Abstract
Sulfate plays an important role in maintaining normal structure and function of tissues, and its content is decreased in certain cancers including lung carcinoma. In this study, we investigated tumor growth in a mouse model of hyposulfatemia (Nas1−/−) and compared it to wild‐type (Nas1+/+) mice. Lung epithelial tumor cells (TC‐1 cell line) were injected subcutaneously into male Nas1−/− and Nas1+/+ mice on a mixed 129Sv and C57BL/6 genetic background. Tumor sections were stained with anti‐glycosaminoglycan antibodies to assess the distribution of proteoglycans and Gomori’s trichrome to detect collagen. After 14 days, tumor weights were markedly increased (by ∼12‐fold) in Nas1−/− mice when compared with Nas1+/+ mice. Histological analyses of tumors revealed increased (by ≈2.4‐fold) vessel content, as well as markedly reduced collagen and immunoreactivity against glycosaminoglycan structural epitopes in the tumors from Nas1−/− mice. No significant differences were found for the growth of cultured TC‐1 cells supplemented with Nas1−/− or Nas1+/+ serum, as determined by 3H‐thymidine incorporation, implying that the cell culture conditions may not reflect the in vivo situation of enhanced tumor growth. This study has revealed increased tumor growth and an altered extracellular tumor matrix in hyposulfatemic Nas1−/− mice. These findings highlight the importance of blood sulfate levels as a possible modulator of tumor growth, and could lead to future cancer studies in humans with altered sulfate homeostasis. (Cancer Sci 2009; 00: 000–000)
Sulfate (SO4 2−) is an abundant anion in the human body and is essential for numerous cellular and metabolic processes.( 1 ) SO4 2− conjugation or sulfonation of glycosaminoglycans (GAGs) is important for maintaining the structure and function of tissues.( 2 ) Heparan sulfate proteoglycans (HSPGs) are a group of variably sulfonated GAGs found at the cell surface and extracellular matrix, which play a major role in regulating growth factor signaling in tumors.( 3 ) In particular, the sulfate content of HSPGs is a critical factor in modulating tumor growth and angiogenesis.( 4 ) Desulfonation of HSPGs by sulfatases promotes the release of growth factors that enhance tumor growth,( 5 ) and increased sulfatase activity is associated with a poor prognosis in patients with hepatocellular carcinoma, breast, and pancreatic cancers.( 6 , 7 , 8 ) In addition, the removal of highly sulfonated regions of HSPGs, using heparanase I, leads to accelerated tumor growth, neovascularization, and decreased apoptosis in a mouse model of lung carcinoma.( 3 ) Highly sulfonated HSPGs or heparins, as well as sulfonated polymers such as laminarin sulfate, act as inhibitors of heparanase activity and have beneficial outcomes in preventing tumor growth and angiogenesis in vivo.( 9 ) These studies highlight the importance of sulfate in modulating tumor growth and angiogenesis.
SO4 2− is obtained directly from the diet and from the oxidation of sulfur containing amino acids, methionine and cysteine.( 2 , 10 ) Importantly, a sufficient supply of sulfate and its universal sulfonate donor, 3′‐phosphoadenosine 5′‐phosphosulfate (PAPS), need to be maintained for sulfonation reactions to function effectively.( 11 ) Circulating sulfate levels are maintained by the NaS1 sulfate transporter, which is expressed in the kidney where it mediates the first step of sulfate reabsorption.( 12 , 13 ) We generated a NaS1 null (Nas1−/−) mouse which exhibits hyposulfatemia, hypersulfaturia, decreased hepatic sulfonation capacity, reduced intestinal sulfomucin content, and decreased circulating dehydroepiandrosterone sulfate (DHEA‐S) levels.( 14 , 15 , 16 , 17 ) This mouse model provides important insights into the physiological consequences of hyposulfatemia and the function of the NaS1 gene, which is yet to be linked with human disease. These studies are relevant to single nucleotide polymorphisms (R12X and N174S) in the human NaS1 gene,( 18 ) which lead to 100% and 60% loss of function, respectively.( 19 ) The aim of this study was to determine the growth of tumor cells injected subcutaneously into hyposulfatemic Nas1−/− mice and compare them to wild‐type (Nas1+/+) normosulfatemic mice. Our findings show increased tumor growth and vascularization, as well as reduced tumor core necrosis, collagen content, and immunoreactivity to heparan sulfate (HS), HS‐, and chondroitin 4‐sulfate‐ (C4S) epitopes in tumors from Nas1−/− mice when compared to Nas1+/+ mice.
