Abstract
A tumor‐specific microenvironment is characterized by hypoxia, in which oxygen tension is considerably lower than in normal tissues. The hypoxic status of various solid tumors has been attributed as an indicator of adverse prognosis due to tumor progression toward a more malignant phenotype with increased metastatic potential and resistance to treatment. Various exogenous and endogenous markers for hypoxia are currently available and studied in relation to each other, tumor architecture, and tumor microenvironment. Over the last few decades, various methods have been suggested to assess the level of oxygenation in solid tumors. Among them, nitroimidazole compounds have provided promising information on tumor hypoxia. To quantify the extent of hypoxia requires that nitroimidazole binding be primarily dependent on oxygen concentration as well as nitroreductase levels in the tumor cells. Furthermore, recent progress in molecular biology has highlighted a transcription factor, hypoxia‐inducible factor (HIF)‐1, whose activity is induced by hypoxia. HIF‐1 plays a central role in malignant progression by inducing the expression of various genes, whose functions are strongly associated with malignant alteration of the entire tumor. The cellular changes induced by HIF‐1 are extremely important therapeutic targets of cancer therapy, particularly in the therapy against refractory cancers. In this review, we will discuss the significance of pimonidazole and HIF‐1 as exogenous and endogenous hypoxia markers, respectively, as well as their evaluation and imaging of tumor hypoxia. (Cancer Sci 2009)
Most solid tumors contain a tumor‐specific microenvironment that is completely different from that inside normal tissues. The microenvironment of a solid tumor is characterized by low pO2 and low pH, which are well below physiological levels.( 1 , 2 ) This is due to the generation of areas within the solid tumors that do not receive adequate nutrients and oxygen from blood vessels because of the uncontrolled growth of tumor cells and disproportionate and incomplete tumor blood vascular structures formed during angiogenesis. The hypoxic status of various solid tumors has been attributed mainly to a poor prognosis due to tumor progression toward a more malignant phenotype, with increased metastatic potential and treatment resistance.( 1 , 2 , 3 , 4 )
Due to certain physical factors within these hypoxic areas, hypoxic tumor cells are resistant to cancer therapy.( 1 , 2 ) As transport of anticancer agents via blood flow to hypoxic tumor cells, which are located at some distance from blood vessels, is inefficient, there is only a small chance that an anticancer agent reaches hypoxic tumor cells at an effective concentration. In addition, many anticancer agents target dividing cells and, thus, are ineffective in growth‐arrested or slowly growing hypoxic tumor cells. Furthermore, because oxygen molecules enhance the cytocidal effects of radiation and certain types of anticancer agents, their therapeutic effects are reduced under hypoxic conditions. Therefore, there are cases in which hypoxic tumor cells survive after radiotherapy or chemotherapy even though the surrounding well‐oxygenated and proliferating tumor cells die, suggesting that they are the cause of poor treatment outcomes and recurrence of cancers.
Hypoxia‐inducible factor‐1
Hypoxia‐inducible factor (HIF)‐1 is a heterodimer consisting of HIF‐1α and HIF‐1β (Fig. 1).( 5 ) The β subunit (HIF‐1β), also known as the aryl hydrocarbon receptor nuclear translocator (Arnt1), is a constitutively expressed protein. The α subunit (HIF‐1α) is regulated at the translational level and strictly controlled by post‐translational modification. HIF‐1α translocates to the nucleus, forms a heterodimer with HIF‐1β through protein–protein interactions via their Per‐Arnt‐Sim (PAS) domains, and binds to hypoxia‐responsive elements of the target genes. Thus, the HIF‐1 activity depends on the degree of HIF‐1α expression. The control of HIF‐1α via post‐translational modification mainly occurs via oxygen‐dependent proline hydroxylases (PHD). The oxygen‐dependent degradation (ODD) domain is responsible for regulation of the oxygen‐dependent degradation of the HIF‐1α protein, which stabilizes in a hypoxic environment and degrades immediately under normal oxygen conditions (Fig. 1). The details of this control mechanism were clarified at the molecular level by cloning three human proline hydroxylase genes.( 6 ) These genes encode PHD1, PHD2, and PHD3, which have closely related catalytic domains and belong to the superfamily of 2‐oxoglutarate‐dependent oxygenases. The PHD contain Fe(II) in their catalytic center, which is oxidized to Fe(III) via interaction of molecular oxygen during the hydroxylation reaction, and should be regenerated prior to another round of catalysis.( 7 ) Therefore, PHD are considered as oxygen sensors, and HIF‐1 and its target‐gene products are endogenous hypoxia makers,( 8 ) because HIF‐1 transcription activity is eventually regulated by the oxygen sensors.
Figure 1.
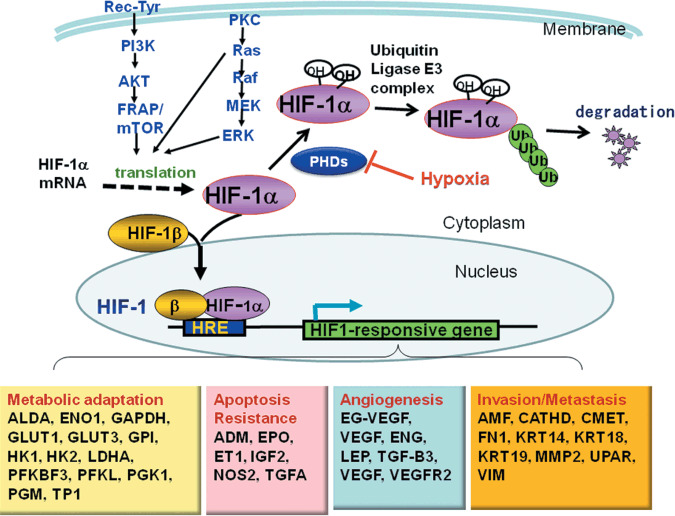
Regulation of hypoxia‐inducible factor (HIF)‐1. In the presence of oxygen (normoxia), prolyl hydroxylase (PHD) hydroxylates proline residues on HIF‐1α, allowing HIF‐1α to interact with a ubiquitin–protein ligase complex (VHL, CLU2, and Elongin‐B and Elongin‐C) through VHL. Ubiquitination of HIF‐1α makes it a target for degradation by the 26S proteasome. Growth signals through receptor tyrosine kinases (Rec‐Tyr) and Ras activate the PI3K–Akt pathway and the Ras–Raf–MAP kinase pathway, respectively, increasing the translation of HIF‐1α. When oxygen supply is not enough to activate PHD or when HIF‐1α expression exceeds the capacity of ubiquitin–proteasome degradation, HIF‐1α binds to ubiquitously expressing HIF‐1β to form a heterodimer. The heterodimer then translocates to the nucleus and binds to hypoxia‐responsive elements in the promoter and enhancer region of target genes, inducing the expression of various HIF1‐responsive genes. HRE, hypoxia‐responsive element; Ub, ubiquitin.
