Abstract
This study examines the molecular pathways of cell–cell communication in chronic inflammatory processes associated with long‐term low‐dose urinary bladder exposure to ionizing radiation in people without major disease living more than 19 years in radio‐contaminated areas of Ukraine after the Chernobyl accident. Patterns of components of the E‐cadherin/β‐catenin complex, and transforming growth factor‐β1 (TGF‐β1) and inducible nitric oxide synthase (iNOS) expression were immunohistochemically evaluated in urinary bladder biopsies from 52 males with benign prostate hyperplasia and 8 females with chronic cystitis (group 1). For comparison, 25 males and 6 females living in non‐contaminated areas of Ukraine were also investigated (group 2). Fourteen patients with primary urothelial carcinomas, which were operated on before the Chernobyl accident, were included as a carcinoma group. Chronic proliferative atypical cystitis (‘Chernobyl cystitis’) was observed in group 1 patients. Foci of dysplasia and carcinoma in situ were found in 51 (85%) and 34 (57%) of the 60 cases, respectively. Chronic cystitis with areas of dysplasia was detected in only 4 (13%) cases of 31 group 2 patients. Statistically significant differences in immunohistochemical scores for TGF‐β1 in the urothelium and lamina propria, iNOS in the urothelium and both β‐catenin and E‐cadherin in the cytoplasm were observed between groups 1 and 2 with marked expression in group 1. Furthermore, TGF‐β1 overexpression and alteration in E‐cadherin/β‐catenin complexes in bladder urothelium might play a crucial role in urinary bladder carcinogenesis in humans exposed to long‐term low‐dose ionizing radiation. (Cancer Sci 2005)
Abbreviations:
- 137Cs
cesium‐137
- 8‐OHdG
8‐hydroxy‐5‐deoxyguanosine
- BPH
benign prostate hyperplasia
- CIS
carcinoma in situ
- COX2, cyclooxygenase 2; H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- IR
ionizing radiation
- NO
nitric oxide
- ROS
reactive oxygen species
- TGF‐β1
transforming growth factor‐β1
- UC
urothelial carcinoma.
Urinary bladder cancer risk from high doses of ionizing radiation (IR) is well known from epidemiological studies of Japanese atomic bomb survivors and of secondary malignancies in radiotherapy patients.( 1 , 2 ) Risks at low doses especially in cases of chronic long‐term radiation exposure cannot be extrapolated directly from the high dose data.( 3 ) Accumulating evidence on the close relationship between chronic inflammatory processes and carcinogenesis in humans stimulated us to elucidate the effects of chronic low dose IR on the urinary bladder urothelium in people living in radio‐contaminated areas of Ukraine. Cesium 137 (137Cs) is known to account for approximately 90% of internal radioactivity, which is concentrated and eliminated through urinary excretion in such people.( 4 ) During the 19‐year period subsequent to the Chernobyl accident the incidence of urinary bladder cancers has gradually increased from 26.2 in 1986 to 50.3 in 2003 per 100 000 of the total population in the Ukraine.( 5 )
The Chernobyl accident introduced for the first time the problem of chronic persistent long‐term human low‐dose exposure to IR. Recent studies by our group showed an increase in the incidences of dysplasia, carcinoma in situ (CIS) and even small developing urothelial carcinomas (UC) pT0–pT1, associated with chronic irradiation cystitis, accompanied by the accumulation of stable p53 protein and specific p53 mutations with G:C to A:T transitions at CpG sites, related to increased oxidative stress in urinary bladder urothelium of patients with benign prostate hyperplasia (BPH).( 6 , 7 )
In our latest study,( 8 ) we have documented for the first time that chronic long‐term (maximum of 17 years after the Chernobyl accident) low‐dose IR leads to the development of a previously unknown urinary bladder disease, radiation‐induced chronic proliferative atypical cystitis, so‐called ‘Chernobyl cystitis’, in humans. This is characterized by multiple areas of dysplasia and CIS of the urinary bladder urothelium in association with sclerosis of connective tissue and strongly increased angiogenesis without a marked inflammatory cell infiltration. It is necessary to note that Chernobyl cystitis is different from the well‐known radiation cystitis induced by high doses of IR.( 9 )
The biologic effects of chronic low doses of IR and their relationship with chronic inflammation and carcinogenesis have received a great deal of attention in the last few years.( 10 ) Low‐dose IR is known to act not only as a mitogen, but also a generator of reactive oxygen species (ROS) and nitric oxide (NO) or its derivatives; these are key contributors to carcinogenesis. NO is a short‐lived free radical, producing many reactive intermediates that account for its bioactivity. Sustained induction of the inducible form of nitric oxide synthase (iNOS) in chronic inflammation may be mutagenic, through NO‐mediated DNA damage or hindrance to DNA repair, and thus potentially carcinogenic.( 11 )
Redox homeostatic processes in the microenvironment, involving signaling pathways and interaction between cells and also with the extracellular matrix, could be targets of chronic low‐dose IR. Cellular interactions are modulated by cytokines and growth factors, which are known to be multifunctional molecules that orchestrate most aspects of the inflammatory response, eliciting their effects locally or systemically in an autocrine or paracrine manner.( 12 , 13 )
One of the transforming growth factor (TGF)‐β family multifunctional cytokines, TGF‐β1, is known to regulate cell growth and differentiation, tissue remodeling, immune response and angiogenesis. Three major isoforms of TGF‐β exist in mammals: TGF‐β1, 2, and 3. TGF‐β1 has been previously reported as a potential extracellular signaling sensor of damage.
