Abstract
The present study was conducted to compare the diagnostic accuracy between carbon‐11 choline (11C‐choline) positron emission tomography (PET)/computed tomography (CT) and conventional imaging for the staging of bone and soft tissue sarcomas. Sixteen patients who underwent 11C‐choline PET/CT prior to treatment were evaluated retrospectively for staging accuracy. Conventional imaging methods consisted of 99,mTc‐hydroxymethylene diphosphonate bone scintigraphy, chest CT and magnetic resonance imaging of the primary site. The images were reviewed and a consensus was reached by two board‐certified radiologists who were unaware of any clinical or radiological information using hard‐copy films and multimodality computer platform. Tumor stage was confirmed by histological examination and/or by an obvious progression in number and/or size of the lesions on follow‐up examinations. Reviewers examining both 11C‐choline PET/CT and conventional imaging classified T stage in all patients. Interpretation based on 11C‐choline PET/CT, the Node (N) stage was correctly diagnosed in all patients, whereas the accuracy of conventional imaging in N stage was 63%. Tumor Node Metastasis (TNM) stage was assessed correctly with 11C‐choline PET/CT in 15 of 16 patients (94%) and with conventional imaging in eight of 16 patients (50%). The overall TNM staging and N staging accuracy of 11C‐choline PET/CT were significantly higher than that of conventional imaging (P < 0.05). 11C‐choline PET/CT is more accurate than conventional imaging regarding clinical staging of patients with bone and soft tissue sarcomas. A whole body 11C‐choline PET/CT might be acceptable for imaging studies of tumor staging prior to treatment. (Cancer Sci 2006; 97: 1125–1128)
The general diagnostic tools for staging bone and soft tissue sarcomas are clinical examination, magnetic resonance imaging (MRI) and X‐ray of the primary tumor site, chest X‐ray or computed tomography (CT), and bone scintigraphy.( 1 )
Positron emission tomography (PET) with [18F]‐fluoro‐2‐deoxy‐d‐glucose (FDG) has been used in the evaluation of patients with bone and soft tissue sarcomas for grading and therapy monitoring.( 2 , 3 , 4 , 5 , 6 , 7 ) Most of these studies reveal that 18FDG‐PET is superior in the assessment of grading and therapy monitoring compared with conventional imaging.
Recently, carbon‐11 choline (11C‐choline) has been introduced as a new oncological positron‐emitting radiopharmaceutical for evaluation of a variety of malignant tumors.( 8 , 9 , 10 , 11 ) Choline is an essential component of the cell membrane, and choline uptake may be via a choline‐specific transporter protein.( 12 ) Choline kinase, which catalyzes the phosphorylation of choline, is upregulated in malignant cells. Some studies have demonstrated additional gains in diagnostic accuracy using 11C‐choline.( 13 ) 11C‐choline uptake is significantly higher in malignant tumors than in benign tumors and correlates well with the degree of 18FDG accumulation with the lesion, while the high background activity owing to excretion via urinary tract interferes with evaluation on 18FDG‐PET.( 14 , 15 ) However, the role of 11C‐choline PET scan in the staging of bone and soft tissue sarcomas has not been clarified. To fully elucidate the role of 11C‐choline PET, the comparison with 18FDG‐PET and conventional imaging modalities are needed.
A new‐modality PET/CT can improve the localization of tumors and accuracy of staging in patients because anatomic and molecular information can be coregistered precisely.( 16 ) The aim of the current study was to compare the diagnostic accuracy between 11C‐choline PET/CT and conventional imaging for the staging of bone and soft tissue sarcomas.
Materials and Methods
Patient. We retrospectively reviewed 11C‐choline PET/CT results from September 2005 to March 2006 for patients with bone and soft tissue sarcomas, who subsequently underwent surgical resection, chemotherapy and/or radiotherapy within 2 weeks. 11C‐choline PET/CT was performed for initial staging in 12 patients and for restaging of recurrent disease in four patients. The study population consisted of 13 men and three women with a mean age of 44 years (range, 13–75 years). The clinical records of all of the patients were available for review. This study was conducted in accordance with the amended Helsinki declaration and the protocol was approved by the Institutional Review Board (National Cancer Center, Research Center for Cancer Prevention and Screening). All of the patients provided their written informed consent to participate in the present study and to review their records and images.
