Abstract
Serum carcinoembryonic antigen (CEA) and CA19‐9, a carbohydrate antigen recognized by the monoclonal antibody NS19‐9, are commonly used as classical tumor markers in colorectal cancer (CRC) clinics. The roles of tumor markers include: (1) diagnostic screening (diagnostic markers); (2) prediction of prognosis after treatment (prognostic markers); and (3) judgment tools for treatment effect (surveillance markers). Tumor markers can be evaluated in serum, stools, or even in tissues depending on the clinical purpose. The American Society for Clinical Oncology recommends that CEA is the only marker of choice for monitoring the response of metastatic disease to systemic therapy at present. In the present paper, we are the first to review the clinical significance of the classical tumor markers CEA and CA19‐9 in serum, allowing for our original data, and present our view on the newly emerging biomarkers in CRC. Novel promising biomarkers for diagnostic, prognostic, and surveillance purposes are reviewed and considered, some of which are anticipated for further validation. For diagnostic markers, urine or serum might replace fecal samples in the near future. On the other hand, prognostic or predictive markers for treatment sensitivity may be identified from the molecular profiles of primary cancer tissues. Selection of patients who are sensitive to chemotherapy will reduce the number of patients who undergo harmful chemotherapy with no effectiveness. The optimal tumor markers would be generalized, easy to assess, and accurate, and such markers are eagerly anticipated to enable personalized tailored therapy for CRC patients. (Cancer Sci 2009; 100: 195–199)
Colorectal cancer (CRC) is the second leading cause of cancer‐related death worldwide. Although current clinical practice in colorectal cancer screening (fecal occult blood test [FOBT] and colonoscopy) has contributed to a reduction in mortality,( 1 , 2 , 3 , 4 , 5 ) 70% of newly discovered CRC are detected at an advanced stage (International Union Against cancer (UICC) stage III/IV), presenting poor patient prognosis. The 5‐year survival rate of stage III CRC, which is defined as involving lymph node metastasis, is known to be below 70%, whereas that of stage I CRC is over 90% after resection( 6 ) (Fig. 1). Thus, in order to eradicate CRC death, early detection is most crucial, and identification of diagnostic markers should be urgently developed. Currently, serum tumor markers such as carcinoembryonic antigen (CEA) and CA19‐9, a carbohydrate antigen recognized by the monoclonal antibody NS19‐9, are commonly used in CRC clinics; however, their clinical usefulness remains controversial from diagnostic, prognostic, and surveillance points of view. As a result, such biomarkers are considered as supplemental tools to determine the therapeutic strategy against CRC. Recently, sophisticated scientific technology has emerged, such as DNA microarray( 7 ) and proteomic techniques,( 8 , 9 , 10 , 11 ) furthering the discovery of novel biomarkers in CRC. In the present paper, we are the first to review the clinical significance of the classical tumor markers CEA and CA19‐9 in serum, allowing for our original data, and present our view on the newly emerging biomarkers in CRC.
Figure 1.
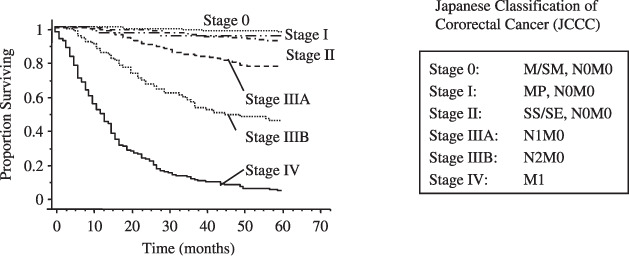
Clinical outcome of colorectal cancers that had been treated at Kitasato University Hospital between 1990 and 2001. Stage was classified according to the Sixth Japanese Classification of Colorectal Cancer. Stage II patients showed excellent prognosis, as did stage 0 and I patients. On the other hand, stage III patients (stage IIIA and IIIB) showed 68% survival during the clinical course. M, mucosa; SM, submucosa; MP, muscularis proprla; SS, subserosa; SE, serosal exposure.
