Abstract
Expression of vascular endothelial growth factor (VEGF) has been reported in renal cell carcinoma (RCC), a highly angiogenic carcinoma. However, little or no information is available on the expression of Ets‐1, which is one of the target molecules of VEGF. In the present study, we examined the expression of Ets‐1 and VEGF in RCC by immunohistochemistry and reverse transcription–polymerase chain reaction (RT‐PCR), and correlations between expression and the microvessel density (MVD) were evaluated. Ets‐1 was immunolocalized to carcinoma cells and endothelial cells of the microvessels in clear cell RCC, but not in papillary RCC. Immunohistochemical Ets‐1 expression and MVD were significantly higher in clear cell RCC than in papillary RCC. Predominant mRNA expression of Ets‐1 in clear cell RCC was confirmed by RT‐PCR. The expression of Ets‐1 correlated directly with MVD in clear cell RCC. Hypoxic treatment upregulated the mRNA expression of Ets‐1 and VEGF in cell lines derived from clear cell RCC, suggesting that hypoxia is a key regulator for these molecules. These results demonstrate the expression of Ets‐1 in human clear cell RCC and suggest the possibility that Ets‐1 is involved in angiogenesis in clear cell RCC. (Cancer Sci 2006; 97: 875–882)
Angiogenesis is the process of new blood vessel formation from preexisting vessels, and is necessary for tumors to grow by receiving nutrition and oxygen. As the center of the tumor mass is hypoxic, the hypoxia is considered to lead to overexpression of hypoxia inducible factor (HIF)‐1α, a component of HIF, and subsequently results in induction of vascular endothelial growth factor (VEGF) expression.( 1 ) VEGF, a potent modulator of tumor angiogenesis, is overexpressed in human renal cell carcinomas (RCC).( 2 ) Clear cell RCC, which is the most common histological variant of RCC, is highly angiogenic partly due to induction of VEGF by activation of HIF through inactivation of von Hippel Lindau (VHL) protein, which degrades HIF‐1α under normoxic conditions.( 3 ) During angiogenesis, VEGF binds to VEGF receptors 1 and 2 and activates the transcription factor Ets‐1 in endothelial cells.( 4 , 5 ) Ets‐1 converts endothelial cells to an angiogenic phenotype by inducing the genes of urokinase‐type plasminogen activator (uPA), matrix metalloproteinase (MMP)‐1, MMP‐3, MMP‐9 and intergin β3.( 5 , 6 ) Therefore, Ets‐1 appears to be a key transcription factor for extracellular matrix remodeling in angiogenesis. Notably, the expression of Ets‐1 has been demonstrated in several malignant tumors and has shown to be associated with their tumor angiogenesis.( 7 , 8 , 9 ) Previous studies on RCC have reported the expression of VEGF (a molecule upstream of Ets‐1)( 10 ) and MMP (molecules downstream of Ets‐1).( 11 ) However, no information is so far available on the expression of Ets‐1 or its correlation with angiogenesis in human RCC.
In the present study, we examined the expression of Ets‐1 and VEGF in surgically removed RCC tissues by immunohistochemistry and reverse transcription–polymerase chain reaction (RT‐PCR). Immunohistochemical expression of Ets‐1 and VEGF was compared with microvascular density (MVD) in RCC tissues. In addition, the effects of hypoxia on the expression of Ets‐1 and VEGF mRNA were examined in vitro using four RCC cell lines.
Materials and Methods
Tissue samples
Forty‐six RCC samples were removed surgically at the Keio University Hospital and used in this study. Histologically, they were 33 clear cell, eight papillary and five chromophobe cell RCC. Small pieces of tumor and non‐neoplastic control renal tissues (n = 13) were frozen in liquid nitrogen for RT‐PCR analysis. Thirty‐three patients were male and 13 patients were female and their ages ranged from 36 to 91 years (mean age 60 years). One patient had regional lymph node metastases, and two had regional and distant metastases. None of these patients received chemotherapy or radiation therapy before surgery. Informed consent for experimental use of the samples was obtained from the patients according to the hospital ethical guidelines.