Materials and Methods
Mice. We previously generated Nas1−/− mice in which the NaS1 gene was disrupted by targeted mutagenesis.( 14 ) Nas1−/− mice exhibit hyposulfatemia (0.22 mm, ∼75% decrease) and increased urinary sulfate excretion (Fractional Excretion Index sulfate = 1.02; ∼4‐fold increase). Studies were performed on male mice at 3 months of age with a mixed genetic background (129Sv and C57BL/6). Mice were fed a standard rodent chow (no. AIN93G; Glen Forrest Stockfeeders, Glen Forrest, Australia) and water ad libitum, and were used for approved experiments in accordance with the guidelines of the University of Queensland Animal Ethics Committee.
Tumor growth in vivo. In order to study tumor growth in vivo, this study used a model epithelium tumor cell line, TC‐1, which is derived from primary lung epithelial cells co‐transformed with human papillomavirus type 16 (HPV16) E6/E7 and c‐Ha‐Ras oncogenes.( 20 ) In vivo, TC‐1 cells injected subcutaneously in the mouse neck scruff form a single tumor mass that grows aggressively in synergic C57BL/6 mice.( 21 , 22 ) Tumor cells were cultured in DMEM supplemented with 5% fetal bovine serum. We injected 1 × 106 TC‐1 cells subcutaneously into the neck scruff of anesthetized Nas1−/− and Nas1+/+ mice. Tumors were excised from mice at 14 days after tumor cell injection, photographed and weighed, and then pieces of tumor were taken for histological and immunohistological analyses. Tumors were digitally measured (ScionImage; Scion Corp., Frederick, MD, USA) and tumor cross‐sectional areas calculated by the formula (length/2 × width/2) × π as previously described.( 23 )
Histopathological and immunohistochemical analyses. Tumors were dissected into approximately 50 volumes of 10% buffered formalin and fixed for 3 days prior to paraffin embedding. Embedded tissues were sectioned, stain with Giemsa, or by the Gomori’s trichrome method to detect collagen,( 24 ) and examined by light microscopy. The vessel density of 10 randomly selected high‐powered (magnification, ×200) tumor sections, excluding the necrotic cores, was scored blinded to genotype (n = 5 per group). The immunohistochemistry was performed using the mouse‐on‐mouse (MOM) immunodetection kit (Vector Laboratories, Burlingame, CA, USA). Antigen epitopes were unmasked by immersing the tissue sections in 0.01 M sodium citrate buffer (pH 6.0) in a pressurized high‐temperature (121°C) decloaking chamber (Applied Medical, Rancho Santa Margarita, CA, USA) for 4 min and rinsing with deionized water. The slides were then incubated with 3% v/v H2O2 to block endogenous enzyme activity, followed by two washes of 50 mm Tris‐HCl (pH 7.2) containing 0.15 M NaCl (TBS). The tissues were left either undigested or digested chondroitinase ABC (0.05 U/mL) or heparinase III (0.01 U/mL) in phosphate buffer (pH 7.2) at 37°C for 2 h. The tissue sections were incubated with the MOM blocking reagent at room temperature (RT) for 1 h then washed twice for 2 min with TBS. The sections were incubated with 5 μg/mL anti‐HS (Mab10E4) or HS stub (Mab3G10) antibodies (Seikagaku, Tokyo, Japan), or the anti‐C4S stub antibody (Mab2B6) diluted 1/500 (a gift from Prof. Bruce Caterson, Cardiff University, UK) at 4°C for 16 h prepared in MOM diluent. The sections were washed twice with TBS for 2 min before and after incubating with biotinylated antimouse Ig diluted 1/500 (GE, Piscataway, NJ, USA) at RT for 1 h followed by incubation with streptavidin–horseradish peroxide diluted 1/250 (GE) at RT for 30 min. The slides were washed four times with TBS before developing with Nova Red (Vector Laboratories) and counterstaining with hematoxylin.