Hypoxia‐inducible factor‐1 activity and tumor hypoxia
Cells in hypoxic areas contribute to malignant alteration of tumors and HIF‐1 contributes to this. Compared with the actively growing tumor cells, which are exposed to an aerobic environment, tumor cells in hypoxic regions are ‘impaired tumor cells’ and are not normally considered as serious targets for cancer therapy. However, recent research has shown that these ‘impaired tumor cells’ increase the malignancy of the entire tumor.( 1 , 2 , 3 , 4 , 5 ) Although these hypoxic tumor cells are in a ‘moribund state’, they try to adapt to their poor environment. HIF‐1 supports their adaptation by inducing the expression of genes that are related to glucose metabolism, glucose transport, and angiogenic and growth factors, and helps to improve the nutritional environment (Fig. 1). HIF‐1 helps prevent apoptosis and death by inducing the expression of genes that induce mutations (Fig. 2). At the same time, it induces the expression of genes that are involved in metastasis and invasion (Fig. 2). These chain‐of‐survival actions are linked to malignant alteration of the entire tumor. Therefore, HIF‐1 is an excellent marker for tumor malignancy as well as hypoxia.
Figure 2.
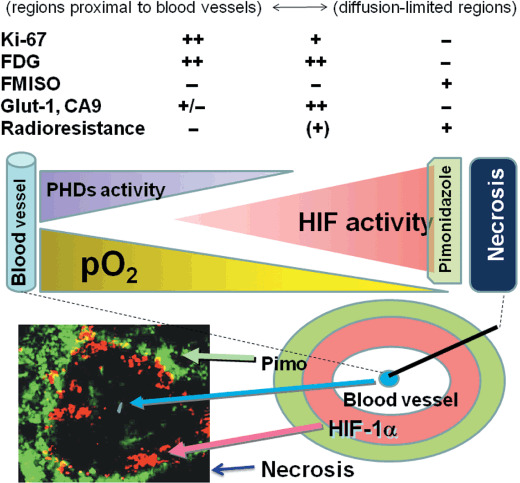
Tumor microenvironment. Tumor hypoxia arises in regions with impaired oxygen delivery. The regions proximal to blood vessels are Ki‐67‐, FDG‐, and glucose transporter (Glut)‐1‐positive but radiosensitive, whereas the diffusion‐limited regions are Ki‐67‐, FDG‐, and Glut‐1‐negative and radioresistant. In hypoxic regions, HIF‐1‐active regions (red/pink) are located closer to the blood vessels than pimonidazole (Pimo)‐positive regions (light green). Pimo‐positive regions are located next to necrotic regions (dark blue) and barely express HIF‐1α; they possess little HIF‐1 activity. CA9, carbonic anhydrase 9; FMISO, fluoromisonidazole.
Hypoxia‐inducible factor‐1 activity and genetic alterations in tumor cells
Many genetic alterations that inactivate tumor suppressors or activate oncoproteins also increase the HIF‐1 activity in tumor cells through a variety of molecular mechanisms that are independent of hypoxia.( 5 ) In tumor cells that have sustained the loss‐of‐function of a tumor suppressor (such as PTEN) or gain‐of‐function of an oncoprotein (such as HER2, IGF1R, or PI3K), increased signal transduction through the phosphatidylinositol‐3‐kinase–AKT pathway leads to increased mTOR activity, which in turn results in increased synthesis of the HIF‐1α protein and increased HIF‐1 activity (Fig. 2). Loss‐of‐function of the von Hippel‐Lindau tumor suppressor interrupts the recruitment of a ubiquitin ligase complex that targets HIF‐1α for ubiquitination and proteasomal degradation, resulting in constitutive activation of HIF‐1. The binding of p53 inhibits HIF‐1‐dependent transactivation and facilitates Mdm2‐dependent degradation of HIF‐1α. Thus loss‐of‐function of p53 increases HIF‐1 activity.( 9 ) Independent of any specific mechanism, increased HIF‐1 activity is associated with increased patient mortality in many different tumors. Therefore, although HIF‐1 and its target‐gene products are excellent markers for tumor malignancy, they are not always accurate markers for hypoxia.
Nitroimidazole compounds and HIF‐1 as hypoxia markers
Its beneficial impact on cancer management has motivated efforts to exploit hypoxia imaging for elucidation of the role of hypoxia in solid tumors. Consequently, over the last decade, various methods have been suggested to assess the level of oxygenation in solid tumors, including invasive measurement using pO2 electrodes and fiber‐optic probes,( 10 , 11 ) immunohistochemical detection of exogenously administered drugs that label hypoxic tumor cells,( 12 ) and of proteins overexpressed in response to hypoxia as endogenous markers.( 8 ) More recently, non‐invasive techniques for imaging tissue function have been established to accelerate the development of new applications.( 13 )
Currently, pimonidazole (Pimo) is considered to be the ‘standard’ exogenous hypoxic marker. It is commercially available and injectable 2‐nitroimidazole derivative. The binding of exogenous hypoxia markers such as Pimo to cellular macromolecules has been shown to increase dramatically below an oxygen concentration of 10 mmHg and is considered to indicate chronic hypoxia.( 14 ) Recently, it was reported that the intratumor regions in which endogenous hypoxia marker, HIF‐1α is expressed (HIF‐1‐active regions) hardly overlapped with the Pimo‐positive regions (Fig. 2); HIF‐1‐active regions were more closely distributed to blood vessels than Pimo‐positive regions.( 15 ) Janssen et al. extensively studied surgical specimens from patients and reported that although typical Pimo‐positive regions were observed at a distance from blood vessels with peaks around 80 µm, the HIF‐1‐positive regions were more variable without clear peaks and there was no correlation between the percentage of positive tumor tissue for both markers; although the median values of the positive area were similar for both (5.8 vs 5.6%), the median percentage of the regions positive with the both markers was below 5% (range 0.2–2.3%).( 16 ) These reports indicate that Pimo is a specific marker for severe hypoxia and hardly crosses HIF‐1‐active regions.
Correlation between hypoxia markers and other markers
However, there are many studies on the correlation of the exogenous 2‐nitroimidazole hypoxia markers, such as Pimo, EF5, and fluoromisonidazole (FMISO), with endogenous hypoxia‐related markers, such as carbonic anhydrase 9 (CA9) and glucose transporter‐1, that are HIF‐1 target‐gene products. A statistically significant correlation (P < 0.05) was obtained between the hypoxic volumes identified by FMISO‐PET and the volumes detected with Pimo and CA9 staining in rat rhabdomyosarcomas.( 17 ) More recently, a novel tumor xenograft model was established by transplanting the hypoxia‐inducible HT29–9 HE‐TKeGFP tumor into nude mice to study tumor hypoxia imaging. They demonstrated that intratumoral distributions of [124I]FIAU and [18F]FMISO were similar, and that hypoxia‐responsive element‐controlled enhanced GFP, Pimo, EF5, and CA9 colocalized in the same areas but not in well‐perfused regions by means of either optical or nuclear imaging techniques.( 18 )
Although the reasons for the controversial reports described above are still unclear, one possible explanation is that the biological status of tumors, which includes the levels of HIF‐1α expression, ODD regulation, and reductase activity, differs among cell types and the tissues they originated from.