Immunohistochemistry
One representative paraffin block from each case was selected by observing the sections stained with hematoxylin and eosin, and paraffin sections on aminopropyltrimethoxysilane‐coated slides were used for immunohistochemistry. The nuclear grading and pathological (p), primary tumor (T), regional lymph nodes (N) and distant metastases (M) factors were determined according to the UICC TNM system for pathological staging.( 12 ) After deparaffinization of the sections, the slides were heated in 10 mM citrate buffer (pH 6.0) at 120°C for 20 min for antigen retrieval. Endogenous peroxidase was quenched by incubation with 0.3% hydrogen peroxide in methanol for 15 min. Non‐specific binding was blocked with 10% normal goat serum. They were then reacted with rabbit polyclonal anti‐Ets‐1 antibodies (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti‐VEGF antibodies (1:200 dilution; Santa Cruz) or mouse monoclonal anti‐CD31 antibody (1:50 dilution; clone JC/70 A; Nichirei, Tokyo, Japan) for 60 min at room temperature. As a negative control, the primary antibody was replaced with non‐immune rabbit serum (1:2000 dilution) or mouse serum (1:50 dilution) in phosphate‐buffered saline (PBS). After washing with PBS, the slides were incubated with antirabbit IgG or antimouse IgG conjugated to peroxidase‐labeled dextran polymer (no dilution; EnVision + Rabbit or EnVision + Mouse; Dako Japan, Tokyo, Japan) for 15 min, and color was developed with 3,3′‐diaminobenzamine tetrahydrochloride in 50 mM Tris‐HCl (pH 7.5) containing 0.005% hydrogen peroxide. The sections were counterstained with hematoxylin.
To evaluate the staining of Ets‐1 and VEGF, positively stained carcinoma cells were counted in at least 10 representative fields (×400 magnification), and staining was scored according to the method of Khatun et al.( 8 ) Namely, a mean percentage of positive cancer cells was calculated and staining intensity was stratified from 0 to 3 (0, no staining; 1 slight staining; 2, medium staining; 3, strong staining). A histoscore was measured by applying the formula:
| Mean percentage × (intensity + 1). |
Microvessel density was determined in the five most intense vascularization areas (hotspots) of each section by observing at ×200 magnification. All discrete clusters or single cells stained positive for CD31 were counted as one vessel. The average count was regarded as MVD (number of microvessels/0.95 mm2) for each case.( 13 )
Cell cultures
The human RCC cell lines 786‐O, 769‐P, ACHN and A‐498 were purchased from the American Type Culture Collection (Manassas, VA, USA). All of the cell lines were derived from clear cell RCC. Cells were cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum and penicillin and streptomycin (Sigma‐Aldrich, St Louis, MO, USA) in a well‐humidified incubator with 20% O2 (atmospheric air) and 5% CO2 at 37°C. For hypoxic treatment, they were cultured in an anaerobic environmental chamber maintained with 1% O2, 94% N2 and 5% CO2.( 13 ) To study the association between VEGF and Ets‐1 expression, 786‐O and ACHN cell lines, both of which were reactive with the hypoxic treatment, were treated for 2 days with or without neutralizing anti‐VEGF antibody (clone MAB293, 2.5 µg/mL; R&D Systems, Minneapolis, MN, USA) and/or recombinant human VEGF165 protein (50 ng/mL; R&D Systems).
RT‐PCR
Total RNA was extracted from RCC and control non‐neoplastic renal tissues remote from the carcinomas (n = 13) and RCC cell lines (n = 4) using ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. Total RNA (1 µg) was reverse transcribed with ReveTra Ace reverse transcriptase (Toyobo, Tokyo, Japan) in a 20‐µL reaction volume containing Oligo (dT)12−18 primers. Reaction mixture (1 µL) was then subjected to 25 cycles of PCR for amplification of cDNA for Ets‐1, VEGF and β‐actin. PCR was carried out in a 50‐µL reaction volume containing 200 nM of each primer, 200 nM of dNTPs and 2.5 units Taq DNA polymerase (Takara Bio, Shiga, Japan) with a thermal controller (MiniCycler; MJ Research, Watertown, MA, USA). The thermal cycle was 1 min at 94°C, 1 min at either 55°C for Ets‐1, 64°C for VEGF or 67°C for β‐actin, and 1 min at 72°C, followed by final extension for 10 min at 72°C. Primer sequences were: 5′‐GTTAATGGAGTCAACCCAGC‐3′ (forward) and 5′‐GGGTGACGACTTCTTGTTTG‐3′ (reverse) for Ets‐1; 5′‐TGCCTTGCTGCTCTACCTCC‐3′ (forward) and 5′‐TCACCGCCTCGGCTTGTCAC‐3′ (reverse) for VEGF; and 5′‐TGACGGGGTCACCCACACTGTGCCCATGTA‐3′ (forward) and 5′‐CTAGAAGCATTTGCGGTGGACGATGGAGGG‐3′ (reverse) for β‐actin. Expected sizes of amplified cDNA for Ets‐1, VEGF121, VEGF145, VEGF165, VEGF189, VEGF206 and β‐actin were 0.26, 0.41, 0.48, 0.54, 0.61, 0.66 and 0.66 kb, respectively. Each PCR product (5 µL) was electrophoresed on a 2% agarose gel, and then stained with ethidium bromide. The nucleotide sequence of the PCR products was confirmed by cycle sequencing using a DYEnamicTM ET dye termination cycle sequencing kit and MegaBASE 1000 DNA sequencer (Amersham Biosciences, Piscataway, NJ, USA).( 14 ) Similarly, the expression of Ets‐1 and β‐actin in the cell lines treated with anti‐VEGF antibody and/or VEGF165 was examined by RT‐PCR as described above.