TC‐1 cell growth in culture. We cultured TC‐1 cells in DMEM supplemented with 2% or 5% serum from Nas1−/− or Nas1+/+ mice and cell growth was determined by 3H‐thymidine incorporation. Briefly, 1 × 104 log‐phase TC‐1 cells were cultured in 96‐well flat bottom plates containing 2 or 5% mouse serum in 200 μL DMEM. Each sample was tested in triplicate. After 24 h of culture, 1 μCi of 3H‐thymidine was added per well and incubated for 48 h. Cells in individual wells were harvested on an automated FilterMate harvester (PerkinElmer, Waltham, MA, USA) and counts per min determined using a TopCount analyzer (PerkinElmer).
Statistical analyses. The statistical significance of differences between Nas1−/− and Nas1+/+ groups was assessed by an unpaired Student’s t‐test with P < 0.05 considered significant.
Results
Enhanced TC‐1 tumor growth and vascularization in Nas1−/− mice. To compare the growth of tumors, mice received a subcutaneous injection of TC‐1 cells and were then sacrificed 14 days after injection, with the tumors removed for analysis. Nas1−/− mice showed markedly larger tumors (0.25 ± 0.16 cm2, mean ± SD) when compared to Nas1+/+ mice (0.05 ± 0.01 cm2) (Fig. 1a,b). Tumor weights were increased by ∼12‐fold in Nas1−/− mice (147.4 ± 60.2 mg) when compared to Nas1+/+ mice (12.6 ± 1.5 mg) (Fig. 1c), demonstrating that tumor growth is enhanced in the hyposulfatemic NaS1 null mice. Our initial observations revealed macroscopically visible blood vessels on the surface of tumors from Nas1−/− mice (Fig. 1a) but not in Nas1+/+ mice. Blood vessel formation is essential for tumor growth and development, and increased vessel density is a hallmark of enhanced tumor growth ( 25 ). In order to ascertain whether vessel density was altered in Nas1−/− mice, we quantitated the number of vessels in tumor sections from Nas1−/− and Nas1+/+ mice (Fig. 1d,e). Tumor vessel density was significantly increased by ∼2.4‐fold in Nas1−/− mice (15.1 ± 3.2 vessels/mm2) when compared to Nas1+/+ mice (6.3 ± 1.6 vessels/mm2) (Fig. 1f), which is consistent with the increased tumor growth in Nas1−/− mice.
Figure 1.
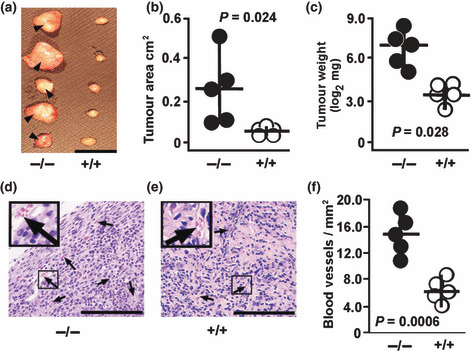
TC‐1 tumor growth in Nas1−/− and Nas1+/+ mice. (a) Representative photograph showing increased tumor size in Nas1−/− mice (n = 5) when compared to Nas1+/+ mice (n = 5). Blood vessels were observed on the tumors from Nas1−/− mice (arrowhead) but not from Nas1+/+ mice. Bar, 1 cm. The individual (n = 5 per group) (b) tumor cross‐sectional areas and (c) weights and medians, ranges, and P‐values from the Student’s t‐test are shown. (d,e) Representative photomicrographs showing an increased number of tumor vessels in Nas1−/− mice (n = 5) when compared to Nas1+/+ mice (n = 5). Bar, 200 μm. (f) Individual (n = 5 per group) vessel densities, medians, ranges, and P‐values from the Student’s t‐test are shown. Photographs and data are representative of three experiments.
Histological assessment of tumors. Since tumor growth and vessel density was increased in Nas1−/− mice, we aimed to determine any differences in the gross histological structure of the tumors from Nas1−/− and Nas1+/+ mice. We observed a necrotic core in all tumors from Nas1+/+ mice (Fig. 2b), but not in Nas1−/− mice (Fig. 2a), implying that the increased vessel content in tumors from Nas1−/− mice was providing sufficient nutrients for cell survival in the tumor core. In addition, we observed markedly reduced collagen staining in tumors from Nas1−/− mice (Fig. 2c) when compared to Nas1+/+ mice (Fig. 2d). Tumors from Nas1−/− mice showed collagen staining predominantly in the peripheral region (Fig. 2c), whereas collagen was detected throughout the tumors from Nas1+/+ mice (Fig. 2d), suggesting a reduced collagen content in the extracellular matrix of tumors from Nas1−/− mice.