Clinical significance of the classical tumor markers CEA and CA19‐9 in the serum of CRC patients
Serum CEA and CA19‐9 are commonly used as classical tumor markers in CRC patients. The roles of tumor markers include: (1) diagnostic screening (diagnostic markers); (2) prediction of prognosis after treatment (prognostic markers); and (3) judgment tools for treatment effect (surveillance markers). Tumor markers can be evaluated in serum, stools, or even in tissues, depending on the purpose of the attending physicians. The American Society for Clinical Oncology recommends that serum CEA testing be ordered preoperatively if it would assist in staging and surgical planning.( 12 ) Postoperative CEA levels should also be assessed every 3 months for stage II and III disease for at least 3 years if the patient is a potential candidate for surgery or chemotherapy of metastatic disease. CEA is the marker of choice for monitoring the response of metastatic disease to systemic therapy. However, data are insufficient to recommend the routine use of other markers, including serum CA19‐9, in the management of patients with CRC.( 12 )
Diagnostic significance of preoperative CEA and preoperative CA19‐9 in CRC. Ideal diagnostic markers must be characterized by both high sensitivity and high specificity. Presently, prostate‐specific antigen (PSA) in prostate cancer is the most relevant diagnostic marker approved by the Federal Drug Administration (sensitivity of PSA, positive rate of disease is ~80%; specificity of PSA, negative rate of non‐disease is ~60%). In CRC patients, preoperative CEA (preCEA) and preoperative CA19‐9 (preCA19‐9) showed various degrees of sensitivity depending on the stage of disease, whereas specificity was as high as ~90%. In our institute between 1990 and 2001, the rates of CRC patients with elevated preCEA levels were: stage 0, 5%; stage I, 10%; stage II, 33%; stage IIIA, 33%; stage IIIB, 45%; and stage IV, 78%. The corresponding rates of patients with elevated preCA19‐9 levels were: stage 0, 5%; stage I, 4%; stage II, 11%; stage IIIA, 10%; stage IIIB, 13%; and stage IV, 52%. The numbers of patients examined at each stage were: stage 0, 13; stage I, 65; stage II, 102; stage IIIA, 143; stage IIIB, 135; and stage IV, 192 (Fig. 2). Because most stage II CRC are potentially curable (Fig. 1), the most beneficial diagnostic markers for screening would be able to detect the disease at stage II or earlier. However, the sensitivities of both tumor markers are insufficient for stage II CRC (33 and 11%, respectively). These results indicate that preCEA and preCA19‐9 do not show satisfactory sensitivity as screening (diagnostic) markers in CRC.
Figure 2.
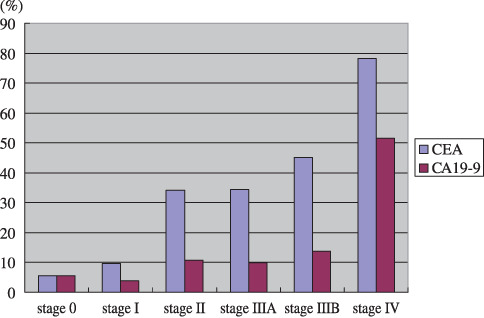
Positive rate of the preoperative serum tumor markers carcinoembryonic antigen (CEA) and CA19‐9 in colorectal carcinoma according to stage. Stage classification is based on the Japanese Classification of Colorectal Cancer.