Statistical analysis
Mann–Whitney's U‐test was used to analyze the relationship between the expression of Ets‐1 or VEGF and the clinicopathological parameters. A simple regression test was applied to analyze the association between Ets‐1 staining and MVD, VEGF staining and MVD, or Ets‐1 staining and VEGF staining. P‐values less than 0.05 were considered to be significant.
Results
Immunohistochemical expression of Ets‐1 and VEGF in RCC tissues
Immunohistochemical staining showed that Ets‐1 protein is expressed preferentially in the nuclei of clear cell RCC (Fig. 1A). In contrast, no apparent staining of Ets‐1 was observed in papillary RCC (Fig. 1B). Ets‐1 was also expressed in chromophobe RCC, but its intensity was much weaker than that in clear cell carcinomas (Fig. 1C). In most cases of clear cell RCC, Ets‐1 expression was also observed in the nuclei of endothelial cells of microvessels adjacent to the carcinoma cell nests (Fig. 1D, arrowheads), and the interstitial cells, including macrophages and fibroblasts (data not shown). However, Ets‐1‐positive interstitial cells were not observed in areas distant from Ets‐1‐positive cancer cell nests nor in Ets‐1‐negative RCC (data not shown). No Ets‐1 staining was obtained in the renal glomeruli or renal tubular cells of normal renal tissue (Fig. 1E).
Figure 1.
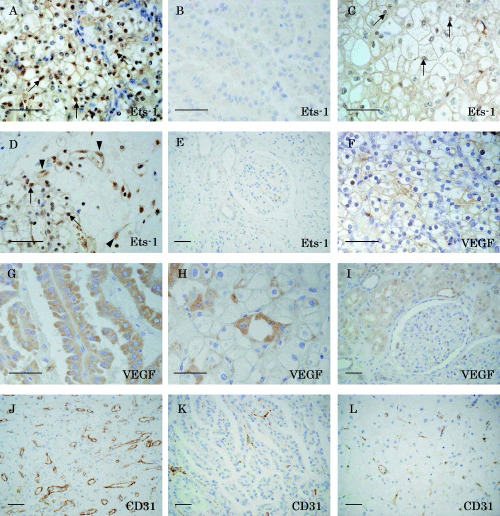
Immunohistochemical staining of (A–E) Ets‐1, (F–I) vascular endothelial growth factor (VEGF) and (J–L) CD31 in renal cell carcinoma. Paraffin sections of (A,D,F,J) clear cell carcinomas, (B,G,K) papillary carcinomas, (C,H,L) chromophobe cell carcinomas and (E,I) normal renal tissue were immunostained with anti‐Ets‐1, anti‐VEGF and anti‐CD31 antibodies. Note that Ets‐1 staining was observed mainly in the nuclei of clear cell carcinomas (arrows in A and D) and chromophobe cell carcinomas (arrows in C), but no definite staining was present in papillary carcinomas (B). The endothelial cells of microvessels adjacent to Ets‐1‐positive cancer cell nests were also positive for Ets‐1 (arrowheads in D). Scale bars = 50 µm.
