Abstract
We examined the expression of sVEGFR1 in colorectal cancer tissue and corresponding normal colorectal mucosa to assess the clinical significance of sVEGFR1 in colorectal cancer. We also assessed the relationship between sVEGFR1 levels and various clinicopathologic factors and prognoses. sVEGFR1 and VEGF levels were measured in fresh‐frozen tumor extracts from 84 primary colorectal cancer tissues and 27 corresponding normal mucosa using ELISA. Mean of sVEGFR1 levels were 3.17 ng/mg protein. sVEGFR1 levels increased significantly in cancer tissue compared with normal mucosa. Although VEGF levels increased in cancer tissues, the ratio of sVEGFR1/VEGF in cancer tissue was significantly lower than that in normal tissue. No significant correlation between sVEGFR1 or VEGF levels and any clinicopathologic parameter was found. Overexpression of sVEGFR1 was significantly associated with a favorable prognosis. Based on sVEGFR1 levels in colorectal cancer without distant metastases, patients with higher sVEGFR1 levels (≥1.5 ng/mg protein) demonstrated significant longer recurrence‐free survival than patients with lower sVEGFR1 levels (<1.5 ng/mg protein) (P = 0.0017). Multivariate analysis showed that the sVEGFR1 levels in cancer tissue were an independent prognostic indicator of disease progression. sVEGFR1 expression was significantly elevated in colorectal cancer tissue compared with normal mucosa and the intratumoral balance between sVEGFR1 and VEGF was significantly different between tumor tissue and normal controls. Furthermore, sVEGFR1 levels showed a significant prognostic value. Further studies concerning the biologic and therapeutic significance of sVEGFR1 in colorectal cancer are warranted. (Cancer Sci 2007; 98: 405–410)
Abbreviations:
- ELISA
enzyme‐linked immunosorbent assay
- PlGF
placental growth factor
- sVEGFR1
soluble vascular endothelial growth factor receptor 1
- VEGF
vascular endothelial growth factor.
Vascular endothelial growth factor, also known as VEGF‐A, is one of the most important angiogenic factors.( 1 ) It has been reported that overexpression of VEGF is an independent factor predicting poor prognosis in various types of malignant tumors.( 2 , 3 , 4 , 5 , 6 , 7 ) VEGF binds to VEGFR1 and VEGFR2, which are tyrosine kinase receptors.( 8 , 9 ) VEGFR1 mediates critical effects on physiologic and pathologic neovascularization; however, the function of VEGFR1 in the process of angiogenesis remains unclear. Some authors have reported that VEGFR1 functions as a positive regulator of angiogenesis,( 10 , 11 , 12 ) whereas others have reported that it is a negative regulator of angiogenesis.( 13 , 14 ) In contrast, VEGFR2 is widely accepted as an angiogenic receptor. VEGFR2 activates a phospholipase C gamma‐protein kinase C‐Raf‐MAP kinase signaling pathway, which results in endothelial cell migration, proliferation, and vascular permeability.( 15 )
In addition to these receptors, a natural soluble form of the VEGF receptor (sVEGFR1) has been identified.( 16 , 17 ) sVEGFR1, which was first cloned from the human umbilical vein endothelial cell cDNA library, is an alternative splicing variant of the VEGFR1 gene. It binds to VEGF with high affinity and inhibits its mitogenic response. Not only VEGF‐A, but also other VEGF family ligands such as placenta growth factor, have the ability to bind to sVEGFR1.( 18 ) sVEGFR1 is believed to be a modulator of or negative regulator of VEGF activity. Recently it has been reported that sVEGFR1 is expressed in breast cancer and astrocytic tumors.( 19 , 20 ) In these tumors, sVEGFR1 correlated significantly with tumor growth and prognosis. In animal experimental models, transfer of sVEGFR1 genes resulted in suppression of angiogenesis and regression of tumors.( 21 ) Experimental data indicate strongly that sVEGFR1 is an important and intrinsic counterpart of VEGF and of angiogenesis, and the clinical importance of serum sVEGFR1 levels has been reported in some kind of tumors, including leukemia, lung cancer, and colorectal cancer.( 22 , 23 , 24 ) However, the clinical significance of sVEGFR1 level in colorectal cancer tissue is largely unknown. We investigated the expression of sVEGFR1 in human colorectal cancer, as well as normal colorectal mucosa and explored the clinical significance of this receptor.