Prognostic significance of preCEA and preCA19‐9 in CRC. Even so, preCEA and preCA19‐9 have been reported to be promising prognostic markers in CRC, mostly for patients treated before the 1990s. PreCEA was reported to be an important independent variable in predicting prognostic outcome, especially in stage III CRC,( 13 , 14 , 15 , 16 , 17 , 18 ) and it was even discussed whether it should be included in the staging system;( 19 ) however, there have been few studies on its prognostic significance in more recent CRC cases. In our institute's data, preCEA was a potent prognostic factor for stage III CRC patients who were treated through the 1990s; however, its prognostic significance was eliminated in the latest decade (2001–04)( 20 ) (Fig. 3a). As a result, we concluded that preCEA has no significant role in predicting the prognosis of stage III (Dukes C) CRC at present( 20 ) where the result may not involve a change of adjuvant chemotherapy. Although we do not know the real reason why preCEA, frequently reported as a significant prognostic factor in stage III CRC,( 13 , 14 , 15 , 16 , 17 , 18 ) is no longer prognostically relevant in recent cases, we speculate that it might involve recent diagnostic advancements and subsequent changes in patient stage distribution. In any case, the absence of prognostic relevance of preCEA in stage III uncovers the need to identify novel prognostic markers for stage III CRC patients for treatment decisions such as determination of adjuvant chemotherapy.
Figure 3.
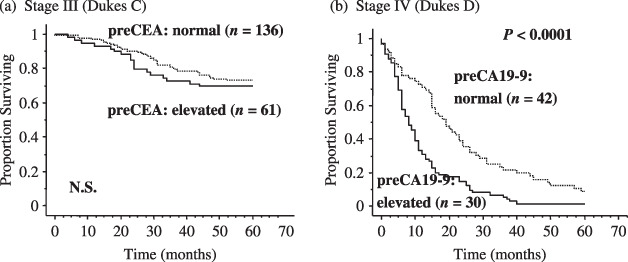
Five‐year survival rate according to the preoperative serum tumor markers carcinoembryonic antigen (preCEA) or CA19‐9 (preCA19‐9) in subgroups (stage III or stage IV) of colorectal cancer between 2001 and 2004. (a) preCEA is no longer of a prognostic relevance in stage III colorectal cancer, whereas patients with elevated preCEA showed significantly poorer prognosis than those with normal preCEA.( 20 ) (b) In stage IV colorectal patients, cases with elevated preCA19‐9 showed significantly poorer prognosis than those with normal preCA19‐9 (P < 0.0001).
On the other hand, preCA19‐9 was proven to be useful in predicting patient prognosis in stage IV CRC patients,( 21 ) but not in other stages (data not shown), according to our institute's data. In this study,( 21 ) our multivariate analysis revealed that the most dismal phenotype of CRC is exhibited when preCA19‐9 (putatively immune), H factor (distant metastatic), and P factor (local invasive capacity) are recognized in CRC patients (Fig. 4). A previous report that included a multivariate prognostic analysis also revealed that preCA19‐9 is an independent prognostic factor in stage IV CRC;( 22 ) however, our multivariate analysis for the first time examined all three tumor factors simultaneously to predict patient prognosis in stage IV CRC. Recently, we further validated this result in patients (20–75 years old with postoperative chemotherapy; Yamashita K. et al., 2008), where preCA19‐9 again remained an excellent prognostic marker even in the latest set (2001–04) of stage IV CRC patients (Fig. 3b). In the validation study, both preCA19‐9 and H factor were independent prognostic factors in stage IV CRC, but P factor was eliminated, putatively suggesting that chemotherapy is most effective to P factor.
Figure 4.
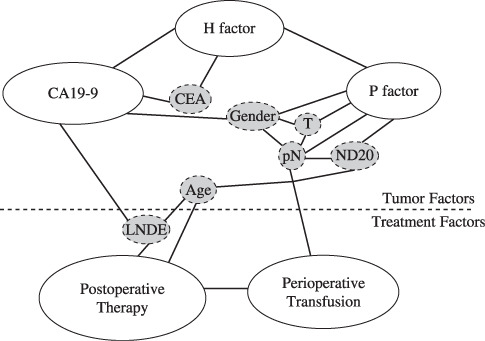
Interrelationship of independent prognostic factors according to logistic regression analyses. White boxes are independent prognostic factors. Gray boxes are dependent prognostic factors. These factors reflect associated independent prognostic factors. CEA, carcinoembryonic antigen; LNDE, Iymph node dissection extent; ND20, lymph node metastasis density over 20%; pN, lymph node mitastasis status; T, tumor factor.