Radiopharmaceuticals. Carbon‐11 choline was synthesized with a commercial module essentially using the method described by Hara and Yuasa.( 17 ) 11CO2 was converted to 11C‐methyl iodide by LiAlH4/HI reaction. 11C‐methyl iodide was trapped in dimethylaminoethanol. After a washing step with ethanol and water, 11C‐choline retained on a cation exchange resin was eluted with saline. Radiochemical purity of the solution was evaluated by liquid chromatography radiodetector. The organic solvents were analyzed by gas chromatography. Endotoxin was assayed by the lysosomal acid lipase method.
PET/CT. Scans were acquired with a PET/CT device (Aquiduo; Toshiba Medical Systems, Tokyo, Japan) that consisted of a PET scanner (ECAT HR+; CTI, Knoxville, TN, USA) and 16‐section CT scanner (Aquilion V‐detector; Toshiba Medical Systems) with a whole‐body mode implemented as the standard software. Prior to the 11C‐choline PET/CT study, the patients fasted for at least 6 h. CT was performed from the head to the mid‐thigh according to a standardized protocol with the following setting: axial 3.0‐mm collimation × 16 modes; 120 kVp; 100 mAs; and a 0.5‐second tube rotation, pitch 11.0. Patients maintained normal shallow respiration during the three‐dimensional acquisition of CT scans. No iodinated contrast material was administered. Emission scans from the base of the skull to the leg were obtained starting 5 min after the intravenous administration of 350–573 MBq of 11C‐choline. The acquisition time for PET was 2 min per table position. Images were reconstructed with attenuation‐corrected ordered‐subset expectation maximization with two iterations and eight subsets using emission scans and CT data.
Positron emission tomography, CT and coregistered PET/CT images were analyzed with dedicated software (e‐soft; Siemens). The initial review of the attenuation‐corrected PET images was performed using transaxial, coronal and sagittal planes. The images were reviewed and a consensus was reached by two board‐certified radiologists who were unaware of any clinical or radiological information using a multimodality computer platform. 11C‐choline uptake was considered to be abnormal when it was substantially greater than the surrounding normal tissue. For 11C‐choline PET/CT, tumor sizes and T staging were determined by the CT part of PET/CT. 11C‐choline‐avid lymph nodes or distant metastases on PET/CT were interpreted as positive for metastases regardless of size. Lymph nodes with abnormal uptake were deemed positive for metastases even when they were smaller than 10.0 mm in short axis nodal diameter. Lung nodules without abnormal uptake but highly suggestive of lung metastases on 11C‐choline PET/CT were considered to be positive for metastases. A pixel region of interest (ROI) was outlined within regions of increased 11C‐choline uptake and measured on each slice. For quantitative interpretations, standardized uptake value (SUV) was determined according to the standard formula, with activity in the ROI given in Bq per mL/injected dose in Bq per weight (kg). However, time decay correction for whole‐body image acquisition was not conducted. A SUV of more than 2.5 was considered to characterize malignancy.
Conventional imaging. Conventional imaging methods, performed within 2 weeks of 11C‐choline PET/CT, either before or after, were 99,mTc‐hydroxymethylene diphosphonate (HMDP) bone scintigraphy, chest CT and MRI of the primary site. 99,mTc‐HMDP bone scintigraphy was performed 2 h after intravenous injection of 740 MBq of 99,mTc‐HMDP. Both anterior and posterior whole‐body planar images were obtained simultaneously with a dual‐headed gamma camera (E.CAM; Siemens). Chest CT was performed using a multidetector scanner (Aquilion V‐detector; Toshiba Medical Systems) with the following setting: axial 4.0‐mm × 4 modes; 120 kVp, automated electric current; 0.5‐second tube rotation; and pitch 5. Images were reconstructed with 10.0‐mm slice thickness by means of a standard algorithm. MRI of the primary site was performed using a 1.5 Tesla system (Signa Horizon; GE Medical Systems, Milwaukee, WI, USA or Visart; MRI produced by Toshiba Medical Systems, Tokyo, Japan). Pulse sequences comprised T1‐weighted spin echo (SE) images, T2‐weighted fast spin echo (FSE) images, as well as post‐contrast T1‐weighted SE images with fat suppression after injection of contrast material. Pulse sequence parameters and slice orientation varied with the examined anatomic site. The images were reviewed and a consensus was reached by two board‐certified radiologists who were unaware of any clinical or radiological information using hard‐copy films and multimodality computer platform. The two readers for 11C‐choline PET/CT and those for conventional imaging were not the same persons.