Recently, alterations to cadherin–catenin complexes has attracted interest as an important step in the progression of many carcinomas in humans. The cadherin gene superfamily contains more than 40 members that encode for transmembrane proteins regulating calcium‐dependent cell–cell adhesion.( 14 ) Epithelial (E)‐cadherin is known to play major roles in intercellular homophilic Ca2+ dependent adhesion. This is mediated by a group of cytoplasmic proteins, including the α‐, β‐ and γ‐catenins that link E‐cadherin to the actin cytoskeleton. β‐catenin, a key regulator located within the Wingless signal transduction signaling cascade, is involved in the control of gene expression, cell behavior, cell adhesion and cell polarity.( 15 ) Several studies have shown that loss or reduction of either E‐cadherin or catenin expression is linked to clinicopathological features of bladder tumors, and E‐cadherin expression might in fact constitute a prognostic factor.( 16 )
The present study was carried out in order to examine the extracellular matrix alterations associated with the development of Chernobyl cystitis induced by chronic low‐dose IR effects in urinary bladders in different patient groups with or without chronic exposure to irradiation. Components of the E‐cadherin/β‐catenin complex, as well as TGF‐β1 and iNOS, were evaluated in urinary bladder specimens in an attempt to detect molecular lesions of cellular membranes with a possible role in urothelial changes in patients chronically exposed to low‐dose IR after the Chernobyl accident in Ukraine.
Materials and Methods
Patients and urinary bladder samples
Our subjects were selected according to the contamination level of the inhabited area (Ci/km2) The BPH patients in group 1 lived for many years in radio‐contaminated areas with densities of 137Cs contamination of 0.5–30 Ci/km2 (Table 1). The controls, group 2 of the analog BPH patients, were from clean areas with no indicated radio‐contamination but with possible chemical contamination, as all of Ukraine is considered to be an ecologic disaster area. Importantly, BPH patients might have had urinary retention, which radiation exposure to the urothelium would have been enhanced. Additionally, a group of 14 cases of CIS and UC (group 3) was studied from patients who lived in non‐contaminated areas. These patients were operated on in Kiev before the Chernobyl accident. Unfortunately, we have no means of determining what radiation dose our patients received. Recently, however, we obtained results for 137Cs measurements in 1‐day urine of the analog patients with BPH in groups 1 and 2. We found a significant elevation of 137Cs levels (5.15 Bq/L) compared with the control patients (0.29 Bq/L) from so‐called clean areas.( 17 )
Table 1.
Characteristics of patients who took part in this study
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| No. of patients (women) | 60 (8) | 31 (6) | 14 (0) |
| Median age (range) | 63 (24–76) | 65 (48–75) | 68 (48–92) |
| Cigarette smokers (%) † | 21 (35.0) | 18 (58.0) | 8 (57.0) |
| Year of surgery | 2002–04 | 2002–04 | 1999–2003 |
| Contamination levels in soils (Ci/km2) ‡ | 0.5–30 | NC | NC |
More than 20 cigarettes per day for more than 10 years.
Data from Raes et al. ( 26 ) NC, not contaminated.