Figure 2.
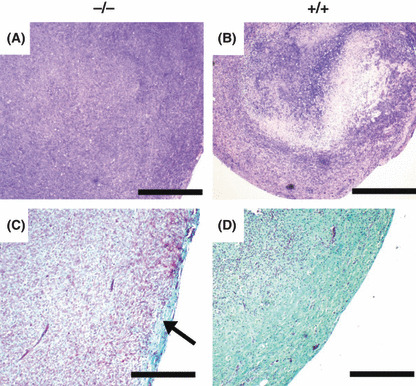
Histological assessment of tumors from Nas1−/− and Nas1+/+ mice. (a,b) Representative photomicrographs (n = 5 per group) showing necrosis in the core of tumors from Nas1+/+ mice, but not in Nas1−/− mice. Bar, 2 mm. (c,d) Representative photomicrographs (n = 5 per group) showing collagen staining primarily in the peripheral region (arrow) of tumors from Nas1−/− mice, whereas abundant staining was detected throughout the tumors from Nas1+/+ mice. Bar, 1 mm.
Immunohistochemical determination of GAG distribution in tumors. Since GAGs play an important role in modulating tumor growth and angiogenesis (reviewed in ref.( 26 )), we compared the abundance and distribution of GAGs in tumors from Nas1−/− and Nas1+/+ mice. Tumors from Nas1−/− mice showed weak immunostaining to native heparan sulfate (HS), or the HS‐stub (remaining after heparinase digestion) and chondroitin 4‐sulfate‐ (C4S) stub (remaining after chondroitinase digestion), which were predominantly distributed to the peripheral region of tumors in Nas1−/− mice, whereas they were immunolocalized throughout the tumors from Nas1+/+ mice (Fig. 3). These data demonstrate a markedly reduced GAG‐content in the extracellular matrix of the tumors in Nas1−/− mice.
Figure 3.
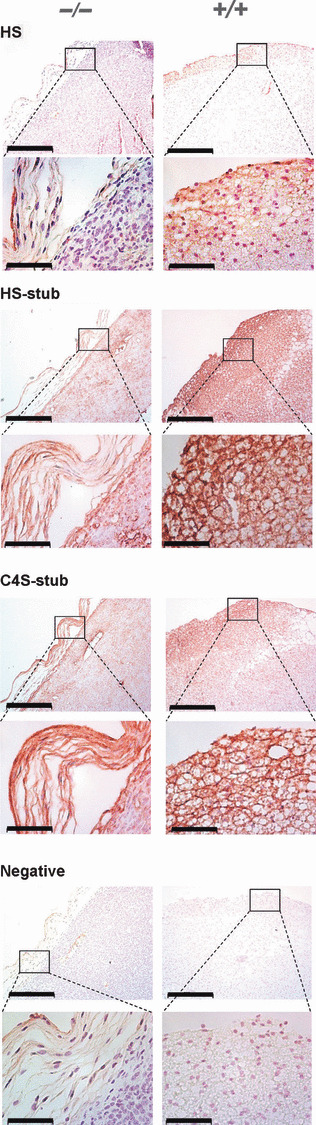
Distribution of native HS, and HS‐, and C4S‐stubs in tumors from Nas1−/− and Nas1+/+ mice. Representative photomicrographs showing that HS, HS‐stub (following heparintinase digestion) and C4S‐stub (following digestion with chondroitinase ABC) immunoreactivity was markedly reduced and localized to the periphery of tumors from Nas1−/− mice when compared to Nas1+/+ mice. Bar, 500 μm (top panels) and 100 μm (bottom panels).
Assessment of cultured TC‐1 cell growth supplemented with serum from Nas1−/− mice. Cultured TC‐1 cells were supplemented with serum obtained from Nas1−/− or Nas1+/+ mice to determine whether the hyposulfatemia in Nas1−/− mice (0.2 mm, ∼75% decrease compared to Nas1+/+ mice) leads to enhanced cell growth in cell culture. No significant differences were found for 3H‐thymidine incorporation in TC‐1 cells supplemented with either 2% or 5% serum from Nas1−/− or Nas1+/+ mice (Fig. 4), implying that serum derived from hyposulfatemic Nas1−/− mice did not lead to increased TC‐1 cell growth and that such cell culture conditions may not reflect the in vivo situation of enhanced tumor growth in Nas1−/− mice.