Materials and Methods
Patient population. We randomly selected tissue from 84 patients with operable colorectal cancer who underwent surgical resection at the Tokyo Metropolitan Komagome Hospital from January to December 2003. As controls, we obtained corresponding normal mucosa from 27 colorectal cancer patients. All patients signed an informed consent according to a protocol approved by the ethics committee of the hospital. Representative samples of tumor specimens and normal mucosa tissue were immediately frozen in liquid nitrogen after surgical resection and stored at −80°C until preparation for ELISA. Pathologic examinations were carried out on formalin‐fixed, paraffin‐embedded specimens.
Histopathologic analysis. Representative sections from all primary tumors were reviewed and analyzed by pathologists. Morphologic features examined included grade, lymph vessel/blood vessel involvement, and number of lymph nodes involved.
Sample preparation. Colorectal tumor tissue and normal mucosa samples were treated as previously reported.( 22 ) Briefly, tissue samples were homogenized in a solution of 10 mM Tris‐HCl buffer (pH 7.4) containing 15 mM NaCl, 1.5 mM MgCl2, 50 µM potassium phosphate, and a protease‐inhibitor cocktail. The supernatants were then stored at −80°C until use. The protein concentration of the supernatant extracted from tumor tissues was determined using a DC protein assay kit (Bio‐Rad Laboratories, Hercules, CA, USA).
ELISA. Total VEGF protein concentrations in the tumor cytosols were measured using VEGF ELISA kits (R&D Systems, Minneapolis, MN, USA). Measurements were made according to the methods recommended by the manufacturer. The minimal detection limit for total VEGF was 31 pg/mL.
ELISA for sVEGFR1 was carried out as previously reported.( 3 , 25 ) A human sVEGFR1 ELISA kit (Bender MedSystems, Vienna, Austria) was used according to the manufacturer's protocol. The minimum detection limit was 100 pg/mL.
All protein level measurements made by ELISA were carried out in duplicate.
Statistical analysis. The correlation between two factors was evaluated using the Spearman's correlation coefficient by rank. Differences between groups were evaluated using the Student's t‐test for continuous variables and the Kruskal–Wallis test for categorical values. Fisher's exact test was used to evaluate the relationship between two discrete and dichotomous variables. The analysis of disease‐free survival was intended for colorectal cancer patients without distant metastases at the time of their operation. Survival curves were drawn using the Kaplan–Meier method and the log‐rank test. Multivariate analyses were performed using logistic regression analysis. Significance was defined as a P < 0.05. All statistical evaluations were carried out by StatView (Abacus Concepts, Inc., Berkley, CA, USA).
Results
Clinicopathologic characteristics. Clinical and pathologic characteristics of the patients are shown in Table 1. All patients underwent surgical resection of the primary tumor and the diagnosis of adenocarcinoma was made microscopically. Of the 84 patients, 10 had distant metastases, including six with liver metastases, four with distant lymph node metastases, one with peritoneal dissemination, and one with lung metastases. Two patients had more than one distant metastasis. Of the 74 patients without distant metastases, 21 received adjuvant chemotherapy. The median follow‐up period for all patients was 29.6 months (range, 1.0–35.3 months).
Table 1.
Clinicopathological characteristics
| Characteristics | Frequencies | Percentage (%) |
|---|---|---|
| Age | ||
| Mean (years) | 64 (37–83) | |
| Gender | ||
| Male | 48 | 57.1 |
| Female | 36 | 42.9 |
| Location | ||
| Colon | 41 | 48.8 |
| Rectum | 43 | 51.2 |
| T factor | ||
| T1 | 3 | 3.6 |
| T2 | 11 | 13.1 |
| T3 | 54 | 64.3 |
| T4 | 16 | 19.0 |
| Size | ||
| Mean (cm) | 4.8 (1.8–11.5) | |
| Lymph node status | ||
| Negative | 46 | 54.8 |
| Positive | 38 | 45.2 |
| Differentiation | ||
| well | 38 | 45.2 |
| non‐well | 46 | 54.8 |
| Dukes stage | ||
| A | 12 | 14.3 |
| B | 33 | 39.3 |
| C | 29 | 34.5 |
| D | 10 | 11.9 |
| Chemotherapy | ||
| Negative | 53 | 63.1 |
| Positive | 31 | 36.9 |
| Recurrence | ||
| Negative | 64 | 86.5 |
| Positive | 10 | 13.5 |
sVEGFR1 and VEGF concentrations. sVEGFR1 levels were detectable in 82 of 84 colorectal cancer tissues and ranged from 0.00 to 7.11 ng/mg protein. Mean sVEGFR1 and VEGF concentrations in colorectal cancer tissue were 3.17 ng/mg protein and 1.26 ng/mg protein, respectively, compared with 0.93 ng/mg protein and 0.25 ng/mg protein, respectively, in normal mucosa (P < 0.0001 for both comparisons, Wilcoxon signed rank test; Fig. 1a,b). The median sVEGFR1/VEGF ratio was 3.6 in colorectal cancer tissue and 11.8 in normal mucosa with a significant difference between groups (P = 0.018, Wilcoxon signed rank test; Fig. 1c). There was a significant correlation between sVEGFR1 and VEGF levels in colorectal cancer tissue (ρ = 0.52, P < 0.0001, Spearman's rank correlation test), whereas no correlation was seen between sVEGFR1 and VEGF levels in normal mucosa (ρ = 0.24, P = 0.23, Spearman's rank correlation test; Fig. 2a,b).