All patients gave their written informed consent and the study was approved by the Institute of Urology Ethics Committee (Kiev, Ukraine). All subjects resided in the same areas before and after the Chernobyl accident. Multiple mapping biopsies (four biopsies from each patient between 0.1 and 0.4 cm in diameter) of bladder urothelium including lamina propria were taken from areas of the bladder neck, both orifices and erythematous sites if they were detected. All biopsy samples from groups 1 and 2 were of suitable quality for diagnosis and investigation. It is important to note that the primary urothelial cancer in urinary bladder or in renal pelvis was never diagnosed in any patient of either group.
A total of 364 formalin‐fixed, paraffin‐embedded specimens were histologically investigated, including 308 from 77 male patients without hematuria and any symptoms of bladder disease who underwent a suprapubic prostatectomy for BPH and 56 from 14 female patients with symptoms of chronic cystitis treated at the Institute of Urology in Kiev during 2002–04. All patients who were treated in the Institute during this period were included in our study, without exception, and they were never included in our previous reports. It is necessary to add that, from 1994, the Institute of Urology in Kiev has collected all BPH patients who underwent suprapubic prostatectomy. We have a computerized register of the patients enrolled from 1994 until now. Unfortunately, the number of patients in control group 2 was lower because the so‐called clean areas of Ukraine are small compared with the radio‐contaminated areas.
Histopathology and immunohistochemical (IHC) staining
Sections (4–5 µm thick) of bladder samples were stained with hematoxylin and eosin (H&E) and bladder lesions were classified according to the histological typing defined by the new classification of the World Health Organization.( 9 ) IHC staining was carried out for all 46 patients, using the standard avidin‐biotin‐peroxidase complex method and a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, USA).
Serial sections were deparaffinized and microwaved in citrate buffer (pH 6.1) for 30 min for antigen retrieval, except for those used for TGF‐β1. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in distilled water for 5 min. Nonspecific binding was blocked with 5% normal goat serum for TGF‐β1, or horse serum for E‐cadherin, β‐catenin and iNOS type II in phosphate‐buffered saline at room temperature for 30 min. Incubation was carried out with antirabbit TGF‐β1 polyclonal antibody (sc 146; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:1000 dilution, antimouse E‐cadherin (sc 8426) monoclonal antibody (Santa Cruz Biotechnology) at 1:100 dilution, antimouse β‐catenin (c 19220) monoclonal antibody (Transduction Laboratories, Lexington, KY, USA) at 1:500 dilution and antimouse iNOS/NOS type II monoclonal antibody (Transduction Laboratories) at 1 300 dilution, overnight at 4°C. Tissue sections known to be positive were used as one set of controls; for negative controls, exposure to the primary antibody was omitted. Color was developed with 3,3′‐diaminobenzidine, and counterstaining was performed with Mayer's hematoxylin for 1 min. IHC was conducted in a blind fashion without the knowledge of the patient group. Consecutive serial sections were used for IHC and H&E staining. All specimens were evaluated independently by at least two pathologists. Approximately 14–16 sections per case were analyzed.
Quantitative analysis
Quantitative estimation of IHC staining was performed according to a system for evaluating and grading immunostaining patterns, with multiple values for extent and intensity, using a scoring system of 0–9.( 18 ) The extent of staining was scored on a semiquantitative scale of 0–3, using the following criteria: 0, no detectable staining; 1, <10% scattered cells; 2, >10% but <50% stained cells; 3, homogeneous staining in >50% of cells. The intensity of staining was scored using the following criteria: 0, no detectable staining; 1, weakly stained cytoplasm and/or nuclei; 2, moderately stained cytoplasm and/or nuclei; 3, strongly stained cytoplasm and/or nuclei. Final scores were derived by multiplying the extent score by the intensity score. Estimation of IHC was performed in a blind fashion without the knowledge of the patient group. Consecutive serial sections were used for IHC and H&E staining.
Statistical analysis
The significance of differences between groups for incidences of urothelial lesions was analyzed using the χ2 test or Fisher's exact probability test (Statview SE + Graphics, version 4.5 (1996); Abacus Concepts, Berkeley, CA, USA). IHC scores for each case were evaluated separately using the non‐parametric Mann–Whitney U‐test with the same software.