Surveillance by serum CEA and CA19‐9 in CRC. As stated earlier, preCEA and preCA19‐9 were positive in 78 and 52% of stage IV CRC, respectively, and higher sensitivity of both markers is anticipated in recurrent CRC through the whole clinical course than in preoperative stage IV CRC cases. This finding suggests that serum CEA may be useful for monitoring disease spread, as recommended by the American Society for Clinical Oncology.( 12 )
A recent study further confirmed a potential surrogate role of these classical tumor markers in predicting prolonged survival, putatively due to their reflection of the therapeutic effect of chemotherapy against recurrent or far‐advanced CRC.( 23 ) In this report, the positive predictive values (PPV) for the response of a decrease in marker levels were 53.8 for CEA and 41.7 for CA19‐9 using a 30% decrease threshold, and 60 for CEA and 52.2 for CA19‐9 using a 50% decrease threshold. Meaningful PPV values (>90%) for progression of an increase in marker levels were only obtained using the 200% increase threshold for CEA alone or a combination of CEA and CA19‐9. A 100% CEA increase between baseline and the 8‐week evaluation was correlated with overall survival (P = 0.0023), suggesting that CEA, CA19‐9, or both may be used with caution for tumor response evaluation to chemotherapy in metastatic CRC.
Newly developing biomarkers in CRC
Diagnostic markers for CRC. The FOBT is currently used as a diagnostic marker for CRC. However, it detects blood in the stool, and potential benign diseases are also included among the positive cases. FOBT actually showed 70% specificity for CRC; thus, it is not very specific to CRC. Moreover, its sensitivity for detecting CRC is reported to be approximately 40–80%, so many CRC may be missed by this screening. Nevertheless, FOBT is currently the most prevalent marker used in CRC clinics for the purpose of screening. It is not surprising that the most relevant biomarker at present is from stools, not serum, because stools can directly reflect CRC.
As an alternative to FOBT, molecular detection was reported to be promising for calprotectin in the stool, which showed 50 positivity among 53 cases of CRC.( 24 ) However, validation and subsequent reports have not followed; thus, it has not yet been approved as a diagnostic molecular marker of stools. Traverso et al. treated stools for DNA extraction, which were subsequently examined for mutation detection derived from the DNA; they revealed that adenomateus polyposis coli (APC) mutation showed 63% sensitivity for Dukes B (stage II) CRC with 100% specificity in the stools of CRC patients.( 25 ) This sensitivity was much higher than that of preCEA (33%) or preCA19‐9 (11%), probably due in part to the likely superiority of fecal samples to serum samples for diagnostic purposes. This result suggested that the sensitivity of CRC detection by molecular detection of DNA in stools could be equivalent to that by FOBT. Moreover, molecular detection overcame the problem of low specificity exhibited by FOBT, making APC mutation a promising diagnostic marker for CRC. However, APC mutation detection was evaluated by the authors’ original methodology, which is not yet generalized worldwide, and the mutation analysis cannot cover all patients. Moreover, the actual mutation site is needed for its feasibility; therefore, the analysis is not a simple procedure and is not practical at present.