Figure 1.
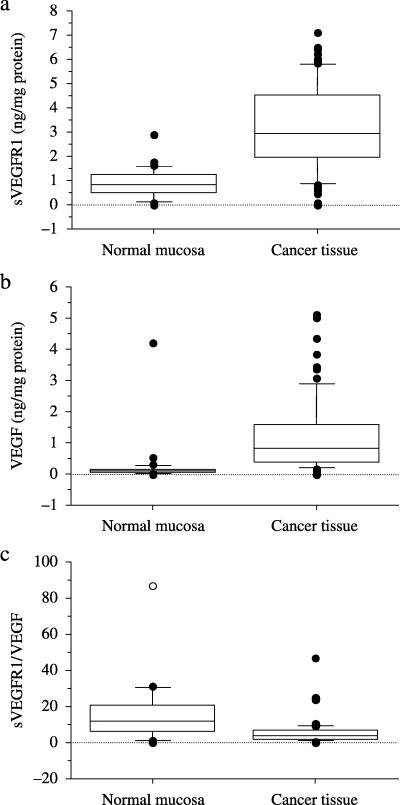
Comparison of the concentration of soluble vascular endothelial growth factor receptor 1 (sVEGFR1) and vascular endothelial growth factor (VEGF) in colorectal cancer and normal mucosa. sVEGFR1 and VEGF levels were significant higher in colorectal cancer than in normal mucosa (Wilcoxon signed rank test; P < 0.0001; Fig. 1a,b). The sVEGFR1/VEGF ratio was significant lower in colorectal cancer than in normal mucosa (Wilcoxon signed rank test; P = 0.018; Fig. 1c).
Figure 2.

Correlation between soluble vascular endothelial growth factor receptor 1 (sVEGFR1) and vascular endothelial growth factor (VEGF) concentrations in colorectal cancer (Fig. 2a) and in normal mucosa (Fig. 2b). There was a significant correlation between sVEGFR1 and VEGF levels in colorectal cancer (Spearman's rank correlation test; ρ = 0.52, P < 0.0001). There was no significant correlation between sVEGFR1 and VEGF levels in normal mucosa (Spearman's rank correlation test; ρ = 0.24, P = 0.23). •, no recurrence; ○, recurrence.
Predictive factor for recurrence of colorectal cancer. Table 2 shows the results of univariate analyses of angiogenic factors in colorectal cancer without distant metastases. sVEGFR1 levels were significantly higher in tissue from patients with colorectal cancer who did not experience a recurrence, compared with tissue from patients with colorectal cancer who did experience a recurrence (P = 0.038). However, there was no significance in VEGF levels between tissue from patients with colorectal cancer who did or did not experience a recurrence (P = 0.46). Based on recurrence status, lymph node metastases and sVEGFR1 levels were significantly different in tissues from patients with colorectal cancer that recurred and tissue from patients with colorectal cancer that did not recur (P = 0.011 and P = 0.038, respectively; Table 3). However, there was no correlation between VEGF levels and recurrence status (P = 0.46).
Table 2.