Results
Patients and histopathology
Table 1 lists the characteristics of patients in all three groups. The gender ratio and the average age were not significantly different between the groups, although the number of female patients was few in both cases. Table 2 shows the significant differences in incidences of urinary bladder dysplasia and urothelial carcinomas in patients in groups 1 and 2. Urinary bladder biopsies from group 1 patients demonstrated typical features of chronic proliferative atypical cystitis, or Chernobyl cystitis. Multiple areas of dysplasia (low‐grade intraurothelial neoplasia) and CIS, or high‐grade intraurothelial neoplasia, were observed in 51 (85%) and 34 (57%), respectively, of the 60 group 1 patients. Areas of CIS in eight (24%) of 34 cases had a nested pattern with microinvasion into the lamina propria in three cases. Grade I papillary pTa (three cases) and invasive pT1 (three cases) small urothelial carcinomas were also detected incidentally in group 1 patients. Proliferative forms of chronic cystitis with von Brunn's nests and cystitis cystica as well as cystitis glandularis were detected in 28 (47%) patients with frequent dysplasia. Large areas of sclerosis and fibrosis in the bladder lamina propria were observed in 41 (68%) of the 60 patients. Along with these lesions, definite new vascularization, sometimes with the development of dilated vessels full of erythrocytes and hemorrhage, was detected in 51 (85%) of the cases. Scattered inflammatory cellular infiltration consisting of macrophages, lymphocytes, histiocytes and plasma cells was also present in 16 (27%). Three (37%) of the 8 females exhibited areas of dysplasia and CIS in association with large areas of sclerosis and some inflammatory infiltration with patchy hemorrhage in the lamina propria. Grade I papillary urothelial carcinoma pTa, leucoplakia with areas of dysplasia and nephrogenic adenoma without malignancy were also detected in three group 1 females. Remarkable urothelial proliferative changes or neoplastic lesions were not present in group 2 patients, although clear cells in the urothelium and urothelial hyperplasia were evident in 5 (16%) of the 31 patients. Areas of mild dysplasia were detected in four (13%) of the group 2 male patients. The majority of UC (64%) from the additional group were superficial (pTa‐pT1), grade 1–2. Invasive UC (pT1–pT3), grade 1–2 were detected in five (35%) of 14 patients.
Table 2.
Incidence of urinary bladder dysplasias and carcinomas in patients from contaminated (group 1) and non‐contaminated (group 2) areas of Ukraine
| Group 1 | Group 2 | |
|---|---|---|
| No. of cases | 60 | 31 |
| No. with dysplasia (%) | 51 (85) ‡ | 4 (13) † |
| No. with carcinoma (%) | ||
| Carcinoma in situ | 34 (57) § | 0 |
| Urothelial carcinoma | 6 (10) | 0 |
| Total no. of cases (%) | 40 (67) | 0 |
Mild dysplasia.
Significantly different versus group 1 at P < 0.01 (χ2 or Fisher's exact probability test).
Significantly different versus group 1 at P < 0.001 (χ2 or Fisher's exact probability test).
Immunohistopathology
Table 3 shows average IHC scores for TGF‐β1 (separately for urothelial lesions and bladder lamina propria) and iNOS, as well as for E‐cadherin/β‐catenin (separately for urothelial membrane and cytoplasmic localization), respectively, in groups 1 and 2. Histological and IHC findings in areas of urothelial dysplasia and CIS of group 1 patients are illustrated in Fig. 1.
Table 3.
Immunohistochemical scores of proteins and factors in patients from contaminated (group 1) and non‐contaminated (group 2) areas of Ukraine
| Protein/factor | Group 1 | Group 2 |
|---|---|---|
| TGF‐β1 (epithelium) | 4.9 ± 3.0 † | 0.5 ± 1.3 |
| TGF‐β1 (lamina propria) | 6.7 ± 2.3 † | 2.4 ± 1.8 |
| Inducible nitric oxide synthase | 5.7 ± 2.5 † | 2.2 ± 1.9 |
| β‐catenin (cytoplasm) | 5.2 ± 2.7 † | 1.6 ± 1.7 |
| β‐catenin (membrane) | 6.5 ± 2.5 | 6.0 ± 2.3 |
| E‐cadherin (cytoplasm) | 4.3 ± 3.2 ‡ | 1.9 ± 2.1 |
| E‐cadherin (membrane) | 6.2 ± 2.4 | 5.7 ± 2.5 |
Significantly different versus group 2 at P < 0.0001.