Recent proteomic techniques have demonstrated that surface‐enhanced laser desorption/ionization (SELDI‐TOF/MS) can discriminate between the serum of CRC patients and healthy persons with 89% sensitivity and 83% specificity.( 26 ) More sophisticated analysis using proteomic technology has identified specific molecules such as α‐defensins( 27 ) and C3a‐anaphylatoxin( 28 ) in the serum of CRC patients with excellent feature as serum tumor makers, showing discrimination of cancer from healthy persons with 100% sensitivity and 69% specificity for α‐defensins, and 97% sensitivity and 96% specificity for C3a‐anaphylatoxin. Hiramatsu et al. also reported diacetylspermine in the urine as a novel sensitive and specific marker for early and late‐stage CRC,( 29 ) where the sensitivity for stage II CRC was as high as 70% and the specificity was over 90%. These markers have great potential for use in CRC screening; however, final validation and confirmation of this potential is needed in the near future.
Prognostic markers in CRC
Stage II (Dukes B) CRC. Prognostic markers for stage II CRC patients are critical because survival is excellent and clinicians have always wondered if such patients really need any adjuvant chemotherapy. A large portion of stage II CRC patients survive without recurrence for over 5 years. To enhance patient selection for such adjuvant chemotherapy, prognostic markers are needed to identify patients who are anticipated to undergo recurrence and predicted to be sensitive to chemotherapy.
Nori et al. compared the DNA content in patients with stage II and no evidence of relapse versus stage II with relapse, and a total of 80% of patients with recurrence showed aneuploidy compared to only 40% of patients in the control group.( 30 ) Furthermore, aneuploidy was associated with a significantly higher tumor recurrence rate and a shorter survival in stage II CRC( 30 , 31 ) and the results were validated by meta‐analysis of the published data.( 32 ) With increased resolution of cytogenetic techniques it became clear that, in addition to nuclear aneuploidy, specific non‐random chromosomal imbalances also exist. Zhou et al. showed that counting the alleles of 8p and 18q can predict recurrence of stage II CRC, which presented a potential method of patient selection for adjuvant chemotherapy.( 33 )
These findings also suggested that recurrent cases of stage II CRC harbor different molecular features compared with those without recurrence, an idea supported by data from transcriptome analysis by Wang et al. who revealed that gene expression profiling by cDNA microarray identified a 23‐gene signature that predicts recurrence in Dukes B patients.( 34 ) This signature was validated in 36 independent patients. The overall performance accuracy was 78%. Thirteen of 18 relapse patients and 15 of 18 disease‐free patients were predicted correctly, giving an odds ratio of 13 (95% confidence interval 2.6–65; P = 0.003). The log‐rank test indicated a significant difference in disease‐free time between the predicted relapse and disease‐free patients (P = 0.0001). Nevertheless, comparison of the often different results by expression array proved to be difficult because of the different array platforms used. So far, no identified gene expression markers have been implemented for clinical application.
For prediction of sensitivity to chemotherapy, a potential marker is microsatellite instability, which is seen in approximately 15–20% of CRC.( 35 ) Microsatellite instability tumors are refractory to chemotherapy.( 36 ) Such information would also help avoid ineffective adjuvant chemotherapy for patients with stage II CRC.
Stage III (Dukes C) CRC. Prognostic markers in stage III CRC do not exist. Serum CEA in stage III, which had been the most promising,( 13 , 14 , 15 , 16 , 17 , 18 ) was proven to be disappointing as a prognostic factor in our recent study.( 20 ) On the other hand, the RASCAL‐2 study by Andreyev et al. revealed that K‐ras mutation in primary cancer tissues showed prognostic significance in stage III CRC cancer.( 37 ) RASCAL‐2 was the second and larger version of RASCAL, the largest (at the time) survey of K‐ras mutation in primary tumor tissues, which comprised a collection of data collected by groups from 13 countries concerning the question of the prognostic importance of K‐ras mutation.( 38 ) We also found that the prognostic relevance of K‐ras mutation is recognized in stage III young colon cancer patients (Ann Sug Ohonato et al.). In stage II patients, recurrence requires direct systemic dissemination from the primary site, which may need more powerful oncoproperties than in stage III, and K‐ras mutation might not be sufficient for this.