The quantitation of soluble vascular endothelial growth factor receptor 1 (sVEGFR1) and vascular endothelial growth factor (VEGF) in colorectal cancer without distant metastases
| Characteristics | sVEGFR1 (ng/mg protein) | P‐value | VEGF (ng/mg protein) | P‐value |
|---|---|---|---|---|
| Age | ||||
| ≤60 | 3.48 ± 1.62 | 0.37 | 1.06 ± 1.08 | 0.34 |
| >60 | 3.11 ± 1.79 | 1.38 ± 1.54 | ||
| Gender | ||||
| Male | 3.46 ± 1.54 | 0.30 | 1.40 ± 1.65 | 0.36 |
| Female | 3.03 ± 1.91 | 1.10 ± 0.99 | ||
| Location | ||||
| Colon | 3.12 ± 1.83 | 0.49 | 1.44 ± 1.63 | 0.24 |
| Rectum | 3.40 ± 1.64 | 1.07 ± 1.07 | ||
| T factor | ||||
| T1 | 2.90 ± 0.53 | 0.76 | 1.08 ± 0.66 | 0.77 |
| T2 | 3.29 ± 2.31 | 1.33 ± 1.09 | ||
| T3 | 3.13 ± 1.56 | 1.12 ± 1.18 | ||
| T4 | 3.88 ± 2.05 | 1.82 ± 2.35 | ||
| Lymph node status | ||||
| Negative | 3.44 ± 1.70 | 0.25 | 1.35 ± 1.48 | 0.47 |
| Positive | 2.97 ± 1.76 | 1.11 ± 1.22 | ||
| Differentiation | ||||
| well | 3.38 ± 1.89 | 0.56 | 1.50 ± 1.67 | 0.15 |
| non‐well | 3.15 ± 1.59 | 1.04 ± 1.04 | ||
| Dukes stage | ||||
| A | 3.27 ± 2.01 | 0.44 | 1.27 ± 0.96 | 0.38 |
| B | 3.50 ± 1.61 | 1.38 ± 1.64 | ||
| C | 2.97 ± 1.76 | 1.11 ± 1.22 | ||
| Adjuvant therapy | ||||
| Negative | 3.37 ± 1.79 | 0.36 | 1.29 ± 0.36 | 0.77 |
| Positive | 2.97 ± 1.55 | 0.18 ± 0.47 | ||
| Recurrence | ||||
| Negative | 3.42 ± 1.73 | 0.038 | 1.30 ± 1.35 | 0.46 |
| Positive | 2.21 ± 1.32 | 0.96 ± 1.59 | ||
Intratumoral sVEGFR1 and VEGF levels were determined by enzyme‐linked immunosorbent assay (ELISA). The results reflect the mean values and P‐value. The correlations between each biological factor and clinicopathological parameters were analyzed using Student's t‐test and Kruskal–Wallis test. The data shown are the mean values ± standard deviation.
Table 3.
The differences of clinicopathological factors in colorectal cancer patients without distant metastases according to their recurrence status
| Characteristics | Absence of recurrence | Presence of recurrence | P‐value |
|---|---|---|---|
| Age | |||
| ≤60 | 25 | 4 | >0.99 |
| >60 | 39 | 6 | |
| Gender | |||
| Male | 32 | 7 | 0.32 |
| Female | 32 | 3 | |
| Location | |||
| Colon | 32 | 5 | >0.99 |
| Rectum | 32 | 5 | |
| Depth of invasion | |||
| T1 | 3 | 0 | 0.26 |
| T2 | 11 | 0 | |
| T3 | 42 | 7 | |
| T4 | 8 | 3 | |
| Size | |||
| Mean (cm) | 4.5 + 2.0 | 5.5 + 2.1 | 0.19 |
| Lymph node status | |||
| Negative | 43 | 2 | 0.011 |
| Positive | 21 | 8 | |
| Differentiation | |||
| well | 33 | 2 | 0.091 |
| non‐well | 31 | 8 | |
| Dukes stage | |||
| A | 12 | 0 | 0.015 |
| B | 31 | 2 | |
| C | 21 | 8 | |
| Adjuvant therapy | |||
| Negative | 45 | 19 | 0.71 |
| Positive | 8 | 2 | |
| sVEGFR1 | |||
| Mean (ng/mg protein) | 3.42 + 1.73 | 2.21 + 1.32 | 0.038 |
| VEGF | |||
| Mean (ng/mg protein) | 1.30 + 1.35 | 0.96 + 1.59 | 0.46 |
The Fisher's exact test was used to evaluate the relationship between two discrete and dichotomy variables, and the Student's t‐test was used to evaluate the differences between the two groups for continuous variables. sVEGFR1, soluble vascular endothelial growth factor receptor 1; VEGF, vascular endothelial growth factor.
