients met the eligibility criteria and their demographic data are presented in Table 1. The majority of patients were male (64%), had a smoking history (69%), adenocarcinoma histology (76%), stage IV disease (70%) and PS 0 (57%). The median duration of first‐line carboplatin/paclitaxel therapy was 3 cycles (range, 1–6 cycles). The median follow‐up time was 24.8 months (range: 4.2–43.9). 57 patients died. 41 patients were still alive.
Table 1.
Patient demographics (n = 98)
| Gender n (%) | |
| Male | 63 (64) |
| Female | 35 (36) |
| Median (range) age, years | 61 (34–78) |
| ECOG PS, n (%) | |
| 0 | 56 (57) |
| 1 | 42 (43) |
| Smoking history, n (%) | |
| Smoker | 68 (69) |
| Non‐smoker | 30 (31) |
| Histology, n (%) | |
| Adenocarcinoma | 74 (76) |
| Other | 24 (24) |
| Stage, n (%) | |
| IIIB | 29 (30) |
| IV | 69 (70) |
ECOG, European Cooperative Oncology Group; PS, performance status.
Efficacy. The overall response rate to first‐line carboplatin/paclitaxel therapy was 20% (20/98), with outcomes similar in patients with adenocarcinoma and other histological subtypes (20% versus 21%, respectively) (Table 2). In the overall population, median PFS was 4.8 months and median OS 16.5 months, with a 1‐year survival rate of 64%.
Table 2.
Best overall objective response, n (%)
| Total population (n = 98) | By histology | ||
|---|---|---|---|
| Adenocarcinoma (n = 74) | Other (n = 24) | ||
| Partial response | 20 (20) | 15 (20) | 5 (21) |
| Stable disease | 53 (54) | 42 (57) | 11 (46) |
| Progressive disease | 25 (26) | 17 (23) | 8 (33) |
For PFS, only disease stage was a significant prognostic factor (Table 3). For OS, histology, smoking history and PS were significant prognostic factors (Table 3).
Table 3.
Efficacy among patient subgroups: Cox regression analysis
| Factor | Variable | PFS P‐value HR (95% CI) | OS P‐value HR (95% CI) |
|---|---|---|---|
| Histology | Adenocarcinoma versus other | 0.2045 | 0.0020 |
| − | 0.410 (0.233–0.723) | ||
| Smoking | Non‐smoker versus smoker | 0.1351 | <0.0001 |
| − | 0.222 (0.109–0.450) | ||
| Gender | Female versus male | 0.2206 | 0.2691 |
| − | − | ||
| PS | 0 versus 1 | 0.9575 | 0.0109 |
| − | 0.499 (0.292–0.852) | ||
| Stage | IIIB versus IV | 0.0074 | 0.2024 |
| 0.536 (0.339–0.847) | − |
PFS, progression‐free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; NS, not significant; PS, performance status.
Multivariate analyses assessing the effects of histology and smoking history on PFS and OS were performed. No significant difference was observed for PFS between adenocarcinoma versus other histology (P = 0.40; Fig. 1) or non‐smokers versus smokers (P = 0.22; Fig. 2). In contrast, OS differed significantly between adenocarcinoma versus other histology (P = 0.0017) and between non‐smokers versus smokers (P < 0.0001). Of particular note, median OS of smokers was 12.3 months, and non‐smokers was 27.3 months. Two‐year survival rates were 17% and 52% in smokers and non‐smokers, respectively (3, 4).
Figure 1.
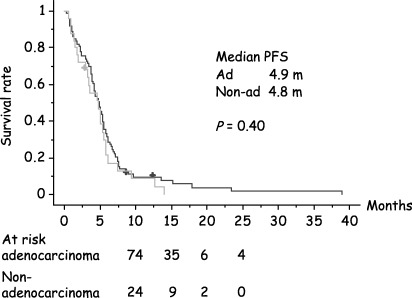
Kaplan‐Meier plot of progression‐free survival (adenocarcinoma versus nonadenocarcinoma histology).
Figure 2.

Kaplan‐Meier plot of progression‐free survival (smoker versus non‐smoker).
Figure 3.

Kaplan‐Meier plot of overall survival (adenocarcinoma versus nonadenocarcinoma histology).