Each tumor was staged according to the Tumor Node Metastasis (TNM) classification of the International Union Against Cancer for sarcoma of bone and the American Joint Committee staging protocol for sarcoma of the soft tissue.( 18 , 19 ) T, N and M stages were assigned for both PET/CT and conventional imaging. T staging was confirmed by pathological evaluation using specimens obtained from surgical resection of the primary tumors. N staging was confirmed by pathological examinations in two patients using specimens obtained from sampling of regional nodes. In terms of extraregional nodes in two patients, nodal staging was confirmed by an obvious progression in number and/or size of the lesions on follow‐up examinations. The mean follow‐up period was 172 days (range, 44–322 days).
Statistical analysis. All valuables were assessed on a patient‐by‐patient basis. The McNemar test was used for paired comparisons between 11C‐choline PET/CT and conventional imaging. Statistical analysis was performed with the SPSS version 11 software program (SPSS, Chicago, IL, USA).
Results
There were eight bone sarcomas and eight soft tissue sarcomas (Table 1). The primary sites included shoulder (n = 2), chest wall (n = 2), retroperitoneum (n = 2), iliac bone (n = 2), leg (n = 2), thigh (n = 1), perineum (n = 1), tibia (n = 1), femur (n = 1), mandible (n = 1) and spine (n = 1). Pathological diagnoses were osteosarcoma (n = 4), Ewing sarcoma (n = 3), leiomyosarcoma (n = 3), clear cell sarcoma (n = 1), chondrosarcoma (n = 1), pleomorphic malignant fibrous histiocytoma (n = 1), myxoid liposarcoma (n = 1), rhabdomyosarcoma (n = 1), and alveolar soft part sarcoma (n = 1). Histological grade of tumors was grade 1 (n = 1), grade 2 (n = 1), grade 3 (n = 11) and grade 4 (n = 3).
Table 1.
Summary of patients and confirmed staging
| Patient no. | Diagnosis | SUV | Size (mm) | Staging type | Location | TNM | Metastasis | Grade | Stage |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Leiomyosarcoma | 4.63 | 110 | Initial | Retroperitoneum | T2bN0M1 | Soft tissue | High | IV |
| 2 | Rhabdomyosarcoma | 3.03 | 60 | Initial | Perineum | T2bN1M0 | Lymph node | High | IV |
| 3 | Pleomorphic malignant Fibrous histiocytoma | 15.05 | 133 | Initial | Chest wall | T2bN0M1 | Bone, pleura, lymph node | High | IV |
| 4 | Leiomyosarcoma | 4.10 | 80 | Initial | Retroperitoneum | T2bN0M,P | Lung | Low | IV |
| 5 | Osteosarcoma | 6.70 | 110 | Initial | Iliac bone | T2N0M1b | Bone, lung | High | IVB |
| 6 | Clear cell sarcoma | 13.03 | 80 | Initial | Chest wall | T2bN0M1 | Bone, lung, pleura, lymph node | High | IV |
| 7 | Myxoid liposarcoma | 2.15 | 50 | Initial | Leg | T1aN1M0 | Lymph node | Low | IVB |
| 8 | Osteosarcoma | 5.31 | 110 | Initial | Tibia | T2N1M0 | Lymph node | High | IV |
| 9 | Ewing sarcoma | 3.46 | 95 | Initial | Leg | T2bN0M0 | N/A | High | III |
| 10 | Ewing sarcoma | 9.86 | 102 | Initial | Shoulder | T2N0M0 | N/A | High | IIB |
| 11 | Ewing sarcoma | 6.14 | 16 | Initial | Spine | T1N0M0 | N/A | High | IA |
| 12 | Chondrosarcoma | 5.99 | 110 | Initial | Iliac bone | T2N0M1b | Bone | High | IVB |
| 13 | Leiomyosarcoma | 3.18 | 50 | Restaging | Thigh | T1bN1M1 | Bone, soft tissue, lymph node | High | IV |
| 14 | Osteosarcoma | 4.95 | 75 | Restaging | Jaw | T1N0M1a | Lung | High | IVA |
| 15 | Osteosarcoma | 3.60 | 50 | Restaging | Femur | T1N0M1b | Lung, bone | High | IVB |
| 16 | Alveolar soft part sarcoma | 3.60 | 25 | Restaging | Shoulder | T2N0M1 | Bone | High | IV |
N/A, not applicable; SUV, standardized uptake value; TNM, Tumor Node Metastasis.