To assess the predictive value of sVEGFR1 status, we determined a cut‐off level according to a step‐wise method that provided the optimal separation between a low and high risk of recurrence. Table 4 shows the relationship between the increasing cut‐off level and the statistical prognostic value by logrank test. The cut‐off value of sVEGFR1 was determined as 1.5 ng/mg protein. When we compared recurrence‐free survival based on sVEGFR1 levels in colorectal cancer, patients with higher sVEGFR1 levels (≥1.5 ng/mg protein) demonstrated significant longer recurrence‐free survival than patients with lower sVEGFR1 levels (<1.5 ng/mg protein) (P = 0.0017; Fig. 3).
Table 4.
Univariate prognostic value of soluble vascular endothelial growth factor receptor 1 (sVEGFR1)
| Cut‐off level (ng/mg protein) | P‐value |
|---|---|
| 1.0 | 0.0161 |
| 1.1 | 0.0161 |
| 1.2 | 0.0161 |
| 1.3 | 0.0161 |
| 1.4 | 0.0161 |
| 1.5 | 0.0017 |
| 1.6 | 0.0057 |
| 1.7 | 0.0087 |
| 1.8 | 0.0532 |
| 1.9 | 0.0110 |
| 2.0 | 0.0201 |
| 2.5 | 0.1242 |
| 3.0 | 0.0655 |
| 3.5 | 0.1531 |
| 4.0 | 0.1704 |
| 4.5 | 0.1974 |
| 5.0 | N.D. |
sVEGFR1 status showed a significant statistical prognostic value by univariate analysis. The cut‐off value of sVEGFR1 was determined as 1.5 ng/mg protein. N.D., not determined.
Figure 3.
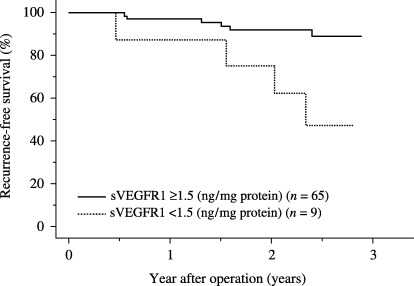
Kaplan–Meier curve for recurrence‐free survival in patients with colorectal cancer by soluble vascular endothelial growth factor receptor 1 (sVEGFR1) level. When we compared recurrence‐free survival based on sVEGFR1 levels in colorectal cancer, patients with higher sVEGFR1 levels (≥1.5 ng/mg protein) demonstrated significant longer recurrence‐free survival than patients with lower sVEGFR1 levels (<1.5 ng/mg protein) (log‐rank test; P = 0.0017).
In the multivariate analysis, lymph node status and sVEGFR1 levels were independent predictive factors of recurrence in colorectal cancer (Table 5).
Table 5.
Multivariate analysis for recurrence‐free survival
| SE | χ2 | HR | P‐value | ||
|---|---|---|---|---|---|
| Lymph node status | Negative : Positive | 0.86 | 4.89 | 6.7 | 0.027 |
| sVEGFR1 (ng/mg protein) | <1.5 : ≥1.5 | 0.85 | 4.13 | 0.17 | 0.042 |
HR, hazard ratio; SE, standard error; sVEGFR1, soluble vascular endothelial growth factor receptor 1.
We investigated the correlation between angiogenic factors and overall survival in colorectal cancer. The two‐year survival was 94.2% in the patients with high sVEGFR1 levels and 80.0% in patients with low sVEGFR1 levels. There was no significant correlation between the two groups (P = 0.25).
Discussion
Vascular endothelial growth factor overexpression is known to be associated with the progression of cancer. In many types of human cancers, VEGF concentrations increase significantly in tumor tissues and correlate with prognosis. In this study, we found that sVEGFR1 levels were elevated in colorectal cancer tissue compared with corresponding normal mucosa. Previously, we reported that the means of VEGF and sVEGFR1 levels in breast cancer tissue were 0.53 ng/mg protein and 0.95 ng/mg protein, respectively.( 25 ) Comparing those data with the current data measured by the same method, both VEGF and sVEGFR1 levels are significantly higher in colorectal cancer than breast cancer (P < 0.001 and P < 0.001, respectively). On the other hand, it has been reported that serum sVEGFR1 level was more often elevated in breast cancer than colorectal cancer.( 24 ) The reasons for the difference between breast cancer and colorectal cancer and between tissue and serum levels are not clear, yet. Further studies are required to clarify the difference. In addition, there is a significant correlation between VEGF expression and sVEGFR1 expression in colorectal cancer tissues. The finding that VEGF and its intrinsic negative counterpart, sVEGFR1, tend to be coexpressed in a positive association was also observed in a study of primary breast cancer.( 3 , 25 ) These similar findings indicate a possibility that a common regulatory mechanism exists between these molecules and that the mechanism is activated in the process of carcinogenesis or cancer progression. Interestingly, the ratio of sVEGFR1 and VEGF was significantly lower in cancer tissue compared with normal tissue, although little is known about the mechanism of this finding. According to accumulated experimental and clinical data, it is likely that the induction of sVEGFR1 in tumor tissue inhibits VEGF‐induced tumor angiogenesis and retards tumor growth.( 16 , 17 , 19 , 20 ) Thus, it is speculated that the shift in the tumor microenvironment from a sVEGFR1‐dominant state to a relatively VEGF‐dominant state helps tumors form new vessels, grow, and progress.