Significantly different versus group 2 at P < 0.001. TGF, transforming growth factor.
Figure 1.
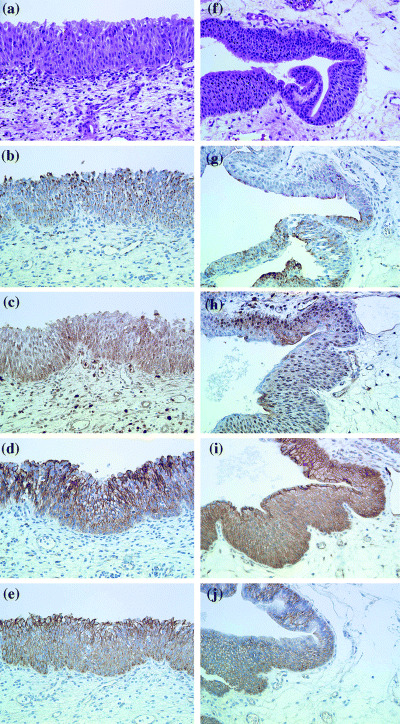
Immunohistochemical findings in group 1 male patients with so‐called Chernobyl cystitis, areas of urothelial dysplasia (a–j). (a, f) hematoxylin and eosin; (b, g) inducible nitric oxide synthase; (c, h) transforming growth factor‐β1; (d, i) E‐cadherin; (e, j) β‐catenin. Magnification × 400.
Statistically significant differences in TGF‐β1 in both urothelial lesions and bladder lamina propria, and for iNOS, cytoplasmic β‐catenin and E‐cadherin in urothelial lesions were observed between groups 1 and 2 (P < 0.0001, < 0.0001, < 0.0001, < 0.0001 and < 0.008, respectively). However, no significant variation was found for β‐catenin or E‐cadherin expression in membranes. Group 1 specimens showed moderate to strong (scores 6–9) cytoplasmic and membrane iNOS and TGF‐β1 immunostaining, mainly in superficial layers of urothelial lesions in 38 (63%) and 30 (50%) of the 60 patients, respectively. TGF‐β1 expression (scores 9 and 6) was elevated in endothelial cells and fibroblasts in the lamina propria. Strong homogeneous staining was also noted with large areas of sclerosis and fibrosis, especially close to the urothelium, detected in 88.4% of group 1 patients.
Strong immunoreactivity for β‐catenin with urothelial cytoplasmic immunostaining was found in group 1 patients. Moreover, β‐catenin had accumulated in the urothelial cytoplasm in 51 (85%) out of the 60 group 1 patients. In accordance, increased levels of this protein were observed in 46 (77%) patients. The same correlation was obtained with E‐cadherin immunoreactivity (scores 9 and 6), both membrane and to a lesser extent cytoplasmic, irregular staining being present in 48 (80%) and 41 (68%) group 1 patients, respectively. However, negative or very slight E‐cadherin cytoplasmic staining was detected in 16 (27%) patients of the same group. In addition, membrane β‐catenin and E‐cadherin immunostaining (scores 6 and 9) was also evident in 26 (83%) and 24 (77%) patients, respectively, in group 2, although regions with normal immunostaining of E‐cadherin and β‐catenin were seen more frequently in the urothelium of this group, with localization predominantly in the basal cell layers.
The expression pattern of β‐catenin was similar to that of E‐cadherin in the different areas of urothelial dysplasia and CIS. These lesions were strongly positive (score 9) for predominantly cytoplasmic staining, with lower expression (scores 4–6) observed for TGF‐β1 and iNOS. Papillary and invasive small urothelial carcinomas were scored from 0 to 2 for TGF‐β1 and iNOS and for β‐catenin and E‐cadherin with predominantly cytoplasmic localization. Reduced expression of E‐cadherin and β‐catenin was particularly evident in the invasive borders of these tumors. All tumors were negative for iNOS and TGF‐β1 immunostaining. Blood vessels, endothelial cells, macrophages, plasma cells and histiocytes demonstrated positive membrane iNOS, β‐catenin and E‐cadherin immunostaining.