Figure 4.
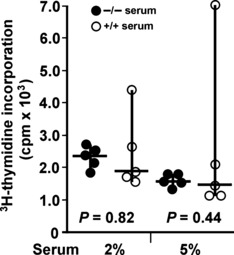
Growth assessment of cultured TC‐1 cells supplemented with 2 or 5% serum from Nas1−/− and Nas1+/+ mice. Individual cpm values (mean of triplicate cultures), median, range, and P‐values from the Student’s t‐test are shown.
Discussion
Nas1−/− mice show increased tumor growth and vascularization, as well as reduced tumor core necrosis, collagen content, and immunoreactivity to the native HS, and HS‐, and C4S‐ epitopes. These abnormal features may be a result of reduced sulfate availability in the tumor, secondary to hyposulfatemia that is characteristic of the Nas1−/− mice.( 14 ) However, we cannot completely exclude the possibility that the altered steroid and Insulin‐like growth factor 1 (IGF‐1) homeostasis in Nas1−/− mice( 14 , 16 ) may contribute to the tumor phenotype that we describe. The increased tumor growth in Nas1−/− mice is of high relevance to human cancers associated with altered sulfate homeostasis, including hepatic, gastric, colorectal, and lung carcinomas( 27 , 28 , 29 , 30 , 31 ) and suggests that hyposulfatemia plays a role in modulating tumor growth.
Interest in the oncogenetics of sulfate metabolism has expanded following the association of human sulfotransferase polymorphisms with numerous neoplasias, including lung, mouth, breast, gastric, colorectal, and bladder cancers.( 27 , 32 , 33 , 34 , 35 , 36 ) Several proteoglycan sulfotransferase‐deficient mice have been characterized,( 37 ) but tumor growth in these models has yet to be studied. A sufficient supply of sulfate is required for sulfotransferases to function effectively.( 11 ) This finding led us to investigate sulfonated proteoglycans in tumors from hyposulfatemic (∼0.2 mm serum sulfate) Nas1−/− mice and compare those to normosulfatemic (∼1.0 mm serum sulfate) Nas1+/+ mice. We found reduced immunoreactivity against HS, HS‐, and C4S‐stubs in the tumors from Nas1−/− mice, which is of particular relevance to tumor biology because the sulfonated proteoglycans play an important role in mediating tumor growth and angiogenesis.( 3 ) This is consistent with our data, showing increased tumor growth and vessel density in the Nas1−/− mice. Whilst decreases in the content of sulfonated proteoglycans have been well documented in human cancers,( 3 ) it is not clear whether the reduced sulfonated proteoglycan content in tumors from Nas1−/− mice are primary or secondary responses to enhanced tumor growth. Nonetheless, our murine model shows that hyposulfatemia may contribute to decreased sulfonated proteoglycan content in tumors with enhanced growth.
There is evidence that decreased collagen levels in the extracellular matrix (ECM) can decrease the mechanical stiffness of tumors, which leads to increased tumor growth.( 38 , 39 ) This finding led us to compare the collagen content of tumors in Nas1−/− and Nas1+/+ mice. Histological analyses revealed an abundant distribution of collagen throughout the tumors from Nas1+/+ mice, whereas tumors from Nas1−/− mice showed markedly reduced collagen staining, which was restricted to the periphery of the tumor. These findings, together with the decreased content of proteoglycans, demonstrate an altered tumor ECM in Nas1−/− mice that is more permissive of enhanced growth.
Although tumor growth was markedly enhanced in our hyposulfatemic mice, we were unable to show increased growth of cultured TC‐1 cells supplemented with serum from Nas1−/− mice. It is likely that the tumor microenvironment in Nas1−/− mice does not mimic the cell culture conditions used in our study. Indeed, numerous studies have shown that the host microenvironment plays an active role in mediating tumor growth, as well as cancer metastasis.( 40 ) There was no evidence of TC‐1 cell metastasis in the Nas1−/− mice, and this issue warrants further investigation. Taken together, our findings indicate the importance of the host microenvironment when studying tumor growth.