In terms of the up‐regulation mechanism of sVEGFR1, Barleon et al. showed that media conditioned by various cancer cell lines grown under hypoxic conditions were able to up‐regulate expression of VEGFR1 and sVEGFR1 but not of VEGFR2.( 26 ) Theses effects were completely inhibited by VEGF‐neutralizing extracellular VEGF receptor domains, indicating that expression of sVEGFR1 might be regulated by VEGF. Consequently, VEGF as well as hypoxia might play a significant role in the regulation of sVEGFR1 expression in the tumor microenvironment.
Recently, circulating sVEGFR1 levels were discovered to increase remarkably in patients with preeclampsia.( 27 ) It is thought that sVEGFR1 is made by the placenta and acts by neutralizing VEGF and PlGF. Higher concentration of sVEGFR1 and lower concentrations of PlGF and VEGF have been observed in the serum of patients with preeclampsia. Therefore, a reciprocal regulatory mechanism can be considered not only in the preeclampsia state, but also in malignant tumors.
There were two opposite reports regarding the serum sVEGFR1 levels in colorectal cancer patients. Kumar et al. reported that sVEGFR1 was detected in the serum of colorectal cancer patients, and after surgery, it was markedly decreased. On the other hand, Chin et al. showed that serum sVEGFR1 levels in colorectal cancer patients before surgery were significantly lower than those in normal controls, and after curative surgery, serum sVEGFR1 levels became equivalent to those in normal controls.( 28 ) As in our study sVEGFR1 levels were significantly higher in colorectal cancer tissue than in normal mucosa, our data supported the former report.
To our knowledge, the current study is the first to show the clinical significance of sVEGFR1 expression in colorectal cancer tissue and normal mucosa. VEGF and sVEGFR1 levels had no correlation with any clinical or pathologic factors in colorectal cancers; however, overexpression of sVEGFR1 was significantly associated with absence of recurrence. Intratumoral VEGF concentrations showed no prognostic value in this study. Several reports have failed to demonstrate the prognostic value of tumor VEGF expression,( 29 , 30 ) whereas many other studies have shown that VEGF has a significant value as a prognostic marker.( 31 , 32 , 33 , 34 , 35 ) This discrepancy might be due to the difference in the measurement methodology of VEGF. We measured VEGF protein concentrations in fresh‐frozen tumor materials by ELISA after confirming a significant relationship between VEGF protein levels measured by ELISA and those measured by Western‐blot analysis.( 25 )
To characterize the prognostic value of sVEGFR1, we assessed a cut‐off value and the ratio between sVEGFR1 and VEGF levels. A significant prognostic value was observed between 1.0 ng/mg protein and 2.0 ng/mg protein, and the highest value was seen at 1.5 ng/mg protein; thus, we used 1.5 ng/mg protein as the cut‐off value for prognostic assessment. Patients with high sVEGFR1 levels (≥1.5 ng/mg protein) showed a significantly favorable prognosis compared with those with low sVEGFR1 levels (<1.5 ng/mg protein). In the analysis of the sVEGFR1/VEGF ratio, we failed to demonstrate a significant prognostic value in the overall population. These results were different from results of our primary breast cancer study where we found a potent prognostic value of the sVEGFR1/VEGF ratio.( 3 , 25 ) It is difficult to explain why the sVEGFR1/VEGF ratio was significant for breast cancer but not for colorectal cancer. However the difference in steroid hormone dependency between these two tumor types might contribute to these different findings, as recent studies have shown that estrogen is a significant regulator of VEGF and sVEGFR1 in human breast cancer cells.( 36 , 37 )
Recently, bevacizumab, which is a recombinant humanized monoclonal antibody of VEGF, has been demonstrated to improve the survival of metastatic colorectal cancer patients undergoing chemotherapy.( 38 ) Bevacizumab blocks VEGF by inhibiting the VEGF signaling pathway, resulting in suppression of tumor angiogenesis and in retardation of tumor growth. Nevertheless, a recent report showed that VEGF levels had no significant correlation with the therapeutic effect of bevacizumab plus chemotherapy.( 39 ) In a future study, it might be interesting to explore the value of sVEGFR1 and VEGF levels for predicting the therapeutic impact of bevacizumab‐containing treatments.