The staining patterns in the additional UC group showed significantly decreased levels of TGF‐β1 (predominantly negative epithelial and lamina propria staining) as well as β‐catenin cytoplasmic and membranous expression with average scores of 1.5 (+ 1.6) and 5.0 (+ 2.1), respectively, compared with group 1 cases (P < 0.0001; Fig. 2). E‐cadherin expression in the epithelial membranes and cytoplasm was significantly stronger in the areas of dysplasia and CIS of group 1 cases, compared with the CIS and UC of the additional UC group (P < 0.0001). iNOS expression was not significantly different between these two groups. Moreover, β‐catenin and E‐cadherin expressions in group 2 and the additional UC group were not changed significantly, with predominantly cytoplasmic expression in UC.
Figure 2.
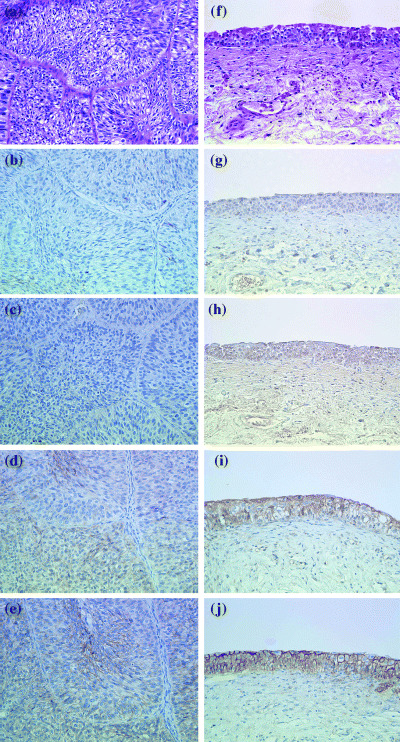
Immunohistochemical findings in group 1 male patients with urothelial carcinoma (a–e) and group 2 male patients with dysplasia (f–j). (a, f) hematoxylin and eosin; (b, g) inducible nitric oxide synthase; (c, h) transforming growth factor‐β1; (d, i) E‐cadherin; (e, j) β‐catenin. Magnification × 400.
Discussion
In this report, we have documented for the first time that Chernobyl cystitis with foci of urothelial dysplasia and CIS, and fibrosis of the lamina propria, is associated with activation of TGF‐β1, as well as disruption of E‐cadherin/β‐catenin expression, which might be a result of chronic long‐term low‐dose IR exposure. It is important to note, as also confirmed in our recent studies, that preneoplastic urothelial lesions occur in men living in radio‐contaminated areas with BPH, who might presumably be suffering from urinary retention and demonstrate increased 137Cs levels in the urine.( 17 ) Chronic inflammation of the urinary tract is a significant risk factor for the development of bladder cancer( 19 ) and chronic long‐term exposure to IR leads to extensive tissue damage, due to continuous production of potent oxidants and generation of ROS and NO.( 19 ) Our previous study showed markedly elevated levels of iNOS, 8‐OHdG and COX2 expression in bladder urothelium lesions from the analog group 1 patients associated with p53 accumulation together with the frequent G:C to A:T transitions at CpG dinucleotides in the p53 gene with a hot spot in codon 245,( 6 ) and this is typical for NO‐mediated DNA deamination. Our present results of elevated levels of iNOS expression in bladder urothelium in patients from radio‐contaminated areas (group 1) support our recent suggestion concerning the possible interaction between toxic peroxynitrites, 8‐OHdG and p53 that offers an explanation of molecular mechanisms of multistep carcinogenesis in human bladder cancer.( 6 , 7 ) These data also support the hypothesis that DNA deamination was induced by oxidative stress in human bladder urothelium induced by long‐term low‐dose IR in people living for more than 19 years in radio‐contaminated (137Cs) areas of Ukraine, which results in the development of preneoplasia in the urinary bladder.
Excess ROS production clearly damages DNA; low levels might affect cell signaling, and in particular, redox modulation. TGF‐β1 is a major extracellular signaling sensor of damage, as it mediates redox homeostasis in cells and contributes to cell–cell communication.( 12 ) Interestingly, it is known as a mediator of tissue response to IR, pointing to a role in orchestrating changes due to oxidative stress.( 20 ) The present results indicate that alteration of TGF‐β1 is a frequent event in background bladder urothelium of group 1 patients, with a decrease observed in areas of dysplasia and CIS and little to no immunoreactivity in urothelial carcinomas. Furthermore, TGF‐β1 immunostaining was here found to be most pronounced in the lamina propria, in particular, in areas of sclerosis and fibrosis, where production of inflammatory cell cytokines is clearly important.( 13 ) IR appears to be one of a few exogenous agents, which can cause TGF‐β1 activation in situ.