The hypoxic subregions that express HIF‐1a and HIF‐1 target genes are overlapped with proliferation markers such as BrdU and Ki‐67, whereas Pimo‐positive cells are hardly stained with them.( 19 ) Therefore, FDG‐PET, which effectively images metabolically active cells including well‐proliferating cancer cells, is able to image HIF‐1‐active cells but poorly images Pimo‐positive quiescent cells (chronic hypoxia), which are located adjacent to the necrotic regions.( 20 ) However, it is well known that local fluctuations in blood perfusion and oxygen supply result in a transient hypoxia (acute hypoxia),( 21 ) which may be long enough for nitroimidazole compounds to bind to macromolecules in hypoxic tumor cells. Because the binding of nitroimidazole compounds is an irreversible reaction and acute hypoxia can be produced in any place including the regions close to blood vessels,( 21 ) it is no wonder that cells stained with both mitotic markers and Pimo exist in tumors.
Nitroreductase for bioreductive activation
In the development of imaging probes for tumor hypoxia, nitroimidazoles have received particular attention( 22 , 23 ) because of their unique behavior in hypoxic environments arising from their high electron affinity. Several 2‐nitroimidazole derivatives have been applied to the imaging of the hypoxic region in both solid tumor tissues and in myocardial and brain ischemia.( 24 ) 2‐Nitroimidazole (azomycin) was originally found to be an antibiotic against anaerobic bacteria and protozoa in 1953.( 25 ) Its selective toxicity was determined by biological reduction to the reactive species in the absence of oxygen.( 26 , 27 ) Over the intervening 40 years, electron‐deficient nitroaromatic compounds have been investigated for use in cancer treatment as chemical modifiers that increase the sensitivity of hypoxic tissue to radiation or chemotherapeutic agents,( 28 , 29 ) and as bioreductive prodrugs or hypoxia selective cytotoxins( 30 , 31 , 32 ) (Fig. 3).
Figure 3.
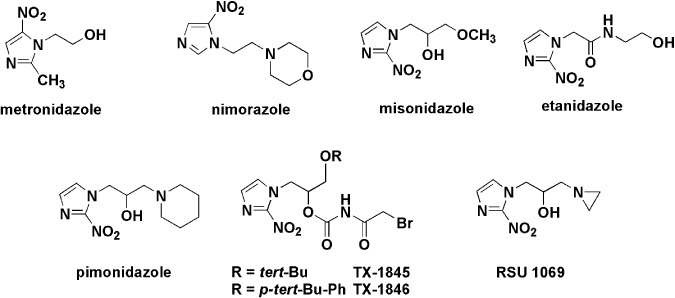
Representative hypoxic cell radiosensitizers and bioreductive prodrugs containing a nitroheterocyclic group.
The bioreductive activation of 2‐nitroimidazoles has also been adapted for the labeling procedure of hypoxic cells in vivo.(
33
) The specificity of nitroimidazole imaging will depend on the oxygen concentration at which bioreductive trapping occurs and the enzyme activity of bioreductive activation. Nitroaromatics are selectively reduced by nitroreductase enzymes under hypoxic conditions to form reactive products that can bind to cellular nucleophiles irreversibly, as shown in Figure 4.(
34
,
35
) In mammals, different nitroreductase enzymes associated with the cytoplasm, mitochondria, and microsomes are capable of the reductive metabolism of nitroheterocycles.(
36
) These enzymes include one‐electron reductases such as a NADPH‐cytochrome P450 reductase, cytochrome b5 reductase, xanthine oxidase, and aldehyde dehydrogenase, and two‐electron reductases such as DT‐diaphorase. It is likely that different specific enzymes, which are able to reduce substrates in two separate one‐electron reaction steps, will be responsible for reductively activating nitroaromatics under hypoxic conditions. Although a specific enzyme for the reductive activation in mammalian cells has not been completely identified, a NADPH‐cytochrome P450 reductase has been shown to be involved in the reductive activation and hypoxic accumulation of various nitroaromatics.(
37
,
38
) In the activation reactions, the addition of the first electron to form a nitro radical anion, 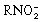 , is reversible in the presence of molecular oxygen, and this step is believed to be the basis of the oxygen dependence of bioreductive activation and binding of nitroaromatics. In contrast to one‐electron reductases, two‐electron reductases such as DT‐diaphorase are able to reduce nitroaromatics in two‐electron steps, which result in the bypassing of the first oxygen‐dependent reduction and thus reduce independently of the oxygenation status of the cell.(
39
) Therefore, DT‐diaphorase does not generally seem to account for the majority of hypoxia‐induced binding of nitroimidazoles.(
40
) In fact, differentiation‐associated and oxygen‐independent staining with anti‐Pimo antibodies was reported in some clinical cancers,(
40
,
41
,
42
) indicating not only that some oxygen‐insensitive reductases might be responsible for the process of exogenous marker reaction but also that other hypoxia‐independent events may occur in exogenous hypoxia markers.
, is reversible in the presence of molecular oxygen, and this step is believed to be the basis of the oxygen dependence of bioreductive activation and binding of nitroaromatics. In contrast to one‐electron reductases, two‐electron reductases such as DT‐diaphorase are able to reduce nitroaromatics in two‐electron steps, which result in the bypassing of the first oxygen‐dependent reduction and thus reduce independently of the oxygenation status of the cell.(
39
) Therefore, DT‐diaphorase does not generally seem to account for the majority of hypoxia‐induced binding of nitroimidazoles.(
40
) In fact, differentiation‐associated and oxygen‐independent staining with anti‐Pimo antibodies was reported in some clinical cancers,(
40
,
41
,
42
) indicating not only that some oxygen‐insensitive reductases might be responsible for the process of exogenous marker reaction but also that other hypoxia‐independent events may occur in exogenous hypoxia markers.
Figure 4.
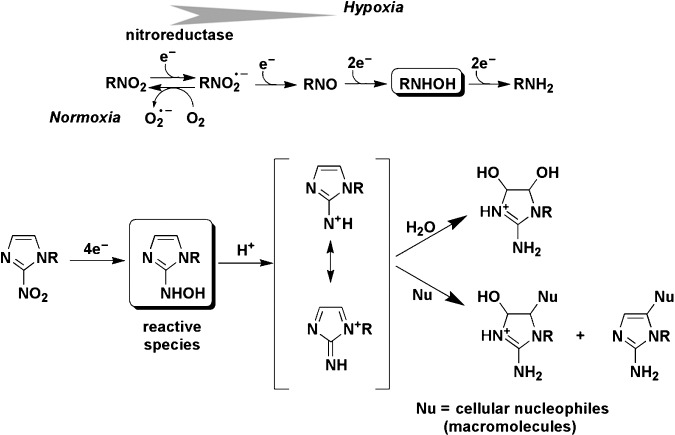
Oxygen‐dependent bioreductive metabolism of nitroimidazoles in cells and proposed mechanism for selective covalent binding reaction of hydroxylamine intermediates with cellular nucleophiles under hypoxic conditions.