Various types of antiangiogenic therapies are being tested clinically. In the future, more novel agents and new combinations will be examined in clinical trials. Combinations consisting of multiple antiangiogenic agents might be also investigated based on preclinical studies that demonstrate additional or synergistic effects. To create more effective antiangiogenic therapies and optimize treatment for patients, it is critical to study cancer biology further, with a particular emphasis on tumor angiogenesis. Because VEGF is regarded as the most important therapeutic target in an antiangiogenesis strategy, it will be important to pay an attention to sVEGFR1 expression in cancer tissues. It would also be interesting to study what regulates the balance between sVEGFR1 and VEGF levels in cancer tissues and in circulation. These investigations could help lead to an efficient individualized antiangiogenesis therapy for cancer.
References
- 1. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–9. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55: 3964–8. [PubMed] [Google Scholar]
- 3. Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res 1994; 85: 1045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maeda K, Chung YS, Ogawa Y et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996; 77: 858–63. [DOI] [PubMed] [Google Scholar]
- 5. George DJ, Halabi S, Shepard TF et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone‐refractory prostate cancer treated on cancer and leukemia group B 9480. Clin Cancer Res 2001; 7: 1932–6. [PubMed] [Google Scholar]
- 6. Fontanini G, Lucchi M, Vignati S et al. Angiogenesis as a prognostic indicator of survival in non‐small‐cell lung carcinoma: a prospective study. J Natl Cancer Inst 1997; 89: 881–6. [DOI] [PubMed] [Google Scholar]
- 7. Gorski DH, Leal AD, Goydos JS. Differential expression of vascular endothelial growth factor‐A isoforms at different stages of melanoma progression. J Am Coll Surg 2003; 197: 408–18. [DOI] [PubMed] [Google Scholar]
- 8. De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms‐like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992; 255: 989–91. [DOI] [PubMed] [Google Scholar]
- 9. Terman BI, Dougher‐Vermazen M, Carrion ME et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial growth factor. Biochem Biophys Res Commun 1992; 187: 1579–86. [DOI] [PubMed] [Google Scholar]
- 10. Clauss M, Weich H, Breier G et al. The vascular endothelial growth factor receptor Flt‐1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996; 271: 17 629–34. [DOI] [PubMed] [Google Scholar]
- 11. Lyden D, Hattori K, Dias S et al. Impaired recruitment of bone‐marrow‐derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001; 7: 1194–201. [DOI] [PubMed] [Google Scholar]
- 12. LeCouter J, Moritz DR, Li B et al. Angiogenesis‐independent endothelial protection of liver: role VEGFR‐1 Science 2003; 299: 890–3. [DOI] [PubMed] [Google Scholar]
- 13. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt‐1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 1998; 95: 9349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunk C, Ahmed A. Vascular endothelial growth factor receptor‐2‐mediated mitogenesis is negatively regulated by vascular endothelial growth factor receptor‐1 in tumor epithelial cells. Am J Pathol 2001; 158: 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veikkola T, Karkkainen M, Claesson‐Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 2000; 60: 203–12. [PubMed] [Google Scholar]
- 16. Kendall RL, Thomas KA. Inhibition of vascular endothelial growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993; 90: 10 705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT‐1, and its heterodimerization with KDR. Biochem Biophys Res Commun 1996; 226: 324–8. [DOI] [PubMed] [Google Scholar]
- 18. Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. J Biol Chem 1994; 269: 25 646–54. [PubMed] [Google Scholar]
- 19. Toi M, Bando H, Ogawa T, Muta M, Hornig C, Weich HA. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor‐1 relationship in breast cancer. Int J Cancer 2002; 98: 14–8. [DOI] [PubMed] [Google Scholar]