Evidence has accumulated that gap junctional intercellular communication is sensitive to oxidative stress.( 21 ) Cadherin/catenin complexes are required for formation of gap junctions and intercellular communications,( 22 ) and mutations in the β‐catenin gene have been described for various human cancers.( 23 ) Our findings indicate that alterations of E‐cadherin/β‐catenin are frequent in bladder urothelium of people living in radio‐contaminated areas. Elevated protein levels and abnormal intracellular localization could be early events in bladder carcinogenesis induced by long‐term low‐dose IR exposure. Several studies have suggested that oxidative stress causes internalization of E‐cadherin from the plasma membrane to the cytosol.( 22 ) Intracellular accumulation of β‐catenin might correspond to an increased level of hypophosphorylated β‐catenin caused by a mutation, in turn leading to transduction of oncogenic signals and cancer progression.( 24 ) Overexpression might result in the premature re‐entry of cells into the cell cycle after γ‐irradiation‐induced DNA damage, and thereby promote the accumulation of oncogene mutations and carcinogenesis.( 25 )
Importantly, aberrant expression of β‐catenin and E‐cadherin, in association with TGF‐β1 upregulation in lamina propria, are the essential molecular alterations in the pathogenesis of urothelial carcinomas in an environment of continued chronic long‐term low‐dose IR exposure, in comparison with CIS and UCs which had developed before the Chernobyl accident.
Altogether, our data favor the conclusion of a crucial role for cellular communication in Chernobyl cystitis due to chronic low‐dose IR. Present findings also support our recent idea of distinct molecular carcinogenic pathways for bladder cancer in Ukraine before and after the Chernobyl disaster.( 27 )
Acknowledgments
We thank K. Touma, Y. Onishi and M. Dokoh for their expert assistance. This investigation was supported by a Grant‐in‐Aid for Scientific Research (B) (12576005) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Pierce D, Shimizu Y, Preston D, Vaeth M, Maubuchi K. Studies of the mortality of atomic bomb survivors. Radiat Res 1996; 146: 1–27. [PubMed] [Google Scholar]
- 2. Prianichnikova MB. Ionizing radiation and bladder cancer. Urol Nefrol (Mosk) 1996; 6: 44–5. [PubMed] [Google Scholar]
- 3. Hall EJ. Radiation, the two‐edged sword: cancer risks at high and low doses. Cancer J 2000; 6: 343–50. [PubMed] [Google Scholar]
- 4. Richmond CR. Accelerating the turnover of internally deposited radio‐cesium. In: Kornberg HA, Norwood WD, eds. Diagnosis and Treatment of Deposited Radionuclides. Battele‐Northwest Richland, WA: Excerpta Medical Foundation, 1968; 465–78. [Google Scholar]
- 5. Pavlova L, Saydacova N, Startzeva L. The state of urologic assistance for the population of Ukraine and the ways to improve it. In: Annual Reports of the Health Care in Ukraine. Kiev: Ukrainian Ministry of Health; 2004. [Google Scholar]
- 6. Yamamoto S, Romanenko A, Wei M et al. Specific p53 gene mutations in urinary bladder epithelium after the Chernobyl accident. Cancer Res 1999; 59: 3606–9. [PubMed] [Google Scholar]
- 7. Romanenko A, Morimura K, Wanibuchi H et al. Increased oxidative stress with gene alteration in urinary bladder urothelium after the Chernobyl accident. Int J Cancer 2000; 86: 790–8. [DOI] [PubMed] [Google Scholar]
- 8. Romanenko A, Morimura K, Wanibuchi H et al. Urinary bladder lesions induced by persistent chronic low‐dose ionizing radiation. Cancer Sci 2003; 94: 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. Pathology and genetics of tumours of the urinary system and male genital organs . In: WHO Classification of Tumors. Lyon: IARC Press, 2004. [Google Scholar]