Figure 4.

Kaplan‐Meier plot of overall survival (smoker versus non‐smoker).
To identify factors influencing OS in patients who received second‐line therapy (n = 76), multivariate analysis was performed with covariates including histology (adenocarcinoma versus other), smoking history (non‐smoker versus smoker), PS (0 versus 1), docetaxel (use versus non‐use) and EGFR‐TKI (use versus non‐use). The use of EGFR‐TKI was identified as a significant prognostic factor associated with longer OS, together with non‐smoking history and PS 0. The use of docetaxel was not associated with an increase in OS in this study (Table 4). When interaction terms between clinical variables and EGFR‐TKI treatment were included in the model, no significant interaction was detected (P = 0.354 and 0.515 for smoking history × EGFR‐TKI and histology × EGFR‐TKI, respectively). In the exploratory Cox analysis, prognostic advantage for non‐smoking history and adnocarcinoma histology was more prominent in patients who received EGFR‐TKI treatment after adjustment for PS, suggesting a potential interaction between these favorable clinical variables and EGFR‐TKI treatment. Compared with smokers, hazard ratio of non‐smokers with or without EGFR‐TKI was 0.961 (95% CI, 0.209–4.420) and 0.193 (0.083–0.449), respectively. Likewise, the hazard ratio of adenocarcinoma patients with or without EGFR‐TKI was 0.429 (0.138–1.334) and 0.387 (0.187–0.800), respectively, compared with patients with other histologies.
Table 4.
Cox regression analysis of prognostic factors for overall survival after second‐line treatment: a stepwise forward procedure
| Factor | Variable | P‐value HR (95% CI) |
|---|---|---|
| Histology | Adenocarcinoma versus other | 0.0639 |
| − | ||
| Smoking | Non‐smoker versus smoker | 0.0052 |
| 0.325 (0.148–0.715) | ||
| PS | 0 versus 1 | 0.0258 |
| (0.258–0.917) | ||
| Docetaxel | – versus + | 0.6720 |
| − | ||
| EGFR‐TKI | – versus + | 0.0084 |
| 2.844 (1.306–1.823) |
PS, performance status; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; HR, hazard ratio; CI, confidence interval.
Discussion
Since the results of a large meta‐analysis revealed that platinum‐based chemotherapy prolonged OS of patients with advanced NSCLC compared with best supportive care (BSC)( 8 ) this therapy has been considered standard first‐line treatment for advanced NSCLC worldwide. Median OS with carboplatin/paclitaxel – the most commonly used standard therapy outside Japan – has been reported to be 8–14 months( 9 , 10 , 11 , 12 , 13 ) similar to the median OS (12.3 months) observed in the FACS trial conducted in Japan.( 2 , 3 )
Comparison of outcomes following carboplatin/paclitaxel treatment in our study with the results obtained with the same regimen in the FACS study, showed there was little difference in median PFS (4.8 versus 4.5 months), but median OS was approximately 4 months longer at our hospital (16.5 versus 12.3 months). With the recent approval of EGFR‐TKIs, the use of these agents as second‐line chemotherapy has increased since the FACS study was performed (30% of patients in this study versus 6% of patients in the FACS study) (Table 5). This observation suggests that the better treatment outcomes obtained in our study compared with those of FACS may be attributable to the effect of anticancer agents used in second‐line and subsequent treatment, especially EFGR‐TKI (gefitinib was used in most cases). In fact, the result of subgroup analysis by patient demographics in our study demonstrated a marked prolongation of OS for non‐smokers and patients with adenocarcinoma, both of which are known to be factors associated with high responsiveness to EGFR‐TKI. Furthermore, the multivariate analysis in patients receiving second‐line treatment, revealed that EGFR‐TKI use was an independent prognostic factor.
Table 5.