All patients of initial staging had increased 11C‐choline uptake of the primary lesion (average maximal SUV ± SD: 5.92 ± 3.68 [range, 2.15–15.05]). Pathological T stages available in patients with initial staging are as follows: T1 (n = 1), T1a (n = 1), T1b (n = 1), T2 (n = 4) and T2b (n = 5). T stages in patients with restaging were T1 (n = 2), T1b (n = 1) and T2 (n = 1). Tumor size of patients for initial staging was 78.5 ± 34.0 mm (mean ± SD [range, 16.0–133.0 mm]). Both 11C‐choline PET/CT and conventional imaging classified the T stage correctly in all patients. Twelve (75%) of the 16 patients had N0 disease. Using 11C‐choline PET/CT, the N stage was correctly assigned in all patients, whereas the accuracy of conventional imaging in N stage was 63% (P = 0.041, Table 2). Understaging occurred in six patients (38%). Three of these patients (19%) had metastasis of inguinal node whose largest diameter was less than 10.0 mm (Fig. 1). The incidence of distant metastases was high in our study population. Both 11C‐choline PET/CT and conventional imaging detected bone metastases in seven patients (44%), lung metastases in five (31%) and pleural dissemination in two (18%, Fig. 2). Using 11C‐choline PET/CT, the M stage was correctly assigned in 15 patients (94%), whereas the accuracy of conventional imaging in M stage was 81% (P = 0.617, Table 2).
Table 2.
Staging of bone and soft tissue sarcoma
| Variables | 11C‐choline PET/CT | Conventional imaging | P‐value |
|---|---|---|---|
| Overall stage | 0.023 | ||
| Correct | 15 (94) | 8 (50) | |
| Understaged | 1 (6) | 8 (50) | |
| Overstaged | 0 | 0 | |
| N stage | 0.041 | ||
| Correct | 16 (100) | 10 (63) | |
| Understaged | 0 | 6 (38) | |
| Overstaged | 0 | 0 | |
| M stage | 0.617 | ||
| Correct | 15 (94) | 13 (81) | |
| Understaged | 1 (6) | 3 (19) | |
| Overstaged | 0 | 0 |
Note: Data are presented as number (n). Numbers in parentheses are percentages. CT, computed tomography; PET, positron emission tomography.
Figure 1.