Bioreductive activation of nitroaromatics
In aerobic conditions, the nitroradical anion is back‐oxidized to the parent nitro compound, resulting in a “futile” cycle without generation of an active intermediate.(
43
) As shown in Figure 4, the “futile” metabolism to afford 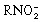 reflects competition between natural radical‐decay pathways (rightward) and a one‐electron transfer reaction from intracellular oxygen to yield superoxide ion,
reflects competition between natural radical‐decay pathways (rightward) and a one‐electron transfer reaction from intracellular oxygen to yield superoxide ion, 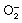 (leftward). The unique biological properties of nitroaryl compounds indicate the ease of nitroreduction, which is estimated by reduction potential as an appropriate index.(
44
,
45
) The most intensively investigated nitroaromatic compounds contain 2‐nitroimidazole, which has a higher reduction potential (
(leftward). The unique biological properties of nitroaryl compounds indicate the ease of nitroreduction, which is estimated by reduction potential as an appropriate index.(
44
,
45
) The most intensively investigated nitroaromatic compounds contain 2‐nitroimidazole, which has a higher reduction potential (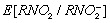 vs normal hydrogen electrode in water at pH 7: –0.39 V) than those of 4‐nitroimidazole (–0.56 V) and 5‐nitroimidazole (–0.47 V). The rate of regeneration of the parent nitro compound is dependent on the intracellular oxygen concentration because the reduction potential of oxygen (approximately –0.15 V) is significantly higher than that of all nitroaryl compounds. If the oxygen concentration is low, subsequent natural radical‐decay steps provide a reactive intermediate such as a hydroxylamine binding to cellular macromolecules, such as proteins or DNA, and nitroaromatic compounds are then retained in the cells. These processes are essential in differentiating normoxic from hypoxic tissue. Thus, it has been suggested that the selective labeling reaction of hypoxic cells by nitroimidazole at non‐toxic doses could be used to detect tumor hypoxia.(
23
)
vs normal hydrogen electrode in water at pH 7: –0.39 V) than those of 4‐nitroimidazole (–0.56 V) and 5‐nitroimidazole (–0.47 V). The rate of regeneration of the parent nitro compound is dependent on the intracellular oxygen concentration because the reduction potential of oxygen (approximately –0.15 V) is significantly higher than that of all nitroaryl compounds. If the oxygen concentration is low, subsequent natural radical‐decay steps provide a reactive intermediate such as a hydroxylamine binding to cellular macromolecules, such as proteins or DNA, and nitroaromatic compounds are then retained in the cells. These processes are essential in differentiating normoxic from hypoxic tissue. Thus, it has been suggested that the selective labeling reaction of hypoxic cells by nitroimidazole at non‐toxic doses could be used to detect tumor hypoxia.(
23
)
Molecular structures and characteristics of nitroaryl hypoxia markers
Many studies have been conducted on the radiosensitizer misonidazole (MISO) and its analogs as hypoxia markers. In studies of the correlation between concentrations of tritiated MISO ([3H]MISO) and oxygen pressures using EMT6/Ro multicell spheroids, accumulation of MISO near the central necrosis was observed where oxygen tensions lower than 10 mmHg were measured.( 46 ) Because bioreduction and subsequent binding of nitroimidazole to cellular macromolecules are inhibited as a function of increasing oxygen concentration but require intact nitroreductase enzymes, the markers can only detect viable hypoxic cells, except in areas of necrosis. The K m of binding inhibition (oxygen concentration for half‐maximal binding) for the tumors varied with the kind of tumor (1000–6000 p.p.m., 0.1–0.6% O2)( 22 , 47 ) whereas similar maximal labeling patterns of binding are obtained at less than 5 p.p.m. (0.0005%) oxygen in a variety of tumors. The most commonly used nitroimidazole hypoxic markers are listed in Table 1. In the structure of nitroimidazole hypoxia markers, generally the nitoroaryl moiety is essential and works as a bioreductive alkylating unit to selectively bind hypoxic tissue. However, the side chains control pharmacokinetics and toxicity (i.e. drug distribution), accumulation, and duration of retention in the target hypoxic site, and rapidity of clearance from normoxic cells. Pharmacokinetics and accumulation rates depend on the presence or absence of dissociable groups and lipophilicity, which is predicted by n‐octanol/water partition coefficient (P or log P).( 48 ) For example, the tumor uptake of Pimo and EF‐5 is expected to improve because of their considerably higher P‐values than the criterion values for less‐toxic radiosensitizers (0.026–0.4).( 49 ) Moreover, because Pimo is weakly basic (pKa = 8.9), the intracellular incorporation was affected by the extracellular pH.( 50 ) The extent of Pimo binding exceeds that for hexafluoromisonidazole (CCI‐103F) by a factor ranging from 1.0 to 1.65, and the binding of Pimo is greater at all pH values.( 51 ) Sustained oral ingestion of Pimo revealed a larger hypoxic fraction than a single injection of an alternative hypoxia marker, CCI‐103F.( 52 , 53 )
Table 1.
Structures, partition coefficients, and detection methods of the most common 2‐nitroimidazoles
| Structure | Partition coefficient (n‐octanol/water) | Imaging method | |
|---|---|---|---|
| Invasive | Non‐invasive | ||
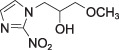 Misonidazole (MISO)
Misonidazole (MISO) |
log P = –0.39 P = 0.43 | Autradiography ([3H]MISO or [14C]MISO) | |
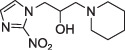 Pimonidazole
Pimonidazole |
P = 8.5 | IHC ELISA Flow cytometry | |
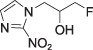 Fluoromisonidazole (FMISO)
Fluoromisonidazole (FMISO) |
log P = 0.40 P = 2.6 | IHC ELISA Flow cytometry | PET [18F] |
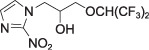 Hexafluoromisonidazole
(CCI‐103F)
Hexafluoromisonidazole
(CCI‐103F) |
P = 20 | IHC ELISA Flow cytometry | MRI [19F] |
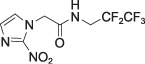 EF5
EF5 |
log P = 0.6 P = 4.0–5.7 | IHC ELISA Flow cytometry | PET [18F] MRI [19F] |
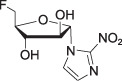 Fluoroazomycin arabinoside
(FAZA)
Fluoroazomycin arabinoside
(FAZA) |
log P = 1.1 | PET [18F] | |
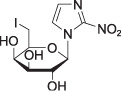 Iodoazomycin galactopyranoside
(β‐D‐IAZGP)
Iodoazomycin galactopyranoside
(β‐D‐IAZGP) |
log P = –0.24 P = 0.63 | PET/computed tomography [124I] | |
IHC, immunohistochemistry.