Such clinical information may help to select patients for whom potent adjuvant chemotherapy that has already proven effective, such as Folic acid Fluorouracil and Oxaliplatin (FOLFOX) in CRC,( 39 ) is truly necessary. In Japan, FOLFOX has not been routinely applied as adjuvant therapy in stage III CRC patients due to social issues such as cost‐performance or insufficient medical supplies (until June 2008). Prognostic markers to predict recurrent stage III cases would enable us to identify good candidates for potent and expensive adjuvant therapy with excellent evidence of effectiveness.
Disease surveillance and patient selection for molecular target therapy against recurrent or far‐advanced CRC. For recurrent or far‐advanced CRC, K‐ras mutation status has been used to predict the treatment effect of epidermal growth factor receptor (EGFR) inhibition by cetuximab or penitumumab.( 40 , 41 ) EGFR is a tyrosine kinase that activates the K‐ras–B‐raf–mitogen‐activated protein kinase (MAPK) or phosphatidylinositol 3‐kinase (PI3K) oncogenic pathways. CRC with K‐ras mutation are unaffected by EGFR targeted therapy, whereas CRC without K‐ras mutation are affected by EGFR inhibition. CEA in the serum is also presently considered a surveillance marker to predict the treatment effect of molecular target therapy, but emerging diagnostic markers may potentially replace serum CEA in the future.
Conclusion and future perspective
Novel promising biomarkers for diagnostic, prognostic, and surveillance purposes are reviewed and considered, some of which are anticipated for further validation (Table 1). For diagnostic markers, urine or serum might replace fecal samples in the near future, and CRC diagnosis may become more convenient than ever. On the other hand, prognostic or predictive markers for treatment sensitivity may be identified from the molecular profiles of primary cancer tissues. Selection of patients who are sensitive to chemotherapy can reduce the number of patients who undergo harmful chemotherapy with no effectiveness. The optimal tumor markers in serum, feces, and tissue samples would be generalized, easy to assess, and accurate, and such markers are eagerly anticipated to enable personalized tailored therapy for CRC patients.
Table 1.
Biomarkers that are used at present and are promising for the future
| Purpose | Present | Future prospective for validation |
|---|---|---|
| Diagnostic marker | Fecal occult blood test, colonoscopy | Serum α‐defensins or C3a‐anaphylatoxin urine diacetyl‐spermine |
| Prognostic marker | ||
| Stage II | – | DNA ploidy in the primary tissues |
| 8p, 18q impalance in the primary tissues | ||
| Microsatellite instability in the primary tissues (for adjuvant therapy) | ||
| Stage III | – | K‐ras mutation in primary tissues |
| Stage IV | – | Serum preoperative CA19‐9 |
| Patient selection for recurrent tumor | – | K‐ras mutation for cetuximab Tx |
| Follow‐up maker | Serum carcinoembryonic antigen | Novel diagnostic marker as above |
References
- 1. Mandel JS, Bond JH, Church TR et al . Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993; 328: 1365–71. [DOI] [PubMed] [Google Scholar]
- 2. Mandel JS, Church TR, Ederer F et al . Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434–7. [DOI] [PubMed] [Google Scholar]
- 3. Kronborg O, Fenger C, Olsen J et al . Randomised study of screening for colorectal cancer with fecal‐occult‐blood test. Lancet 1996; 348: 1467–71. [DOI] [PubMed] [Google Scholar]
- 4. Hardcastle JD, Chamberlain JO, Robinson MHE et al . Randomised controlled trial of fecal‐occult‐blood screening for colorectal cancer. Lancet 1996; 348: 1472–7. [DOI] [PubMed] [Google Scholar]