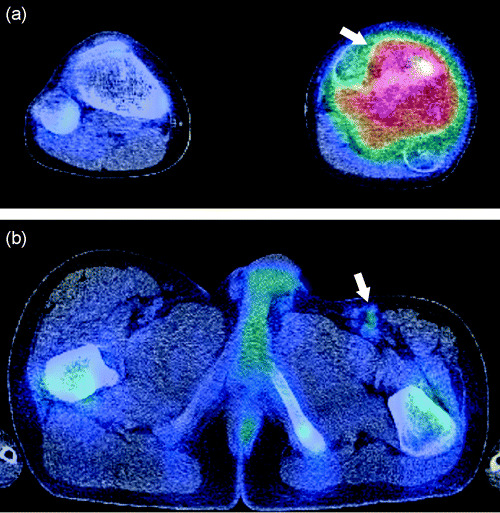
A 13‐year‐old boy with osteosarcoma. (a) Transverse 11C‐choline positron emission tomography (PET)/computed tomography (CT) image revealed hypermetabolic focus in the proximal portion of the left tibia (arrow). PET/CT findings were verified at histopathological analysis. (b) Abnormal uptake of 11C‐choline was also noted in the left inguinal lymph node, which was interpreted as highly suspicious for malignancy (arrow). Subsequent resection revealed metastasis from osteosarcoma.
Figure 2.
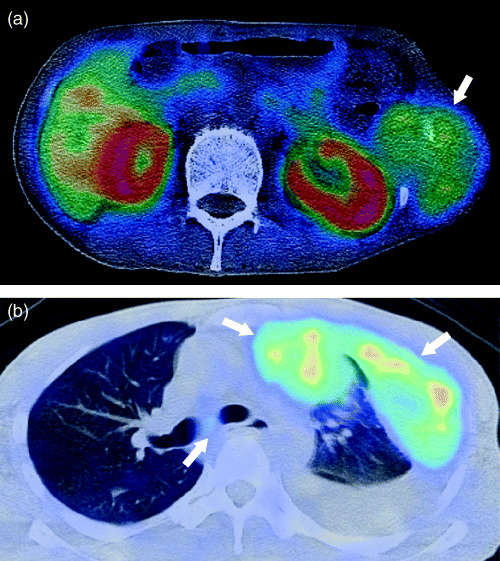
A 34‐year‐old man with clear cell sarcoma. (a) Transverse 11C‐choline positron emission tomography (PET)/computed tomography (CT) image depicting abnormal uptake in the tumor arising from the left lateral chest wall (arrow). (b) PET/CT image also depicts pleural dissemination and mediastinal lymph node (arrows). Follow‐up findings in this patient confirmed the diagnosis.
The complete stages of all patients were stage IA (n = 1), stage IIB (n = 1), stage III (n = 1) and stage IV (n = 13). TNM stage was correctly assessed with 11C‐choline PET/CT in 15 of 16 patients (94%) and with conventional imaging in eight of 16 patients (50%, P = 0.023, Table 2). 11C‐choline PET/CT assigned an incorrect TNM stage in a patient. This patient was understaged due to small metastatic lung tumor which was not clearly visualized by CT part of 11C‐choline PET/CT. Eight patients were understaged by conventional imaging (50%). Of these, skip metastases of soft tissues were identified in two (25%) and small nodal metastases in six (75%). 11C‐choline PET/CT correctly determined TNM stage in seven patients (44%) in whom stage derived from conventional imaging was incorrect.
Discussion
The results of the present study show that 11C‐choline PET/CT improves the accuracy of staging in patients with bone and soft tissue sarcomas compared to conventional imaging. Specifically, 11C‐choline PET/CT has potentially significant implications for detecting nodal and distant metastases at overall staging. Reports about the efficacy of 11C‐choline in the localization and detection of bone and soft tissue sarcomas are still limited.( 15 ) To our knowledge, no study regarding 11C‐choline PET/CT for staging bone and soft tissue sarcomas was found. In our study, seven of the 16 patients had skip metastases of soft tissue or nodal metastases detected by 11C‐choline PET/CT that were not identified by routine clinical and conventional radiological evaluation.
The ability of PET to depict increased metabolism in malignancies has greatly improved the accuracy in detecting neoplasms.( 4 ) However, compared with conventional imaging studies, use of PET alone results in a lack of substantial detail. 20 The PET/CT device permits sequential acquisition of anatomic CT and functional PET images in a single scanning session. Morphological characterization of scintigraphic lesions by PET/CT resulted in a lower percentage of equivocal interpretations compared with that of conventional imaging. Tumor‐detecting PET/CT technology is growing rapidly. However, there are only limited data available on staging of bone and soft tissue sarcomas with PET/CT.