Immunological detection of exogenous hypoxia markers
The stability of the labeled marker in viable hypoxic tissue enables invasive detection, biopsy, and immunological identification to be carried out after the administration of nitroimidazole hypoxia markers. Intracellular Pimo and [18F]FMISO adducts are more stable than an adduct of CCI‐103F.( 54 , 55 ) The turnover rates of hypoxic tumor cells were observed with half‐lives ranging from 17 to 49 h in various solid tumors.( 56 ) Protein adducts of reductively activated nitroimidazoles are effective immunogens for the production of both polyclonal and monoclonal antibodies. For preparation of the immunogens for polyclonal and monoclonal antibodies, Pimo was bound to bovine serum albumin by means of a radiation chemical reduction.( 12 , 57 ) The antiserum was obtained for the Pimo‐bound bovine serum albumin with no cross reactivities to other nitroimidazole derivatives, such as misonidazole and etanidazole. At present, clinically relevant exogenous hypoxia markers are 2‐nitroimidazole derivatives such as Pimo and EF5, which are injected once i.v. before an invasive biopsy and immunohistochemical staining are carried out.( 58 ) Information on the prognostic role of these markers is so far limited because of the low number of patients in the published data.( 59 ) However, it may become a new gold standard for assays detecting hypoxia at the cellular level. The outcome for patients treated with radiotherapy can be predicted by assessment of the hypoxic fraction in a tumor biopsy by means of immunodetection using Pimo or EF5.( 60 )
Noninvasive imaging probes for hypoxia
PET, particularly PET or computed tomography imaging, has been developed to achieve non‐invasive in vivo mapping of tumor hypoxia with anatomical resolution. For the synthesis of hypoxia probes for PET imaging, short‐lived radionuclides such as 123I, 124I, 64Cu, and 18F are been bound to nitroimidazole. FMISO,( 58 )[18F]EF5,( 61 , 62 )[18F]fluoroazomycin arabinoside,( 63 ) and [124I]iodoazomycin galactopyranoside( 64 ) are available for PET. An alternative PET probe for hypoxia consists of radioactive 64Cu(II), 62Cu(II), or 60Cu(II) complexed with diacetyl bis(N 4‐methylthiosemicarbazone), which permeates the cell membrane easily, is reduced biologically, and is trapped in viable cells under low cellular oxygen tension.( 65 ) PET permits the pretreatment assessment of oxygen status non‐invasively by measuring the spatiotemporal distribution of the hypoxia‐specific tracers. Among them, FMISO has been studied most extensively to demonstrate the utility in both basic research and clinical practice.( 66 ) FMISO‐PET promises to predict the response to treatment and provide a prognosis for cancer patients.( 67 ) 2‐Nitroimidaole labeled with multiple 19F atoms has been used for magnetic resonance imaging.( 68 ) So far, the achievable signal‐to‐noise ratio is typically inadequate.( 20 , 69 , 70 )
Development of imaging bioprobes specific to HIF‐1 activity
We have been developing bioprobes with protein transduction domain (PTD)–ODD fusion proteins, which consist of a PTD and part of the human HIF‐1α ODD domain containing a VHL‐mediated protein destruction motif.( 71 , 72 , 73 ) We previously reported that the PTD domain effectively delivers the PTD–ODD fusion protein to hypoxic regions.( 74 , 75 ) The VHL‐mediated protein destruction motif provides a fused protein with hypoxia‐dependent stabilization( 76 ) (Fig. 5a). Based on these studies, we are developing an imaging probe for HIF‐1‐active cells with a PTD–ODD fusion protein. Because PTD–ODD fusion proteins underlie the same ODD control as HIF‐1α, they are expected to be colocated with HIF‐1α, and previous results support the expectation.( 74 , 75 , 76 , 77 ) We first constructed PTD–ODD–enhanced GFP labeled with near‐infrared fluorescent dye Cy5.5 for use as a model protein. When testing the membrane permeability and oxygen‐dependent degradation control of this prototype probe using cultured cells, we found that it permeated cell membranes with high efficiency, and its stability was controlled in an oxygen concentration‐dependent manner. When an optimized and redesigned bioprobe is administered to tumor‐bearing mice, it is delivered to the whole body soon after administration, but it degrades immediately in cells that are under aerobic conditions, and this leads to immediate clearance of the probe. On the other hand, the probe accumulates in hypoxic tumor cells with HIF‐1 activity, and in contrast to the surrounding cells that are under aerobic conditions, the hypoxic tumor cells with HIF‐1 activity can be imaged (Fig. 5b). Currently, we are conducting immunohistochemical analyses to investigate whether the probe accumulates locally in HIF‐1‐active cells. In addition, we are continuing our research into the preparation of a probe with radioactive reagents for clinical PET or SPECT probes. We are also developing novel near infra‐red (NIR) probes for non‐invasive optical imaging of tumor hypoxia, based on a bioreductive activating trigger or trapping mechanism for nitroimidazole imaging.
Figure 5.
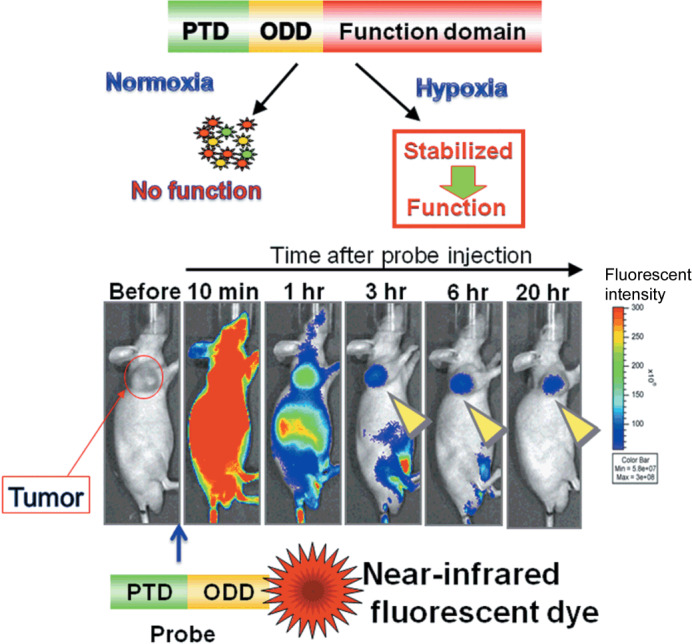
Imaging of hypoxia‐inducible factor (HIF)‐1 active cells. (a) Protein transduction domain (PTD)–oxygen‐dependent degradation (ODD) fusion protein consisting of three domains: PTD, ODD, and a functional domain. PTD enables the fusion protein to diffuse and enter the cell. ODD is derived from ODD548–603 of the HIF‐1α protein and endows the fusion protein with the same oxygen‐dependent degradation regulation as the HIF‐1α protein. Thus, PTD–ODD is degraded quickly in normoxia (aerobic conditions) but is stabilized and functional in hypoxia. (b) The PTD–ODD–enhanced GFP (EGFP) fusion protein (probe) was labeled with a near‐infrared fluorescent dye and injected into a tumor‐bearing mouse. Fluorescence was detected in the whole body shortly after i.v. injection of the labeled probe. By 6 h after probe injection, the fluorescence was predominantly detected in the tumor, suggesting that the PTD–ODD probe could be a potential probe for imaging HIF‐1 activity.