- 5. Sonnenberg A, Delco F, Inadomi JM. Cost‐effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000; 133: 573–84. [DOI] [PubMed] [Google Scholar]
- 6. O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004; 96: 1420–5. [DOI] [PubMed] [Google Scholar]
- 7. Schena M, Shalon D, Davis RW et al . Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995; 270: 467–70. [DOI] [PubMed] [Google Scholar]
- 8. Gorg A, Obermaier C, Boguth G et al . Very alkaline immobilized pH gradients for two‐dimensional electrophoresis of ribosomal and nuclear proteins. Electrophoresis 1997; 18: 328–37. [DOI] [PubMed] [Google Scholar]
- 9. Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 1997; 18: 2071–7. [DOI] [PubMed] [Google Scholar]
- 10. Veenstra TD, Prieto DA, Conrads TP. Proteomic patterns for early cancer detection. Drug Dicov Today 2004; 9: 889–97. [DOI] [PubMed] [Google Scholar]
- 11. Conrads TP, Hood BL, Issaq HJ et al . Proteomic patterns as a diagnostic tool for early‐stage cancer: a review of its progress to a clinically relevant tool. Mol Design 2004; 8: 77–85. [DOI] [PubMed] [Google Scholar]
- 12. Locker GY, Hamilton S, Harris J et al . ASCO: update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006; 24: 5313–27. [DOI] [PubMed] [Google Scholar]
- 13. Wanebo HJ, Rao B, Pinsky CM. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med 1978; 299: 448–51. [DOI] [PubMed] [Google Scholar]
- 14. Scott NA, Wieand HS, Moertel CG. Colorectal cancer: Dukes’ stage, tumor site, preoperative plasma CEA level, and patient prognosis related to tumor DNA ploidy pattern. Arch Surg 1987; 122: 1375–9. [DOI] [PubMed] [Google Scholar]
- 15. Wiggers T, Arends JW, Volovics A. Regression analysis of prognostic factors in colorectal cancer after curative resections. Dis Colon Rectum 1988; 31: 33–41. [DOI] [PubMed] [Google Scholar]
- 16. Harrison LE, Guillem JG, Paty P et al . Preoperative carcinoembryonic antigen predicts outcomes in node‐negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg 1997; 185: 55–9. [DOI] [PubMed] [Google Scholar]
- 17. Marchena J, Acosta MA, Garcia‐Anguiano F. Use of the preoperative levels of CEA in patients with colorectal cancer. Hepatogastroenterology 2003; 50: 1017–20. [PubMed] [Google Scholar]
- 18. Weissenberger C, Von Plehn G, Otto F. Adjuvant radiochemotherapy of stage II and III rectal adenocarcinoma: role of CEA and CA 19‐9. Anticancer Res 2005; 25: 1787–93. [PubMed] [Google Scholar]
- 19. Chen CC, Yang SH, Lin JK. Is it reasonable to add preoperative serum level of CEA and CA19‐9 to staging for colorectal cancer? J Surg Res 2005; 124: 169–74. [DOI] [PubMed] [Google Scholar]
- 20. Katoh H, Yamashita K, Satoh T et al . Validation of prognostic impact of preoperative carcinoembryonic antigen (CEA) in Dukes’ C colorectal cancer. A prospective study. Anticancer Res 2008; 28(3B): 1933–41. [PubMed] [Google Scholar]
- 21. Katoh H, Yamashita K, Kokuba Y et al . Surgical resection of stage IV colorectal cancer and prognosis. World J Surg 2008; 32: 1130–7. [DOI] [PubMed] [Google Scholar]
- 22. Wang WS, Lin JK, Chiou TJ. CA19‐9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepato-Gastroenterology 2002; 49: 160–4. [PubMed] [Google Scholar]
- 23. Trillet‐Lenoir VCF, Touzet S, Barbier JY et al . Any clinical benefit from the use of oncofoetal markers in the management of chemotherapy for patients with metastatic colorectal carcinomas? Clin Oncol (R Coll Radiol) 2004; 16: 196–203. [DOI] [PubMed] [Google Scholar]