Vascular endothelial growth factor staining was observed in RCC regardless of the histological subtype. Clear cell RCC tended to show VEGF staining in the cell membrane (Fig. 1F). In contrast, intense and diffuse cytoplasmic staining was obtained in papillary RCC (Fig. 1G). VEGF was immunolocalized to the cytoplasm and cell membrane in chromophobe RCC (Fig. 1H). In addition, VEGF was also localized to renal tubular cells of normal renal tissue (Fig. 1I). CD31 was positively immunostained in endothelial cells of microvessels within tumors (Fig. 1J–L).
mRNA expression of Ets‐1 and VEGF in RCC tissues
As shown in Fig. 2A, Ets‐1 mRNA expression was observed in nine of 11 clear cell RCC tissues (cases 2–7 and 9–11), but no apparent expression was observed in papillary RCC (cases 12 and 13). In some positive clear cell RCC cases (cases 2, 9, 10 and 11), Ets‐1 expression was also observed in non‐neoplastic renal tissues, although the levels appeared to be weak. mRNA expression of VEGF species was detected in all 11 clear cell RCC and in two papillary RCC, and all of these non‐neoplastic renal tissues also showed weak VEGF expression (Fig. 2A).
Figure 2.
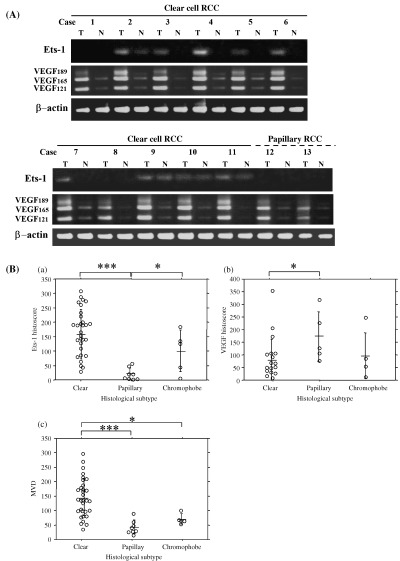
(A) Ets‐1 and vascular endothelial growth factor (VEGF) mRNA expression in renal cell carcinomas (RCC) and control non‐neoplastic tissues. T, tumor tissues; N, non‐neoplastic tissues. Note that Ets‐1 mRNA was observed in nine of 11 clear cell RCC tissues, and no apparent Ets‐1 mRNA was seen in two papillary RCC. VEGF mRNA expression was observed in all 11 clear cell and in two papillary RCC. (B) (a) Ets‐1 histoscore, (b) VEGF histoscore and (c) microvessel density in histological subtypes of RCC. The bars indicate mean ± SD. *P < 0.05; ***P < 0.0001.
Correlations in Ets‐1 expression, VEGF expression and MVD between histological subtypes of RCC
The Ets‐1 immunohistochemical histoscore in clear cell RCC (157 ± 85; mean ± SD) was significantly higher than that in papillary RCC (19 ± 22; P < 0.0001) (Fig. 2Ba). The score in chromophobe RCC (99 ± 72) was also significantly higher than that in papillary RCC (P < 0.05). In contrast, the VEGF immunohistochemical score in clear cell RCC (76 ± 85) was significantly lower than that in papillary RCC (169 ± 99; P < 0.05) (Fig. 2Bb). MVD in RCC varied from 15 to 298, and the mean MVD was 118 ± 70 (mean ± SD). The MVD in clear cell RCC (144 ± 65) was significantly higher than that in papillary RCC (42 ± 24; P < 0.0001) and chromophobe RCC (69 ± 18; P < 0.05) (Fig. 2Bc). However, Ets‐1 expression, VEGF expression and MVD showed no correlation with TNM stage, histological grade or venous invasion (Table 1).
Table 1.