Hypoxia markers and radioresistance
The significance of imaging techniques and markers is useful for diagnosis and treatments. It is known that there is a strong correlation between radioresistance and hypoxic fractions in tumors and that Pimo‐positive cells, which are located in severe hypoxia less than 10 mmHg, are radioresistant. Recently we and other groups reported that the HIF‐1‐active fraction can cause radioresistance.( 77 , 78 ) A therapeutic benefit has been suggested for the appropriate combination of hypoxia imaging to select patients for hypoxia‐targeting treatment, such as treatment with hypoxia‐selective cytotoxins.( 59 , 79 ) Tirapazamin, which is a hypoxic cytotoxin currently undergoing clinical evaluation, exhibited selective toxicity to the Pimo‐positive primitive stem cell subset of bone marrow fractions.( 80 ) However, it does have some limitations in solid tumors, including poor diffusion through hypoxic tissue. It was suggested that the major effect of tirapazamin in vivo may be related to tumor vasculature dysfunction rather than cytotoxicity for the hypoxic cells located distal to functional blood vessels.( 81 )
Conclusion
As gene clusters whose expression is induced by the transcription factor HIF‐1 exert functions that contribute greatly to the malignant alteration of a tumor, the imaging and targeting of ‘hypoxic cells with HIF‐1 activity’ has become important. Nitroimidazoles have been advocated as exogenous makers for imaging hypoxic tissue even though hypoxia‐independent cases, in which specific tissues and situations may have induced the reaction, have been reported. Although the oxygen concentrations at which HIF‐1 is activated differs among tissue cells, nitroimidazole compounds such as Pimo function at absolute oxygen concentrations below 10 mmHg, indicating that nitroimidazole compounds are specific markers for severe hypoxia whereas HIF‐1α and HIF‐1‐induced endogenous markers are indicators for physiological hypoxia. Many biological factors and alterations other than hypoxia activate HIF‐1; however, hypoxia is the major factor for HIF‐1 activation. As long as tumor hypoxia is the crucial factor for tumor malignancy and treatment failures, both nitroimidazole compounds and HIF‐1 are important markers for tumor hypoxia. Further studies are necessary to understand the significance of these exogenous and endogenous markers, and we are going to address this crucial issue with the development of specific in vivo imaging probes for tumor hypoxia.
Abbreviations
| FDG | 2‐fluoro‐2‐deoxy‐D‐glucose |
| FIAU | 2′‐fluoro‐2′deoxy‐1ß‐D‐arabinofuranosyl‐5‐iodouracil |
| HER | human epidermal growth factor receptor |
| IGF1R | insulin‐like growth factor I receptor |
| Mdm‐2 | murine double minute 2 |
| mTOR | mammalian Target Of Rapamycin |
| PETN | pentaerythritol tetranitrate |
| PI3K | phosphatidyl‐inositol 3 kinase |
| SPECT | Single photon emission computed tomography |
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Scientific Research on Priority Areas, Cancer, from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This study is part of joint research focusing on development of core technology for establishing a COE for nanomedicine carried out through the Kyoto City Collaboration of Regional Entities for Advancing Technology Excellence Project of the Japan Science and Technology Agency.
References
- 1. Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Sem Radiat Oncol 2004; 14: 198–206. [DOI] [PubMed] [Google Scholar]
- 2. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4: 437–47. [DOI] [PubMed] [Google Scholar]
- 3. Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 4. Kizaka‐Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 2003; 94: 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. [DOI] [PubMed] [Google Scholar]
- 6. Epstein AC, Gleadle JM, McNeill LA et al . C. elegans EGL‐9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54. [DOI] [PubMed] [Google Scholar]
- 7. Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402. [DOI] [PubMed] [Google Scholar]
- 8. Vordermark D, Brown JM. Endogenous markers of tumor hypoxia predictors of clinical radiation resistance? Strahlenther Onkol 2003; 179: 801–11. [DOI] [PubMed] [Google Scholar]
- 9. Semenza GL. VHL and p53: tumor suppressors team up to prevent cancer. Mol Cell 2006; 22: 437–9. [DOI] [PubMed] [Google Scholar]
- 10. Hockel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res 1991; 51: 6098–102. [PubMed] [Google Scholar]
- 11. Griffiths JR, Robinson SP. The OxyLite: a fibre‐optic oxygen sensor. Br J Radiol 1999; 72: 627–30. [DOI] [PubMed] [Google Scholar]
- 12. Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 1995; 72: 889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer 2008; 8: 94–107. [DOI] [PubMed] [Google Scholar]
- 14. Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res 1999; 151: 580–9. [PubMed] [Google Scholar]
- 15. Sobhanifar S, Aquino‐Parsons C, Stanbridge EJ, Olive P. Reduced expression of hypoxia‐inducible factor‐1alpha in perinecrotic regions of solid tumors. Cancer Res 2005; 65: 7259–66. [DOI] [PubMed] [Google Scholar]
- 16. Janssen HL, Haustermans KM, Balm AJ, Begg AC. Hypoxia in head and neck cancer: how much, how important? Head Neck 2005; 27: 622–38. [DOI] [PubMed] [Google Scholar]
- 17. Dubois L, Landuyt W, Haustermans K et al . Evaluation of hypoxia in an experimental rat tumour model by [(18)F]fluoromisonidazole PET and immunohistochemistry. Br J Cancer 2004; 91: 1947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He F, Deng X, Wen B et al . Noninvasive molecular imaging of hypoxia in human xenografts: comparing hypoxia‐induced gene expression with endogenous and exogenous hypoxia markers. Cancer Res 2008; 68: 8597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janssen HL, Haustermans KM, Sprong D et al . HIF‐1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head‐and‐neck tumors. Int J Radiat Oncol Biol Phys 2002; 54: 1537–49. [DOI] [PubMed] [Google Scholar]