Carbon‐11 choline uptake was significantly higher in malignant soft tissue tumors and was due to the high utilization of cell membranes of these lesions. 11C‐choline uptake is observed physiologically in the liver, pancreas, kidney and duodenum. 11C‐choline is also secreted into phospholipid‐rich pancreatic juice in a non‐fasting state. A potential advantage of 11C‐choline PET/CT might be the assessment of tumors in the skull or retroperitoneum. Blood clearance of 11C‐choline is rapid and radioactive distribution in tissues is constant in 5 min. The accumulation of 11C‐choline in the skull or retroperitoneum is hardly affected by background within the limits of short uptake time. In comparison to 18FDG, physiological background level in the urinary tract is low. This may be due to incomplete tubular reabsorption of the intact tracer, or enhanced excretion of labeled oxidative metabolites like betaine.( 12 )
Limited resolution of the present generation of 11C‐choline PET/CT and the partial volume effect result in failure to detect small lesions. In our study, one patient was understaged due to small metastatic lung tumor, which was not visualized clearly by the CT part of 11C‐choline PET/CT. Faint increase in tracer uptake and motion artifact caused by breathing contribute to false negative results. However, the advantage of 11C‐choline PET/CT is that the whole‐body can be visualized in a single examination. In our study, 50% of patients were understaged by conventional imaging. The inaccuracy of conventional imaging in assessing skip metastases of soft tissues is due to the field of view.
We reported the accurate modality of 11C‐choline PET/CT as a non‐invasive method for staging in patients with bone and soft tissue sarcomas compared to conventional imaging. Choline is an essential component of the cell membrane, and choline uptake may be via a choline‐specific transporter protein. Choline kinase, which catalyzes the phosphorylation of choline, is upregulated in tumor cells.( 12 ) In some types of tumor cells, overexpression of choline‐specific transporter protein and choline kinase were identified by in situ hybridization.( 21 ) 11C‐choline will be phosphorylated by choline kinase as a choline analog and retained in tumor cells.( 21 ) However, the precise pathway of metabolic trapping by tumor cells has not been elucidated, and further studies to clarify the mechanism of imaging by 11C‐choline are needed.
Our study has limitations. Most patients in this study had high‐grade tumors (88%) and may differ from the patient population of previous studies. Our study was intended to examine the staging prior to treatment; therefore, patient population of high‐grade tumors may explain the significant accuracy in overall staging compared to conventional imaging. A study with a larger patient population would clarify the influence of 11C‐choline PET/CT on staging. 11C‐choline is clearly a sensitive PET tracer for staging patients with bone and soft tissue sarcomas. The short half‐life of 11C‐choline necessitates the availability of an on‐site cyclotron, which causes practical restriction. More specific radiotracers will help overcome this limitation in the future.
In summary, the use of 11C‐choline PET/CT in patients with bone and soft tissue sarcomas increases the accuracy of overall staging and N staging compared to conventional staging. Our study suggests that whole‐body 11C‐choline PET/CT should be the preferred modality for staging in patients with bone and soft tissue sarcomas.