Relationship between the clinicopathological parameters and Ets‐1 expression, vascular endothelial growth factor (VEGF) expression or microvessel density (MVD)
| Parameter | Ets‐1 score (mean ± SD) | VEGF score (mean ± SD) | MVD (mean ± SD) |
|---|---|---|---|
| T (primary tumor) | |||
| pT1 (n = 23) | 111 ± 90 | 123 ± 105 | 106 ± 77 |
| pT2 (n = 8) | 130 ± 82 | 62 ± 74 | 139 ± 67 |
| pT3 (n = 15) | 149 ± 101 | 62 ± 65 | 127 ± 60 |
| N (regional lymph nodes) | |||
| pN0 (n = 45) | 123 ± 91 | 96 ± 93 | 117 ± 70 |
| pN1 (n = 1) | 270 | 51 | 178 |
| M (distant metastasis) | |||
| pM0 (n = 44) | 123 ± 91 | 99 ± 94 | 117 ± 71 |
| pM1 (n = 2) | 208 | 35 | 144 ± 16 |
| Stage grouping | |||
| Stage I (n = 23) | 111 ± 90 | 123 ± 105 | 106 ± 77 |
| Stage II (n = 7) | 131 ± 89 | 81 ± 78 | 140 ± 72 |
| Stage III (n = 13) | 128 ± 93 | 63 ± 73 | 121 ± 63 |
| Stage IV (n = 3) | 229 ± 88 | 40 ± 30 | 155 ± 23 |
| Histological grade | |||
| G1 (n = 10) | 136 ± 104 | 56 ± 70 | 127 ± 78 |
| G2 (n = 29) | 130 ± 85 | 120 ± 105 | 117 ± 70 |
| G3 (n = 7) | 97 ± 110 | 57 ± 43 | 111 ± 66 |
| Venous invasion | |||
| v (–) (n = 33) | 117 ± 95 | 124 ± 97 | 120 ± 76 |
| v (+) (n = 13) | 151 ± 81 | 35 ± 38 | 114 ± 53 |
Correlations among Ets‐1 expression, VEGF expression and MVD in RCC
Ets‐1 immunohistochemical histoscore was correlated directly with MVD in all of the RCC samples (r 2 = 0.61, P < 0.0001; n = 46) (Fig. 3A). In addition, a similar positive correlation was observed in clear cell RCC (r 2 = 0.51, P < 0.0001; n = 30) (Fig. 3B). Although there was no correlation between VEGF histoscore and MVD (r 2 = 0.04, P = 0.31; n = 30) (Fig. 3C), or between Ets‐1 histoscore and VEGF histoscore in all of the RCC samples (r 2 = 0.006, P = 0.69; n = 30) (Fig. 3D), a direct correlation was observed between VEGF histoscore and MVD in the clear cell RCC (r 2 = 0.30, P = 0.01; n = 20) (Fig. 3E). Clear cell RCC with high VEGF histoscores tended to have high Ets‐1 histoscores, but no correlation was observed between Ets‐1 and VEGF histoscore (r 2 = 0.02, P = 0.51; n = 20), because a certain proportion of cases with high Ets‐1 histoscores had low VEGF histoscores (Fig. 3F).
Figure 3.
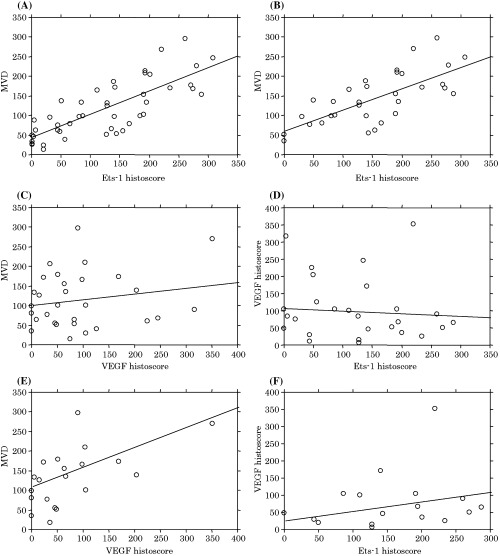
Correlations among Ets‐1 histoscore, vascular endothelial growth factor (VEGF) histoscore and microvessel density (MVD) in renal cell carcinoma (RCC). Correlations between Ets‐1 histoscore and MVD in (A) all of the RCC samples and (B) clear cell RCC. (C) Correlation between VEGF histoscore and MVD in all of the RCC samples. (D) Correlation between Ets‐1 and VEGF histoscores in all of the RCC samples. (E) Correlation between VEGF histoscore and MVD in clear cell RCC. (F) Correlation between Ets‐1 and VEGF histoscores in clear cell RCC.
Stimulation of Ets‐1 and VEGF mRNA expression by hypoxia in RCC cell lines
Steady‐state mRNA expression of Ets‐1 was observed in 786‐O, 769‐P and ACHN cells, but not in A498 cells (Fig. 4A). All of these cell lines showed upregulation of Ets‐1 expression after hypoxia, and expression of the VEGF121, VEGF165 and VEGF189 isoforms also appeared to increase concomitantly (Fig. 4A). In A498 cells, Ets‐1 was induced 30 h after hypoxia, although there were no definite changes in VEGF expression. To examine whether VEGF is upstream of Ets‐1 in the RCC cell lines, neutralizing anti‐VEGF antibody and/or recombinant human VEGF165 protein were added to 786‐O and ACHN cell lines, but the mRNA expression level of Ets‐1 showed no apparent changes (Fig. 4B).
Figure 4.