In summary, this study revealed enhanced tumor growth in hyposulfatemic Nas1−/− mice. This finding suggests that circulating sulfate may play an important role in the modulation of tumor growth. The significance of this study is relevant to humans with hyposulfatemia and prompts future assessment of cancer patients with altered sulfate homeostasis.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank Ms Lisa Kidd and Mr Paul Addison (University of Queensland, Brisbane, Australia) for assistance with mouse anesthesia and tumor histology, respectively. This work was supported by project grants awarded by the Queensland Cancer Fund, National Health and Medical Research Council of Australia, and the Lions Medical Research Foundation.
References
- 1. Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 2001; 81: 1499–534. [DOI] [PubMed] [Google Scholar]
- 2. Mulder GJ, Jakoby WB. Sulfation. In: Mulder GJ, ed. Conjugation Reactions in Drug Metabolism: An Integrated Approach: Substrates, Co‐substrates, Enzymes and Their Interactions In Vivo and In Vitro. London: Taylor and Francis, 1990; 107–61. [Google Scholar]
- 3. Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem 2005; 96: 897–905. [DOI] [PubMed] [Google Scholar]
- 4. Novak K. Signalling Cryptic clues about metastasis. Nature Rev Cancer 2002; 2: 1–2. [Google Scholar]
- 5. Liu D, Shriver Z, Venkataraman G, El Shabrawi Y, Sasisekharan R. Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. Proc Natl Acad Sci U S A 2002; 99: 568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai JP, Sandhu DS, Yu C et al. Sulfatase 2 up‐regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology 2008; 47: 1211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyoshi Y, Ando A, Hasegawa S et al. High expression of steroid sulfatase mRNA predicts poor prognosis in patients with estrogen receptor‐positive breast cancer. Clin Cancer Res 2003; 9: 2288–93. [PubMed] [Google Scholar]
- 8. Nawroth R, Van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PloS One 2007; 2: e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miao HQ, Elkin M, Aingorn E, Ishai‐Michael R, Stein CA, Vlodavsky I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int J Cancer 1999; 83: 424–31. [DOI] [PubMed] [Google Scholar]
- 10. Florin THJ, Neale G, Goretski S, Cummings JH. The sulfate content of foods and beverages. J Food Compos Anal 1993; 6: 140–51. [Google Scholar]
- 11. Klassen CD, Boles J. The importance of 3′‐phosphoadenosine 5′‐phosphosulfate (PAPS) in the regulation of sulfation. FASEB J 1997; 11: 404–18. [DOI] [PubMed] [Google Scholar]
- 12. Beck L, Markovich D. The Mouse Na+‐Sulfate Cotransporter Gene Nas1: cloning, tissue distribution, gene structure, chromosomal assignment, and transcriptional regulation by vitamin D. J Biol Chem 2000; 275: 11880–90. [DOI] [PubMed] [Google Scholar]
- 13. Lee A, Beck L, Markovich D. The human renal sodium sulfate cotransporter (SLC13A1; hNaSi‐1) cDNA and gene: organisation, chromosomal localization, and functional characterization. Genomics 2000; 70: 354–63. [DOI] [PubMed] [Google Scholar]
- 14. Dawson PA, Beck L, Markovich D. Hyposulfatemia, growth retardation, reduced fertility and seizures in mice lacking a functional NaSi‐1 gene. Proc Natl Acad Sci U S A 2003; 100: 13704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dawson PA, Gardiner B, Grimmond S, Markovich D. Transcriptional profile reveals altered hepatic lipid and cholesterol metabolism in hyposulfatemic NaS1 null mice. Physiol Genomics 2006; 26: 116–24. [DOI] [PubMed] [Google Scholar]
- 16. Dawson PA, Gardiner B, Lee S, Grimmond S, Markovich D. Kidney transcriptome reveals altered steroid homeostasis in NaS1 sulfate transporter null mice. J Steroid Biochem Mol Biol 2008; 112: 55–62. [DOI] [PubMed] [Google Scholar]
- 17. Dawson PA, Huxley S, Gardiner B et al. Impaired intestinal function in the hyposulphataemic NaS1 null mouse. Gut 2009; 58: 910–9. [DOI] [PubMed] [Google Scholar]
- 18. Dawson PA, Markovich D. Genetic polymorphisms of human sulfate transporters. Curr Pharmacogenomics 2007; 5: 262–74. [Google Scholar]