References
- 1. Reuther G, Mutschler W. Detection of local recurrent disease in musculoskeletal tumors: magnetic resonance imaging versus computed tomography. Skeletal Radiol 1990; 19: 85–90. [DOI] [PubMed] [Google Scholar]
- 2. Nieweg OE, Pruim J, Van Ginkel RJ et al. Fluorine‐18‐fluorodeoxyglucose PET imaging of soft‐tissue sarcoma. J Nucl Med 1996; 37: 257–61. [PubMed] [Google Scholar]
- 3. Eary JF, Conrad EU, Bruckner JD, Folpe A, Hunt KJ, Mankoff DA, Howlett AT. Quantitative [F‐18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res 1998; 4: 1215–20. [PubMed] [Google Scholar]
- 4. Franzius C, Sciuk J, Daldrup‐Link HE et al. FDG‐PET for detection of osseous metastases from malignant primary bone tumors: comparison with bone scintigraphy. Eur J Nucl Med 2000; 27: 1305–11. [DOI] [PubMed] [Google Scholar]
- 5. Schwarzbach MHM, Dimitrakopoulou‐Strauss A, Willeke F et al. Clinical value of [18‐F]fluorodeoxyglucose positron emission tomography imaging in soft tissue sarcomas. Ann Surg 2000; 231: 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ioannidis JP, Lau J. 18F‐FDG PET for the diagnosis of soft‐tissue sarcoma: a meta‐analysis. J Nucl Med 2003; 44: 717–24. [PubMed] [Google Scholar]
- 7. Tateishi U, Yamaguchi U, Seki K et al. Glut‐1 expression and enhanced glucose metabolism are associated with tumor grade in bone and soft tissue sarcomas: a prospective evaluation by [F‐18]‐fluorodeoxyglucose positoron emission tomography. Eur J Nucl Med Mol Imaging 2006; 33: 683–91. [DOI] [PubMed] [Google Scholar]
- 8. Hara T, Kosaka N, Shinoura N et al. PET imaging of brain tumor with [methyl‐11C]choline. J Nucl Med 1997; 38: 842–7. [PubMed] [Google Scholar]
- 9. Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon‐11‐choline. J Nucl Med 1998; 39: 990–5. [PubMed] [Google Scholar]
- 10. Hara T, Inagaki K, Kosaka N et al. Sensitive detection of mediastinal lymph node metastasis of lung cancer with 11C‐choline PET. J Nucl Med 2000; 41: 1507–13. [PubMed] [Google Scholar]
- 11. Torizuka T, Kanno T, Futatsubashi M et al. Imaging of gynecologic tumors: comparison of 11C‐choline PET with 18F‐FDG PET. J Nucl Med 2003; 44: 1051–6. [PubMed] [Google Scholar]
- 12. Ishidate K. Choline/ethanolamine kinase from mammalian tissues. Biochim Biophys Acta 1997; 1348: 70–8. [DOI] [PubMed] [Google Scholar]
- 13. Maeda T, Tateishi U, Komiyama M et al. Distant metastasis of prostate cancer: Early detection of recurrent tumor with dual‐phase carbon‐11 choline positron emission tomography/computed tomography in two cases. Jpn J Clin Oncol in press. [DOI] [PubMed]
- 14. Zhang H, Tian M, Oriuchi N et al. 11C‐choline PET for the detection of bone and soft tissue tumours in comparison with FDG PET. Nucl Med Commun 2003; 24: 273–9. [DOI] [PubMed] [Google Scholar]
- 15. Tian M, Zhang H, Oriuchi N et al. Comparison of 11C‐choline PET and FDG PET for the differential diagnosis of malignant tumors. Eur J Nucl Med Mol Imaging 2004; 31: 1064–72. [DOI] [PubMed] [Google Scholar]
- 16. Bar‐Shalom R, Yefremov N, Guralnik L et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003; 44: 1200–9. [PubMed] [Google Scholar]
- 17. Hara T, Yuasa M. Automated synthesis of [11C]choline, a positron‐emitting tracer for tumor imaging. Appl radiat Isot 1999; 50: 531–3. [DOI] [PubMed] [Google Scholar]
- 18. Green FL, Page DL, Fleming ID et al. AJCC Cancer Staging Manual, 6th edn. New York: Springer, 2002. [Google Scholar]
- 19. Sobin LH, Wittekind C. UICC TNM Classification of Malignant Tumours, 6th edn. New York: Wiley, 2002. [Google Scholar]
- 20. Franzius C, Daldrup‐Link HE, Wagner‐Bohn A et al. FDG‐PET for detection of recurrences from malignant primary bone tumors: comparison with conventional imaging. Ann Oncol 2002; 13: 157–60. [DOI] [PubMed] [Google Scholar]
- 21. Uchida T, Yamashita S. Molecular cloning, characterization, and expression in Escherichia coli of a cDNA encoding mammalian choline kinase. J Biol Chem 1992; 267: 10 156–62. [PubMed] [Google Scholar]