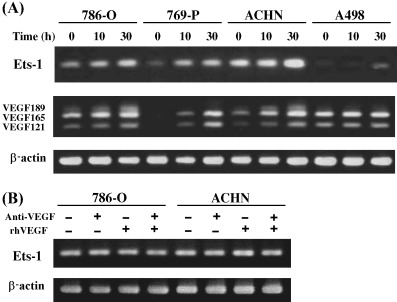
(A) Time‐dependent induction of Ets‐1 and vascular endothelial growth factor (VEGF) expression by hypoxia. Renal cell carcinoma cell lines were incubated under hypoxic conditions (1% O2). Total RNA was isolated at the time points indicated after hypoxic stimulation, and reverse transcription–polymerase chain reaction (RT‐PCR) was carried out. Note that hypoxic treatment increased Ets‐1 and VEGF mRNA expression in 786‐O, 769‐P and ACHN cell lines, and induced Ets‐1 expression in A498 cells. (B) Effect of neutralizing anti‐VEGF antibody and recombinant human VEGF165 protein on Ets‐1 expression. 786‐O and ACHN cells were cultured for 2 days with or without anti‐VEGF antibody (2.5 µg/mL) and/or recombinant human VEGF165 protein (50 ng/mL), and the Ets‐1 expression was analyzed by RT‐PCR.
Discussion
Previous studies have indicated that Ets‐1 is related to angiogenesis in a variety of cancers, including carcinomas of the uterine cervix, ovary and esophagus.( 7 , 8 , 9 ) However, no information was available in RCC. RCC are classified histologically into three subtypes: clear cell, papillary and chromophobe cell. Among them, clear cell RCC is characterized by a remarkable degree of angiogenesis from the beginning of carcinogenesis, showing that MVD is higher in clear cell RCC than in non‐clear cell RCC.( 15 ) In the present study, we have provided the first evidence that Ets‐1 is immunolocalized predominantly to carcinoma cells in the tumor masses of clear cell RCC, with a direct correlation with MVD.
Interestingly, our study showed that Ets‐1 is localized to the carcinoma cell nuclei in the clear cell RCC, suggesting that Ets‐1 functions as a transcription factor in carcinoma cells. In most clear cell RCC, the VHL gene is inactivated by a combination of allelic deletion and either mutation or, less commonly, hypermethylation, whereas no inactivation has been reported in non‐clear cell RCC.( 16 ) The expression of Ets‐1 is induced by HIF‐1α,( 17 ) which is degraded by the action of VHL under normoxic conditions. Thus, HIF‐1α‐mediated transcription of Ets‐1 may be activated constitutively due to impaired degradation of HIF1‐α by VHL in clear cell RCC.
Our immunohistochemical study has demonstrated that Ets‐1 is also expressed in endothelial cells of the microvessels adjacent to carcinoma cell nests in clear cell RCC. Similar findings have been reported for human gastric and ovarian cancers.( 8 , 18 ) Cancer cells are known to release various growth factors, including acidic fibroblast growth factor, basic fibroblast growth factor, VEGF and epidermal growth factor, all of which can induce the expression of Ets‐1 mRNA in human umbilical vein endothelial cells.( 19 ) As clear cell RCC overexpress basic fibroblast growth factor and VEGF,( 20 ) these growth factors may be responsible for the induction of Ets‐1 mRNA expression in endothelial cells in clear cell RCC. Ets‐1 expressed in endothelial cells of microvessels is known to convert endothelial cells to angiogenic phenotypes, probably through the induction of MMP, uPA and integrin β3.( 6 ) Among them, MMP are well known to play a central role in angiogenesis through degradation of the extracellular matrix components by endothelial cells migrating from the original blood vessels.( 21 ) Expression of MMP‐1, MMP‐3 and MMP‐9, all of which are expressed in RCC,( 22 ) is upregulated by Ets‐1.( 6 ) Altogether, it is reasonable to speculate that Ets‐1 expressed by endothelial cells plays a role in angiogenesis through the induction of MMP in clear cell RCC.
In contrast to clear cell RCC, Ets‐1 staining was not observed in the endothelial cells of papillary RCC, although VEGF was immunolocalized to the carcinoma cells of papillary RCC. This finding suggests that VEGF expressed by papillary RCC does not induce Ets‐1 in endothelial cells. Although the molecular mechanism of this finding is not clear, several factors, such as connective tissue growth factor (CTGF) and platelet factor‐4, inhibit VEGF‐induced angiogenesis by inhibiting the binding of VEGF to endothelial cells.( 23 , 24 ) In fact, our preliminary immunohistological study showed that CTGF protein expression was higher in papillary RCC than in clear cell RCC (unpublished data). In addition, Niu et al.( 25 ) have reported recently that the mRNA expression level of nephroblastoma overexpressed gene (NOV), which is a molecule similar to CTGF, is significantly higher in papillary RCC than in clear cell RCC.( 25 ) Thus, it is possible to speculate that VEGF expressed by carcinoma cells can not gain access to the endothelial cells in papillary RCC because of complex formation with CTGF and other molecules.