Historical comparison of outcomes in our study and the FACS study( 2 )
| FACS( 1 ) (n = 145) | This study (n = 98) | |
|---|---|---|
| Response rate (%) | 32 | 20 |
| Median PFS (months) | 4.5 | 4.8 |
| Median OS (months) | 12.3 | 16.5 |
| 1‐year survival rate (%) | 51 | 64 |
| Second‐line therapy, n (%) | 87 (60) | 76 (78) |
| Docetaxel | 25 (17) | 42 (43) |
| EGFR‐TKI | 9 (6) | 29 † (30) |
| Other | 58 (40) | 5 (5) |
25 patients were treated with Gefitinib. FACS, Four‐Arm Cooperative Study; PFS, progression‐free survival; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor.
Generally, the prolongation of OS is the ultimate goal of anticancer therapy and an important clinical outcome in the evaluation of the effect of first‐line treatment for NSCLC. With the emergence of potent anticancer agents in the second‐line setting, therapy administered after the occurrence of progressive disease becomes a confounding factor in the interpretation of OS. To overcome this issue of confounding, there may be value in using prolongation of PFS as the primary outcome of first‐line trials. Currently, the Food and Drug Administration (FDA) requires an applicant to demonstrate prolonged survival as an approval condition for new anticancer agents.( 14 ) However, the European Agency for Evaluation of Medical Products (EMEA) has accepted PFS as the primary endpoint in some instances, and our present study result supports this view.( 15 )
The results of the BR21 trial showed that erlotinib significantly prolonged OS compared with placebo (6.7 versus 4.7 months, hazard ratio [HR] = 0.70). In the multivariate analysis, Asian origin (P = 0.01), adenocarcinoma histology (P = 0.004) and non‐smoking status (P = 0.048) correlated with prolonged OS.( 6 ) In the preplanned subgroup analysis in the ISEL trial, significantly longer survival was seen with gefitinib compared with placebo in patients of Asian origin (9.5 versus 5.5 months, HR = 0.66) and never‐smokers (8.9 versus 6.1 months, HR = 0.67).( 7 ) Although these two studies did not include Japanese patients, the findings might be extrapolated into Japanese populations. Since the reports of Paez et al.( 16 ) and Lynch et al.( 17 ) in April and May 2004, respectively, numerous studies of EGFR mutations have been conducted in a short period and studies conducted in Japan have reported a good correlation between OS and EGFR mutations in patients treated with gefitinib.( 18 , 19 , 20 ) Moreover, the incidence of EGFR mutations is more frequent in women, patients with adenocarcinoma, never‐smokers and Japanese patients( 16 , 17 ) suggesting that there is a correlation between clinical and molecular factors and clinical benefit from EGFR‐TKIs.
Although EGFR mutations are of interest as a biomarker that can be predictive of the effect of gefitinib, especially in patients of Asian or Japanese origin, their immediate clinical application for patient selection is not always possible, due to issues including method determination, cost and convenience. Correlation between response to gefitinib and EGFR copy number determined by fluorescence in situ hybridization (FISH) has attracted attention in the West as an alternative potential biomarker( 21 , 22 ) and this needs to be further investigated in Japan. Acknowledging the need to pay close attention to future research trends, we believe further discussion into how to select those patient populations most likely to benefit from gefitinib in routine clinical practice is required. It is important to establish whether patients could be selected on the basis of biomarker data such as EGFR mutations, or EGFR over‐expression, or clinical characteristics such as histological subtype and smoking history. Nevertheless, selection of appropriate patients for EGFR‐TKI therapy is undoubtedly necessary, and we hope that future research will be able to identify possible methods as soon as possible. Once identified these will require validation in large‐scale prospective clinical studies.