- 24. Roseth AG, Kristinsson J, Fagerhol MK. Faecal calprotectin: a novel test for the diagnosis of colorectal cancer ? Scand J Gastroenterol 1993; 28: 1073–6. [DOI] [PubMed] [Google Scholar]
- 25. Traverso G, Shuber A, Levin B. Detection of APC mutation in fecal DNA from patients with colorectal cancer. N Engl J Med 2002; 346: 311–20. [DOI] [PubMed] [Google Scholar]
- 26. Yu JK, Chen YD, Zheng S. An integrated approach to the detection of colorectal cancer utilizing proteomics and bioinformatics. World J Gastroenterol 2004; 10: 3127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Melle C, Ernst G, Schimmel B. Discovery and identification of α‐defensins as low abundant, tumor‐derived serum markers in colorectal cancer. Gastroenterology 2005; 129: 66–73. [DOI] [PubMed] [Google Scholar]
- 28. Habermann JK, Roblick UJ, Luke BT. Increased serum levels of complement C3a anaphylatoxin indicate the presence of colorectal tumors. Gastroenterology 2006; 131: 1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiramatsu KTK, Yamaguchi T, Matsumoto H et al . N(1),N(12)‐Diacetylspermine as a sensitive and specific novel marker for early‐ and late‐stage colorectal and breast cancers. Clin Cancer Res 2005; 11: 2986–90. [DOI] [PubMed] [Google Scholar]
- 30. Nori D, Merimsky O, Samala E et al . Tumor ploidy as a risk factor for disease recurrence oand short survival in surgically‐treated Dukes B2 colon cancer. J Surg Oncol 1995; 59: 239–42. [DOI] [PubMed] [Google Scholar]
- 31. Lanza G, Gafa R, Santini A et al . Prognostic significance of DNA ploidy in patients with stage II and stage III colon carcinoma: a prospective flow cytometric study. Cancer 1998; 82: 49–59. [DOI] [PubMed] [Google Scholar]
- 32. Araujo SE, Bernardo WM, Habr‐Gama A et al . DNA ploidy status andprognosis in colorectal cancer: a meta‐analysis of published data. Dis Colon Rectum 2007; 50: 1800–10. [DOI] [PubMed] [Google Scholar]
- 33. Zhou W, Goodman SN, Galizia G et al . Counting alleles to predict recurrence of early‐stage colorectal cancers. Lancet 2002; 359: 219–25. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Jatkoe T, Zhang Y et al . Gene expression profiles and molecular markers to predict recurrence of Dukes’ B colon cancer. J Clin Oncol 2004; 22: 1564–71. [DOI] [PubMed] [Google Scholar]
- 35. Ribic CM, Sargent DJ, Moore MJ. Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy. N Engl J Med 2003; 349: 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Branch P, Aquilina G, Bignami M et al . Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature 1993; 362: 652–4. [DOI] [PubMed] [Google Scholar]
- 37. Andreyev HJNA, Cunningham D, Oates J et al . Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer 2001; 85: 692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreyev HJ, Norman AR, Cunningham D et al . Kirsten ras mutations in patients with colorectal cancer: the multicenter ‘RASCAL’ study. J Natl Cancer Inst 1998; 90: 675–84. [DOI] [PubMed] [Google Scholar]
- 39. Andre T, Boni C, Mounedji‐Boudiaf L. Oxaliplatin, fluorouracil, and lecovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. [DOI] [PubMed] [Google Scholar]
- 40. Khambata‐Ford SGC, Meropol NJ, Basik M et al . Expression of epiregulin and amphiregulin and K‐ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007; 25: 3230–7. [DOI] [PubMed] [Google Scholar]
- 41. Benvenuti SS‐BA, Di Nicolantonio F, Zanon C et al . Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti‐epidermal growth factor receptor antibody therapies. Cancer Res 2007; 67: 2643–8. [DOI] [PubMed] [Google Scholar]