Tumors can not grow beyond a few millimeters in diameter without angiogenesis, because the center of the tumor mass is under hypoxic conditions. Therefore, the growth of solid tumors depends on angiogenesis. We have shown that hypoxia upregulates gene expression of Ets‐1 and VEGF in cultured cell lines derived from clear cell RCC, suggesting that hypoxia is a key regulator for these two molecules. Expression of VEGF correlates with MVD in clear cell RCC in the present and previous studies.( 10 ) These findings suggest that VEGF expression is important in angiogenesis in clear cell RCC. As VEGF increases Ets‐1 expression in endothelial cells,( 26 ) it is possible to speculate that upregulation of Ets‐1 in the RCC cells lines is due partly to VEGF. However, no direct correlation was observed between Ets‐1 and VEGF expression in clear cell RCC, because there were cases with high Ets‐1 and low VEGF expression. Furthermore, neutralizing anti‐VEGF antibody and/or recombinant human VEGF165 protein had no apparent effect on Ets‐1 expression in the RCC cell lines. These results suggest that VEGF is not involved in the upregulation of Ets‐1 in the cell lines, probably because they do not express VEGF receptors 1 and 2.( 27 ) Because hypoxia is known to induce Ets‐1 expression through the action of HIF‐1α,( 17 ) it is possible to speculate that HIF‐1α or other factors induced by hypoxia increased Ets‐1 expression in the cell lines used in this study.
Understanding the pathogenesis of angiogenesis in RCC is crucial for developing novel treatment strategies. The treatment of RCC with metastases is challenging for clinicians because RCC are refractory to chemotherapy and radiation therapy. Immunotherapy including interferon‐α and interleukin‐2 is the current treatment modality, with an efficacy rate 10–20%.( 28 ) Anti‐angiogenesis therapy by VEGF antibodies was evaluated recently for clinical use for RCC, but the results of the trial were not satisfactory.( 29 ) As multiple angiogenic signaling pathways converge on Ets‐1, Ets‐1 may be a logical molecular target for RCC.
In conclusion, our present study demonstrated that Ets‐1 is expressed in clear and chromophobe cell RCC, and in the endothelial cells of microvessels within the tumor masses of clear cell RCC. As Ets‐1 expression correlated with MVD in RCC, Ets‐1 may act as an angiogenic factor in RCC. Furthermore, hypoxia may accelerate angiogenesis of RCC through upregulation of Ets‐1 and VEGF. Ets‐1 was expressed predominantly in clear cell RCC, but negligible expression was observed in papillary RCC, suggesting that RCC have a diverse molecular background. The specific inhibition of Ets‐1 may therefore be a potentially effective therapeutic modality for the treatment of clear cell RCC through angiogenic inhibition.
Acknowledgments
This study was partly supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. [DOI] [PubMed] [Google Scholar]
- 2. Paradis V, Lagha NB, Zeimoura L et al. Expression of vascular endothelial growth factor in renal cell carcinomas. Virchows Arch 2000; 436: 351–6. [DOI] [PubMed] [Google Scholar]
- 3. Linehan WM, Zbar B. Focus on kidney cancer. Cancer Cell 2004; 6: 223–8. [DOI] [PubMed] [Google Scholar]
- 4. Valter MM, Hugel A, Huang HJ et al. Expression of the Ets‐1 transcription factor in human astrocytomas is associated with Fms‐like tyrosine kinase‐1 (Flt‐1)/vascular endothelial growth factor receptor‐1 synthesis and neoangiogenesis. Cancer Res 1999; 59: 5608–14. [PubMed] [Google Scholar]
- 5. Sato Y, Kanno S, Oda N et al. Properties of two VEGF receptors, Flt‐1 and KDR, in signal transduction. Ann NY Acad Sci 2000; 902: 201–5. [DOI] [PubMed] [Google Scholar]
- 6. Oda N, Abe M, Sato Y. ETS‐1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin β3. J Cell Physiol 1999; 178: 121–32. [DOI] [PubMed] [Google Scholar]
- 7. Fujimoto J, Aoki I, Toyoki H et al. Clinical implications of expression of ETS‐1 related to angiogenesis in uterine cervical cancers. Ann Oncol 2002; 13: 1598–604. [DOI] [PubMed] [Google Scholar]