- 20. Busk M, Horsman MR, Kristjansen PE, Van Der Kogel AJ, Bussink J, Overgaard J. Aerobic glycolysis in cancers: implications for the usability of oxygen‐responsive genes and fluorodeoxyglucose‐PET as markers of tissue hypoxia. Int J Cancer 2008; 122: 2726–34. [DOI] [PubMed] [Google Scholar]
- 21. Brown JM. Evidence for acutely hypoxic cells in mouse tumors and a possible mechanism of reoxygenation. Br J Radiol 1979; 52: 650–6. [DOI] [PubMed] [Google Scholar]
- 22. Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med 1995; 22: 265–80. [DOI] [PubMed] [Google Scholar]
- 23. Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med 2008; 49 (Suppl 2): 129S–48S. [DOI] [PubMed] [Google Scholar]
- 24. Takasawa M, Moustafa RR, Baron JC. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke 2008; 39: 1629–37. [DOI] [PubMed] [Google Scholar]
- 25. Maeda K, Osato T, Umezawa H. A new antibiotic, azomycin. J Antibiot (Tokyo) 1953; 6: 182. [PubMed] [Google Scholar]
- 26. Mohindra JK, Rauth AM. Increased cell killing by metronidazole and nitrofurazone of hypoxic compared to aerobic mammalian cells. Cancer Res 1976; 36: 930–6. [PubMed] [Google Scholar]
- 27. Edwards DI. Nitroimidazole drugs – action and resistance mechanisms. II. Mechanisms of resistance. J Antimicrob Chemother 1993; 31: 201–10. [DOI] [PubMed] [Google Scholar]
- 28. Nagasawa H, Uto Y, Kirk KL, Hori H. Design of hypoxia‐targeting drugs as new cancer chemotherapeutics. Biol Pharm Bull 2006; 29: 2335–42. [DOI] [PubMed] [Google Scholar]
- 29. Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 2007; 19: 397–417. [DOI] [PubMed] [Google Scholar]
- 30. Bremner JC. Assessing the bioreductive effectiveness of the nitroimidazole RSU1069 and its prodrug RB6145: with particular reference to in vivo methods of evaluation. Cancer Metastasis Rev 1993; 12: 177–93. [DOI] [PubMed] [Google Scholar]
- 31. Denny WA. The role of hypoxia‐activated prodrugs in cancer therapy. Lancet Oncol 2000; 1: 25–9. [DOI] [PubMed] [Google Scholar]
- 32. Jin CZ, Nagasawa H, Shimamura M et al . Angiogenesis inhibitor TX‐1898: syntheses of the enantiomers of sterically diverse haloacetylcarbamoyl‐2‐nitroimidazole hypoxic cell radiosensitizers. Bioorg Med Chem 2004; 12: 4917–27. [DOI] [PubMed] [Google Scholar]
- 33. Hodgkiss RJ. Use of 2‐nitroimidazoles as bioreductive markers for tumour hypoxia. Anticancer Drug Des 1998; 13: 687–702. [PubMed] [Google Scholar]
- 34. Bolton JL, McClelland RA. Kinetics and mechanism of the decomposition in aqueous solutions of 2‐(hydroxyamino) imidazoles. J Am Chem Soc 1989; 111: 8172–81. [Google Scholar]
- 35. Hori H, Nagasawa H, Terada H. Effects of free radicals from hypoxic cell radiosensitizers, hypoxic cell cytotoxins, and bioreductive anticancer drugs on the biological environment, environmental oxidants. In: Nriagu O, Simmons MS, eds. Environmental Oxidants. New York: John Wiley and Sons, 1994: 425–44. [Google Scholar]
- 36. Kedderis GL, Miwa GT. The metabolic activation of nitroheterocyclic therapeutic agents. Drug Metab Rev 1988; 19: 33–62. [DOI] [PubMed] [Google Scholar]
- 37. Melo T, Ballinger JR, Rauth AM. Role of NADPH: cytochrome P450 reductase in the hypoxic accumulation and metabolism of BRU59‐21, a technetium‐99m‐nitroimidazole for imaging tumor hypoxia. Biochem Pharmacol 2000; 60: 625–34. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Role of cytochrome P450 reductase in nitrofurantoin‐induced redox cycling and cytotoxicity. Free Radic Biol Med 2008; 44: 1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skelly JV, Knox RJ, Jenkins TC. Aerobic nitroreduction by flavoproteins: enzyme structure, mechanisms and role in cancer chemotherapy. Mini Rev Med Chem 2001; 1: 293–306. [DOI] [PubMed] [Google Scholar]
- 40. Janssen HL, Hoebers FJ, Sprong D et al . Differentiation‐associated staining with anti‐pimonidazole antibodies in head and neck tumors. Radiother Oncol 2004; 70: 91–7. [DOI] [PubMed] [Google Scholar]
- 41. Azuma Y, Chou SC, Lininger RA, Murphy BJ, Varia MA, Raleigh JA. Hypoxia and differentiation in squamous cell carcinomas of the uterine cervix: pimonidazole and involucrin. Clin Cancer Res 2003; 9: 4944–52. [PubMed] [Google Scholar]
- 42. Cobb LM, Nolan J, Hacker T. Retention of misonidazole in normal and malignant tissues: interplay of hypoxia and reductases. Int J Radiat Oncol Biol Phys 1992; 22: 655–9. [DOI] [PubMed] [Google Scholar]
- 43. Orna MV, Mason RP. Correlation of kinetic parameters of nitroreductase enzymes with redox properties of nitroaromatic compounds. J Biol Chem 1989; 264: 12 379–84. [PubMed] [Google Scholar]
- 44. Adams GE, Flockhart IR, Smithen CE, Stratford IJ, Wardman P, Watts ME. Electron‐affinic sensitization. VII. A correlation between structures, one‐electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res 1976; 67: 9–20. [PubMed] [Google Scholar]
- 45. Wardman P. Some reactions and properties of nitro radical‐anions important in biology and medicine. Environ Health Perspect 1985; 64: 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gross MW, Karbach U, Groebe K, Franko AJ, Mueller‐Klieser W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int J Cancer 1995; 61: 567–73. [DOI] [PubMed] [Google Scholar]
- 47. Franko AJ, Koch CJ, Garrecht BM, Sharplin J, Hughes D. Oxygen dependence of binding of misonidazole to rodent and human tumors in vitro . Cancer Res 1987; 47: 5367–76. [PubMed] [Google Scholar]
- 48. Workman P, Brown JM. Structure–pharmacokinetic relationships for misonidazole analogues in mice. Cancer Chemother Pharmacol 1981; 6: 39–49. [DOI] [PubMed] [Google Scholar]
- 49. Brown JM, Workman P. Partition coefficient as a guide to the development of radiosensitizers which are less toxic than misonidazole. Radiat Res 1980; 82: 171–90. [PubMed] [Google Scholar]
- 50. Watts ME, Jones NR. The effect of extracellular pH on radiosensitization by misonidazole and acidic or basic analogues. Int J Radiat Biol Relat Stud Phys Chem Med 1985; 47: 645–53. [DOI] [PubMed] [Google Scholar]