- 10. Clarke RH. Control of low‐level radiation exposure: what is the problem and how can it be solved? Health Phys 2001; 80: 391–6. [DOI] [PubMed] [Google Scholar]
- 11. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumor progression. Lancet Oncol 2001; 2: 149–56. [DOI] [PubMed] [Google Scholar]
- 12. Barcellos‐Hoff MN, Brooks AL. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effect and genomic instability. Radiat Res 2001; 156: 618–27. [DOI] [PubMed] [Google Scholar]
- 13. Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation‐induced pulmonary fibrosis: examination of cytokine and chemokine receptor families. Radiat Res 2002; 157: 256–65. [DOI] [PubMed] [Google Scholar]
- 14. Parrish AR, Catania JM, Orozco J, Gandolfi AJ. Chemically induced oxidative stress disrupts the E‐cadherin/catenin cell adhesion complex. Toxicol Sci 1999; 51: 80–6. [DOI] [PubMed] [Google Scholar]
- 15. Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of the Wnt signaling through beta‐catenin. Science 2002; 296: 1644–6. [DOI] [PubMed] [Google Scholar]
- 16. Bindels EM, Vermey M, De Both NJ, van Der Kwast TH. Influence of the microenvironment on invasiveness of human bladder carcinoma cell lines. Virchows Arch 2001; 439: 522–9. [DOI] [PubMed] [Google Scholar]
- 17. Romanenko A, Morimura K, Wei M, Zaparin W, Vozianov A, Fukushima S. DNA damage repair in bladder urothelium after the Chernobyl accident in Ukraine. J Urol 2002; 168: 973–7. [DOI] [PubMed] [Google Scholar]
- 18. Malmstrom PU, Busch C, Norlen BJ, Andersson B. Expression of ABH blood group isoantigen as a prognostic factor in transitional cell bladder carcinoma. Scand J Urol Nephrol 1998; 22: 265–70. [DOI] [PubMed] [Google Scholar]
- 19. Wall BM, Dmochowski RR, Malecha M, Mangold T, Bobal MA, Cooke CR. Inducible nitric oxide synthase in the bladder of spinal cord injured patients with chronic indwelling urinary catheter. J Urol 2001; 165: 1457–61. [PubMed] [Google Scholar]
- 20. Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos‐Hoff MH. Latent transforming growth factor beta1 activation in situ: quantitative and functional evidence after low‐dose gamma‐irradiation. FASEB J 1997; 11: 991–1002. [DOI] [PubMed] [Google Scholar]
- 21. Sai K, Upham BL, Kang KS, Hasegawa R, Inoue T, Trosko JE. Inhibitory effect of pentachlorophenol on gap junctional intercellular communication in rat liver epithelial cells in vitro. Cancer Lett 1998; 130: 9–17. [DOI] [PubMed] [Google Scholar]
- 22. Jansen LA, Mesnil M, Jongen WM. Inhibition of gap junctional intercellular communication and delocalization of the cell adhesion molecule E‐cadherin by tumor promoters. Carcinogenesis 1996; 17: 1527–31. [DOI] [PubMed] [Google Scholar]
- 23. Pereira‐Suarez AL, Meraz MA, Lizano M, Estrada‐Chavez C, Hernandez F, Olivera PA. Frequent alterations of the beta‐catenin protein in cancer of the uterine cervix. Tumour Biol 2002; 23: 45–53. [DOI] [PubMed] [Google Scholar]
- 24. Shiina H, Igawa M, Urakami S et al. Alterations of beta‐ and gamma‐catenin in N‐butyl‐N‐(‐4‐hydroxybutyl) nitrosamine‐induced murine bladder cancer. Cancer Res 2001; 61: 7101–9. [PubMed] [Google Scholar]
- 25. Orford K, Orford CC, Byers SW. Exogenous expression of beta‐catenin regulates contact inhibition, anchorage‐independent growth, anoikis and radiation‐induced cell cycle arrest. J Cell Biol 1999; 146: 855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raes F, De Cort M, Graziani G. Multi–fractal nature of radioactivity deposition on soil after the Chernobyl accident. Health Phys 1991; 61: 271–82. [DOI] [PubMed] [Google Scholar]
- 27. Morimura K, Romanenko A, Min W et al. Possible distinct molecular carcinogenic pathways for bladder cancer in Ukraine, before and after the Chernobyl disaster. Oncol Reports 2004; 11: 881–5. [PubMed] [Google Scholar]