- 8. Khatun S, Fujimoto J, Toyoki H et al. Clinical implications of expression of ETS‐1 in relation to angiogenesis in ovarian cancers. Cancer Sci 2003; 94: 769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukherjee T, Kumar A, Mathur M et al. Ets‐1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2003; 129: 430–6. [DOI] [PubMed] [Google Scholar]
- 10. Yagasaki H, Kawata N, Takimoto Y et al. Histopathological analysis of angiogenic factors in renal cell carcinoma. Int J Urol 2003; 10: 220–7. [DOI] [PubMed] [Google Scholar]
- 11. Kallakury BV, Karikehalli S, Haholu A et al. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res 2001; 7: 3113–19. [PubMed] [Google Scholar]
- 12. Sobin LH, Wittkind C. International Union Against Cancer (UICC): TNM Classification of Malignant Tumours. New York: Wiley‐Liss, 1997. [Google Scholar]
- 13. Noda K, Ishida S, Shinoda H et al. Hypoxia induces the expression of membrane‐type 1 matrix metalloproteinase in retinal glial cells. Invest Ophthalmol Vis Sci 2005; 46: 3817–24. [DOI] [PubMed] [Google Scholar]
- 14. Kodama T, Ikeda E, Okada A et al. ADAM12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin‐binding epidermal growth factor. Am J Pathol 2004; 165: 1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology 1995; 46: 27–30. [DOI] [PubMed] [Google Scholar]
- 16. Velickovic M, Delahunt B, Storkel S et al. VHL and FHIT locus loss of heterozygosity is common in all renal cancer morphotypes but differs in pattern and prognostic significance. Cancer Res 2001; 61: 4815–19. [PubMed] [Google Scholar]
- 17. Oikawa M, Abe M, Kurosawa H et al. Hypoxia induces transcription factor ETS‐1 via the activity of hypoxia‐inducible factor‐1. Biochem Biophys Res Commun 2001; 289: 39–43. [DOI] [PubMed] [Google Scholar]
- 18. Nakayama T, Ito M, Ohtsuru A et al. Expression of the Ets‐1 proto‐oncogene in human gastric carcinoma: Correlation with tumor invasion. Am J Pathol 1996; 149: 1931–9. [PMC free article] [PubMed] [Google Scholar]
- 19. Iwasaka C, Tanaka K, Abe M et al. Ets‐1 regulates angiogenesis by inducing the expression of urokinase‐type plasminogen activator and matrix metalloproteinase‐1 and the migration of vascular endothelial cells. J Cell Physiol 1996; 169: 522–31. [DOI] [PubMed] [Google Scholar]
- 20. Wechsel HW, Bichler KH, Feil G et al. Renal cell carcinoma: relevance of angiogenetic factors. Anticancer Res 1999; 19: 1537–40. [PubMed] [Google Scholar]
- 21. Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med 2005; 9: 267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagemann T, Gunawan B, Schulz M et al. mRNA expression of matrix metalloproteases and their inhibitors differs in subtypes of renal cell carcinomas. Eur J Cancer 2001; 37: 1839–46. [DOI] [PubMed] [Google Scholar]
- 23. Inoki I, Shiomi T, Hashimoto G et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF‐induced angiogenesis. FASEB J 2002; 16: 219–21. [DOI] [PubMed] [Google Scholar]
- 24. Gengrinovitch S, Greenberg SM, Cohen T et al. Platelet factor‐4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem 1995; 270: 15 059–65. [DOI] [PubMed] [Google Scholar]
- 25. Niu Z, Ito M, Awakura Y et al. The expression of NOV and WT1 in renal cell carcinoma: a quantitative reverse transcriptase‐polymerase chain reaction analysis. J Urol 2005; 174: 1460–2. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe D, Takagi H, Suzuma K et al. Transcription factor Ets‐1 mediates ischemia‐ and vascular endothelial growth factor‐dependent retinal neovascularization. Am J Pathol 2004; 164: 1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi A, Sasaki H, Kim SJ et al. Identification of receptor genes in renal cell carcinoma associated with angiogenesis by differential hybridization technique. Biochem Biophys Res Commun 1999; 257: 855–9. [DOI] [PubMed] [Google Scholar]
- 28. Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol 2000; 163: 408–17. [PubMed] [Google Scholar]
- 29. Yang JC, Haworth L, Sherry RM et al. A randomized trial of bevacizumab, an anti‐vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