- 51. Kleiter MM, Thrall DE, Malarkey DE et al . A comparison of oral and intravenous pimonidazole in canine tumors using intravenous CCI‐103F as a control hypoxia marker. Int J Radiat Oncol Biol Phys 2006; 64: 592–602. [DOI] [PubMed] [Google Scholar]
- 52. Bennewith KL, Raleigh JA, Durand RE. Orally administered pimonidazole to label hypoxic tumor cells. Cancer Res 2002; 62: 6827–30. [PubMed] [Google Scholar]
- 53. Ljungkvist AS, Bussink J, Kaanders JH, Van Der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res 2007; 167: 127–45. [DOI] [PubMed] [Google Scholar]
- 54. Thrall DE, McEntee MC, Cline JM, Raleigh JA. ELISA quantification of CCI‐103F binding in canine tumors prior to and during irradiation. Int J Radiat Oncol Biol Phys 1994; 28: 649–59. [DOI] [PubMed] [Google Scholar]
- 55. Azuma C, Raleigh JA, Thrall DE. Longevity of pimonidazole adducts in spontaneous canine tumors as an estimate of hypoxic cell lifetime. Radiat Res 1997; 148: 35–42. [PubMed] [Google Scholar]
- 56. Ljungkvist AS, Bussink J, Kaanders JH et al . Hypoxic cell turnover in different solid tumor lines. Int J Radiat Oncol Biol Phys 2005; 62: 1157–68. [DOI] [PubMed] [Google Scholar]
- 57. Raleigh JA, Koch CJ. Importance of thiols in the reductive binding of 2‐nitroimidazoles to macromolecules. Biochem Pharmacol 1990; 40: 2457–64. [DOI] [PubMed] [Google Scholar]
- 58. Troost EG, Laverman P, Philippens ME et al . Correlation of [18F]FMISO autoradiography and pimonodazole immunohistochemistry in human head and neck carcinoma xenografts. Eur J Nucl Med Mol Imaging 2008; 35: 1803–11. [DOI] [PubMed] [Google Scholar]
- 59. Bache M, Kappler M, Said HM, Staab A, Vordermark D. Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem 2008; 15: 322–38. [DOI] [PubMed] [Google Scholar]
- 60. Evans SM, Fraker D, Hahn SM et al . EF5 binding and clinical outcome in human soft tissue sarcomas. Int J Radiat Oncol Biol Phys 2006; 64: 922–7. [DOI] [PubMed] [Google Scholar]
- 61. Komar G, Seppanen M, Eskola O et al . 18F‐EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med 2008; 49: 1944–51. [DOI] [PubMed] [Google Scholar]
- 62. Yapp DT, Woo J, Kartono A et al . Non‐invasive evaluation of tumour hypoxia in the Shionogi tumour model for prostate cancer with 18F‐EF5 and positron emission tomography. BJU Int 2007; 99: 1154–60. [DOI] [PubMed] [Google Scholar]
- 63. Busk M, Horsman MR, Jakobsen S et al . Imaging hypoxia in xenografted and murine tumors with 18F‐fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2‐polarography, and fluorescence microscopy. Int J Radiat Oncol Biol Phys 2008; 70: 1202–12. [DOI] [PubMed] [Google Scholar]
- 64. Engelhardt EL, Schneider RF, Seeholzer SH, Stobbe CC, Chapman JD. The synthesis and radiolabeling of 2‐nitroimidazole derivatives of cyclam and their preclinical evaluation as positive markers of tumor hypoxia. J Nucl Med 2002; 43: 837–50. [PubMed] [Google Scholar]
- 65. Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper‐62‐ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 1997; 38: 1155–60. [PubMed] [Google Scholar]
- 66. Padhani A. PET imaging of tumour hypoxia. Cancer Imaging 2006; 6: S117–21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F‐fluoromisonidazole. Semin Nucl Med 2007; 37: 451–61. [DOI] [PubMed] [Google Scholar]
- 68. Jin GY, Li SJ, Moulder JE, Raleigh JA. Dynamic measurements of hexafluoromisonidazole (CCI‐103F) retention in mouse tumours by 1H/19F magnetic resonance spectroscopy. Int J Radiat Biol 1990; 58: 1025–34. [DOI] [PubMed] [Google Scholar]
- 69. Procissi D, Claus F, Burgman P et al . In vivo 19F magnetic resonance spectroscopy and chemical shift imaging of tri‐fluoro‐nitroimidazole as a potential hypoxia reporter in solid tumors. Clin Cancer Res 2007; 13: 3738–47. [DOI] [PubMed] [Google Scholar]
- 70. Salmon HW, Siemann DW. Utility of 19F MRS detection of the hypoxic cell marker EF5 to assess cellular hypoxia in solid tumors. Radiother Oncol 2004; 73: 359–66. [DOI] [PubMed] [Google Scholar]
- 71. Ivan M, Kondo K, Yang H et al . HIFα targeted for VHL‐mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–8. [DOI] [PubMed] [Google Scholar]
- 72. Jaakkola P, Mole DR, Tian YM et al . Targeting of HIF‐alpha to the von Hippel‐Lindau ubiquitylation complex by O2‐regulated prolyl hydroxylation. Science 2001; 292: 468–72. [DOI] [PubMed] [Google Scholar]
- 73. Maxwell PH, Wiesener MS, Chang GW et al . The tumour suppressor protein VHL targets hypoxia‐inducible factors for oxygen‐dependent proteolysis. Nature 1999; 399: 271–5. [DOI] [PubMed] [Google Scholar]
- 74. Harada H, Hiraoka M, Kizaka‐Kondoh S. Antitumor effect of TAT‐oxygen‐dependent degradation‐caspase‐3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res 2002; 62: 2013–18. [PubMed] [Google Scholar]
- 75. Harada H, Kizaka‐Kondoh S, Hiraoka M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia‐targeting drug in living animals. Mol Imaging 2005; 4: 182–93. [DOI] [PubMed] [Google Scholar]
- 76. Harada H, Kizaka‐Kondoh S, Hiraoka M. Mechanism of hypoxia‐specific cytotoxicity of procaspase‐3 fused with a VHL‐mediated protein destruction motif of HIF‐1α containing Pro564. FEBS Lett 2006; 580: 5718–22. [DOI] [PubMed] [Google Scholar]
- 77. Harada H, Kizaka‐Kondoh S, Li G et al . Significance of HIF‐1‐active cells in angiogenesis and radioresistance. Oncogene 2007; 26: 7508–16. [DOI] [PubMed] [Google Scholar]
- 78. Moeller BJ, Dreher MR, Rabbani ZN et al . Pleiotropic effects of HIF‐1 blockade on tumor radiosensitivity. Cancer Cell 2005; 8: 99–110. [DOI] [PubMed] [Google Scholar]
- 79. Le QT. Identifying and targeting hypoxia in head and neck cancer: a brief overview of current approaches. Int J Radiat Oncol Biol Phys 2007; 69: S56–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 2007; 104: 5431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Huxham LA, Kyle AH, Baker JH, McNicol KL, Minshinton AI. Tirapazamine causes vascular dysfunction in HCT‐116 tumour xenografts. Radiother Oncol 2006; 78: 138–45. [DOI] [PubMed] [Google Scholar]