In conclusion, this retrospective study demonstrated a marked prolongation of overall survival in patients with adenocarcinoma and non‐smoking history who received carboplatin/paclitaxel as first‐line treatment. Our study results suggest that the use of EGFR‐TKI (especially gefitinib) after first‐line treatment may be associated with an improvement in overall survival.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74 – 108. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K, Nishiwaki Y, Ohashi Y et al. The four‐arm cooperative study (FACS) for advanced non‐small‐cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2004; 23: 616a (Abstr 7006). [Google Scholar]
- 3. Watanabe H, Ieki R, Mori K et al. The impact of an independent response evaluation committee (REC) using RECIST guidelines in a Four‐Arm Cooperative Study (FACS) for advanced non‐small cell lung cancer (NSCLC) in Japan. Proc Am Soc Clin Oncol 2004; 23: 629a (Abstr 7065). [Google Scholar]
- 4. Fukuoka M, Yano S, Giaccone G et al. Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer. J Clin Oncol 2003; 21: 2237 – 46. [DOI] [PubMed] [Google Scholar]
- 5. Kris MG, Natale RB, Herbst RS et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non‐small cell lung cancer. A randomized trial. JAMA 2003; 290: 2149 – 58. [DOI] [PubMed] [Google Scholar]
- 6. Shepherd FA, Pererira J, Ciuleanu T et al. Erlotinib in previously treated non‐small‐cell lung cancer. N Engl J Med 2005; 353: 123 – 32. [DOI] [PubMed] [Google Scholar]
- 7. Thatcher N, Chang A, Parikh P et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: results from a randomised, placebo‐controlled, multicentre study (Iressa survival evaluation in lung cancer). Lancet 2005; 366: 1527 – 37. [DOI] [PubMed] [Google Scholar]
- 8. Non‐small Cell Lung Cancer Collaborative Group. Chemotherapy in non‐small cell lung cancer: a meta‐analysis using updated data on individual patients from 52 randomized clinical trials. BMJ 1995; 311: 899 – 909. [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly K, Crowley J, Bunn PAJ et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non‐small‐cell lung cancer: a southwest oncology group trial. J Clin Oncol 2001; 19: 3210 – 8. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R, Gatzemeier U, Betticher DC et al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non‐small‐cell lung cancer: a cooperative multinational trial. Ann Oncol 2002; 13: 1539 – 49. [DOI] [PubMed] [Google Scholar]
- 11. Schiller JH, Harrington D, Belani CP et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002; 346: 92 – 8. [DOI] [PubMed] [Google Scholar]
- 12. Kosmidis P, Mylonakis N, Nicolaides C et al. Paclitaxel plus carboplatin versus gemcitabine plus paclitaxel in advanced non‐small‐cell lung cancer: a phase III randomized trial. J Clin Oncol 2002; 20: 3578 – 85. [DOI] [PubMed] [Google Scholar]
- 13. Chen YM, Perng RP, Lee YC et al. Paclitaxel plus carboplatin, compared with paclitaxel plus gemcitabine, shows similar efficacy while more cost‐effective: a randomized phase II study of combination chemotherapy against inoperable non‐small‐cell lung cancer previously untreated. Ann Oncol 2002; 13: 108 – 15. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Department of Health and Human Services, Food and Drug Administration. Center for Drug Evaluation and Research. Guidance for industry. http://www.fda.gov/cder/guidance/index.htm
- 15. The European Agency for the Evaluation of Medicinal Products (EMEA). Note for guidance on evaluation of anticancer medicinal products in man. http://www.emea.eu.int
- 16. Paez JG, Jänne PA, Lee JC et al. EGFR Mutations in Lung Cancer: correlation with clinical response to gefitinib therapy. Sci 2004; 304: 1497 – 500. [DOI] [PubMed] [Google Scholar]
- 17. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129 – 39. [DOI] [PubMed] [Google Scholar]
- 18. Mitsudomi T, Kosaka T, Endoh H et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non‐small‐cell lung cancer with postoperative recurrence. J Clin Oncol 2005; 23: 2513 – 20. [DOI] [PubMed] [Google Scholar]
- 19. Takano T, Ohe Y, Sakamoto H et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non‐small‐cell lung cancer. J Clin Oncol 2005; 23: 6829 – 37. [DOI] [PubMed] [Google Scholar]
- 20. Tokumo M, Toyooka S, Kiura K et al. The relationship between epidermal growth factor receptor mutation and clinicopathologic features in non‐small cell lung cancers. Clin Cancer Res 2005; 11: 1167 – 73. [PubMed] [Google Scholar]
- 21. Cappuzzo F, Hirsch FR, Rossi E et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non‐small‐cell lung cancer. J Natl Cancer Inst 2004; 96: 1133 – 41. [DOI] [PubMed] [Google Scholar]
- 22. Hirsch FR, Varella‐Garcia M, McCoy J et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a southwest oncology group study. J Clin Oncol 2005; 23: 6838 – 45. [DOI] [PubMed] [Google Scholar]