- 19. Lee S, Dawson PA, Hewavitharana AK, Shaw PN, Markovich D. Disruption of NaS1 sulfate transport function in mice leads to enhanced acetaminophen‐induced hepatotoxicity. Hepatology 2006; 43: 1241–7. [DOI] [PubMed] [Google Scholar]
- 20. Lin KY, Guarnieri FG, Staveley‐O’Carroll KF et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56: 21–6. [PubMed] [Google Scholar]
- 21. Stewart TJ, Smyth MJ, Fernando GJ, Frazer IH, Leggatt GR. Inhibition of early tumor growth requires J alpha 18‐positive (natural killer T) cells. Cancer Res 2003; 63: 3058–60. [PubMed] [Google Scholar]
- 22. Stewart TJ, Fernando GJ, Frazer IH, Leggatt GR. Tumour susceptibility to innate and adaptive immunotherapy changes during tumour maturation. Immunol Cell Biol 2004; 82: 455–61. [DOI] [PubMed] [Google Scholar]
- 23. Chen J, Power KA, Mann J, Cheng A, Thompson LU. Flaxseed alone or in combination with tamoxifen inhibits MCF‐7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Exp Biol Med (Maywood) 2007; 232: 1071–80. [DOI] [PubMed] [Google Scholar]
- 24. Gomori G. A rapid one‐step trichrome stain. Am J Clin Pathol 1950; 20: 661–4. [DOI] [PubMed] [Google Scholar]
- 25. Hasan J, Byers R, Jayson GC. Intra‐tumoural microvessel density in human solid tumours. Br J Cancer 2002; 86: 1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blackhall FH, Merry CL, Davies EJ, Jayson GC. Heparan sulfate proteoglycans and cancer. Br J Cancer 2001; 85: 1094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang G, Miao X, Zhou Y, Tan W, Lin D. A functional polymorphism in the SULT1A1 gene (G638A) is associated with risk of lung cancer in relation to tobacco smoking. Carcinogenesis 2004; 25: 773–8. [DOI] [PubMed] [Google Scholar]
- 28. Lv H, Yu G, Sun L, Zhang Z, Zhao X, Chai W. Elevate level of glycosaminoglycans and altered sulfation pattern of chondroitin sulfate are associated with differentiation status and histological type of human primary hepatic carcinoma. Oncology 2007; 72: 347–56. [DOI] [PubMed] [Google Scholar]
- 29. Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol 2003; 35: 376–90. [DOI] [PubMed] [Google Scholar]
- 30. Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. Distribution and changes of glycosaminoglycans in neoplasias of rectum. Anticancer Res 1995; 15: 2107–12. [PubMed] [Google Scholar]
- 31. Yamori T, Kimura H, Stewart K, Ota DM, Cleary KR, Irimura T. Differential production of high molecular weight sulfated glycoproteins in normal colonic mucosa, primary colon carcinoma, and metastases. Cancer Res 1987; 47: 2741–7. [PubMed] [Google Scholar]
- 32. Boccia S, Sayed‐Tabatabaei FA, Persiani R et al. Polymorphisms in metabolic genes, their combination and interaction with tobacco smoke and alcohol consumption and risk of gastric cancer: a case‐control study in an Italian population. BMC Cancer 2007; 7: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung YT, Hsieh LL, Chen IH et al. Sulfotransferase 1A1 haplotypes associated with oral squamous cell carcinoma susceptibility in male Taiwanese. Carcinogenesis 2009; 30: 286–94. [DOI] [PubMed] [Google Scholar]
- 34. Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, Harper PA. Red meat intake, doneness, polymorphisms in genes that encode carcinogen‐metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2008; 17: 3098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalhoff K, Buus Jensen K, Enghusen Poulsen H. Cancer and molecular biomarkers of phase 2. Methods Enzymol 2005; 400: 618–27. [DOI] [PubMed] [Google Scholar]
- 36. Wang YH, Juang GD, Hwang TI, Shen CH, Shao KY, Chiou HY. Genetic polymorphism of sulfotransferase 1A1, cigarette smoking, hazardous chemical exposure and urothelial cancer risk in a Taiwanese population. Int J Urol 2008; 15: 1029–34. [DOI] [PubMed] [Google Scholar]
- 37. Habuchi H, Habuchi O, Kimata K. Sulfation pattern in glycosaminoglycan: does it have a code? Glycoconj J 2004; 21: 47–52. [DOI] [PubMed] [Google Scholar]
- 38. Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest 2003; 111: 487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9: 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev 2008; 18: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