- 20. Lamszus K, Ulbricht U, Matschke J, Brockmann MA, Fillbrandt R, Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity and VEGF‐A. Clin Cancer Res 2003; 9: 1399–405. [PubMed] [Google Scholar]
- 21. Takayama K, Ueno H, Nakanishi Y et al. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 2000; 60: 2169–77. [PubMed] [Google Scholar]
- 22. Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology 2005; 10: 131–4. [DOI] [PubMed] [Google Scholar]
- 23. Ilhan N, Ilhan N, Deveci F. Functional significance of vascular endothelial growth factor and its receptor (receptor‐1) in various lung cancer types. Clin Biochem 2004; 37: 840–5. [DOI] [PubMed] [Google Scholar]
- 24. Kumar H, Heer K, Greenman J, Kerin MJ, Monson JR. Soluble FLT‐1 is detectable in the sera of colorectal and breast cancer patients. Anticancer Res 2002; 22: 1877–80. [PubMed] [Google Scholar]
- 25. Bando H, Weich HA, Brokelmann M et al. Association between intratumoral free and total VEGF, soluble VEGFR‐1, VEGFR‐2 and prognosis in breast cancer. Br J Cancer 2005; 92: 553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barleon B, Siemeister G, Martiny‐Baron G, Weindel K, Herzog C, Marme D. Vascular endothelial growth factor up‐regulates its receptor fms‐like tyrosine kinase 1 (FLT‐1) and a soluble variant of FLT‐1 in human vascular endothelial cells. Cancer Res 1997; 57: 5421–5. [PubMed] [Google Scholar]
- 27. Levine RJ, Maynard SE, Qian C et al. Circulating angiogenic factors and the risk of preeclampsia. New Eng J Med 2004; 350: 672–83. [DOI] [PubMed] [Google Scholar]
- 28. Chin KF, Greenman J, Reusch P, Gardiner E, Marme D, Monson J. Changes in serum soluble VEGFR‐1 and Tie‐2 receptors in colorectal cancer patients following surgical resections. Anticancer Res 2004; 24: 2353–7. [PubMed] [Google Scholar]
- 29. Karayiannakis AJ, Syrigos KN, Zbar A et al. Clinical significance of preoperative serum vascular endothelial growth factor levels in patients with colorectal cancer and the effect of tumor surgery. Surgery 2002; 131: 548–55. [DOI] [PubMed] [Google Scholar]
- 30. Roumen RM, Slooter GD, Croiset van Uchelen FA, Huib LV. Preoperative serum vascular endothelial growth factor is not a marker for subsequent recurrence during long‐term follow‐up of colorectal cancer patients. Dis Colon Rectum 2005; 48: 1070–5. [DOI] [PubMed] [Google Scholar]
- 31. Kumar H, Heer K, Lee PW et al. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res 1998; 4: 1279–85. [PubMed] [Google Scholar]
- 32. Werther K, Christensen IJ, Brunner N, Nielsen HJ. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. The Danish Ranx05 colorectal cancer study group. Eur J Surg Oncol 2000; 26: 657–62. [DOI] [PubMed] [Google Scholar]
- 33. Nakayama Y, Sako T, Shibao K et al. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res 2002; 22: 2437–42. [PubMed] [Google Scholar]
- 34. Galizia G, Lieto E, Ferraraccio F et al. Determination of molecular marker expression can predict clinical outcome in colon carcinomas. Clin Cancer Res 2004; 10: 3490–9. [DOI] [PubMed] [Google Scholar]
- 35. Ferroni P, Spila A, Martini F et al. Prognostic value of vascular endothelial growth factor tumor tissue content of colorectal cancer. Oncology 2005; 69: 145–53. [DOI] [PubMed] [Google Scholar]
- 36. Elkin M, Orgel A, Kleinman HK. An angiogenic switch in breast cancer involves estrogen and soluble vascular endothelial growth factor receptor 1. J Natl Cancer Inst 2004; 96: 875–8. [DOI] [PubMed] [Google Scholar]
- 37. Seo KH, Lee HS, Jung B et al. Estrogen enhances angiogenesis through a pathway involving platelet‐activating factor‐mediated nuclear factor‐kappaB activation. Cancer Res 2004; 64: 6482–8. [DOI] [PubMed] [Google Scholar]
- 38. Hurwitz HI, Fehrenbacher L, Hainsworth JD et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first‐line metastatic colorectal cancer. J Clin Oncol 2005; 23: 3502–8. [DOI] [PubMed] [Google Scholar]
- 39. Jubb AM, Hurwitz HI, Bai W et al. Impact of vascular endothelial growth factor‐A expression, thrombospondin‐2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006; 24: 217–27. [DOI] [PubMed] [Google Scholar]


