Abstract
Because gastric cancer is still the most common cancer, its prevention is one of the most important aspects of Japan's cancer control strategy. Observations among Japanese immigrants in the USA and Brazil based on the geographic differences, the trend in cancer incidence with time, and the change in incidence patterns indicate that gastric cancer is closely associated with dietary factors, such as the intake of salt and salted food. In international and intra‐Japanese ecological studies, the average salt excretion level, estimated using randomly selected 24‐h urine samples in each population, was closely correlated with gastric cancer mortality. Several case–control and cohort studies, including the author's recent works, have shown that a higher intake of some traditional salt‐preserved food and salt per se, which was estimated using a validated food‐frequency questionnaire, was associated with a risk of gastric cancer. While salted food intake may increase the risk of Helicobacter pylori infection, it can also act synergistically to promote the development of gastric cancer. Based on substantial evidence about the association between salt and salted food intake and the risk of gastric cancer from ecological, case–control, and cohort studies conducted in Japan and other countries, as well as mechanistic plausibility, dietary modification involving less salt and salted food intake is a practical strategy with which to prevent gastric cancer. (Cancer Sci 2005; 96: 1–6)
In 2000, gastric cancer was the second most frequent cause of deaths from cancer and the fourth most common cancer in the world, with an estimated 650 000 deaths and 880 000 new cases per year, almost two‐thirds of which occurred in developing countries. (1) In 2001, gastric cancer caused 50 000 deaths in Japan, and was the second most frequent cause of cancer death. (2) Moreover, it was estimated that in 1998, a total of 100 000 new cases of gastric cancer were detected in Japan, and that it was the most common type of cancer (21% of all cancers). (3) Therefore, the prevention of gastric cancer is one of the most important aspects of any cancer control strategy, both in Japan and across the world.
Geographic and ethnic differences, trends in cancer incidence with time, and changes in incidence patterns observed among immigrants indicate that gastric cancer is closely associated with modifiable factors, such as diet. Substantial evidence from ecological, case–control, and cohort studies strongly suggest that the risk of cancer could increase with a high intake of some traditional salt‐preserved food and salt per se, and that this risk could be decreased with a high intake of fruits and vegetables. 4 , 5 Other established non‐dietary factors include cigarette smoking (6) and infection with the bacterium, Helicobacter pylori. (7)
In experimental studies with rats, ingestion of salt is known to cause gastritis and, when coadministered, enhance the carcinogenic effects of known gastric carcinogens, such as N‐methyl‐N‐nitro‐N‐nitrosoguanidine (MNNG). 8 , 9 A high intragastric salt concentration destroys the mucosal barrier, and leads to inflammation and damage such as diffuse erosion and degeneration. The induced proliferous change might enhance the effect of food‐derived carcinogens. Based on human observational and animal experimental data, as well as mechanistic plausibility, a recent report from a joint World Health Organization/Food and Agriculture Organization Expert Consultation concluded that salt‐preserved food and salt ‘probably’ increase the risk of gastric cancer. (10) The reasons for using the grade ‘probable’ instead of ‘convincing’ is partly due to the limited amount of prospective data.
In the present review, epidemiological evidence on the association between salt and salted food intake and gastric cancer will be presented, with special reference to the author's recent works as well as to related articles.
Evidence from Descriptive Epidemiology and Ecological Studies
Geographic and ethnic differences. In Japan, the age‐standardized (world population) incidence rate (per 100 000) of gastric cancer in the 1990s ranged from 60 to 92 in men and from 24 to 39 in women, (11) and these rates as well as those in Korea (67–73 in men and 20–30 in women) were among the highest in the world. Among the white population in the USA, the incidence rate was 6.6 in men and 2.6 in women, which is approximately one‐tenth of the rate observed in Japan. Relatively higher rates, approximately half of those in Japan, were observed in several areas in Asia, South America, and Eastern Europe. In the USA, the rates among the black population (13 in men and 5.3 in women) were twice as high as those among the white population. The rates in the Japanese (22 in men and 12 in women) and the Korean (43 in men and 18 in women) ethnic groups were relatively higher than those in other ethnic groups such as the non‐Hispanic and Hispanic whites, blacks, Chinese, and Filipinos in California, USA. There were also approximately three‐fold differences in age‐standardized mortality rates within Japan: higher rates in Akita and Yamagata, lower rates in the Kyushu district in prefectures such as Kagoshima and Miyazaki, and a particularly low rate in Okinawa (Fig. 1).
Figure 1.
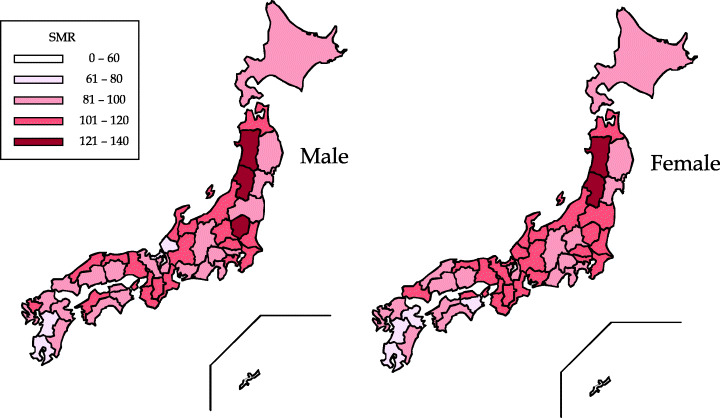
Geographic difference in gastric cancer distribution within Japan: standardized mortality ratios in 2000. There were approximately three‐fold differences in age‐standardized mortality rates within Japan.
Using data from the INTERSALT study, in which randomly selected 24‐h urine samples were taken from 39 populations from 24 countries (N = 5756), the median sodium levels in samples from subjects aged 20–49 years were analyzed in relation to the national gastric cancer mortality rates. (12) For the 24 countries, Pearson's correlation coefficient for gastric cancer mortality with sodium was 0.70 in men and 0.74 in women (both P < 0.001). In an ecological study of 65 rural counties in China, the consumption of salt‐preserved vegetables was correlated with gastric cancer mortality (r = 0.26 in men and 0.36 in women). (13)
While the geographic variation in gastric cancer mortality in Japan is not correlated with the per capita salt consumption estimated in the national nutrition survey, (14) the ecological study by Tsugane et al. of five selected areas in Japan showed an almost linear correlation between the cumulative mortality rate of gastric cancer up to 75 years of age (%) and the urinary salt excretion level in 24‐h urine samples (Fig. 2). 15 , 16 However, the salt consumption levels that were estimated using a 3‐day dietary record survey, which is identical to the method used in the national nutrition survey, did not correlate with the level of gastric cancer mortality in the same study. (17) This suggests the inadequacy of using the uniform composition table to evaluate the level of salt intake in areas with different dietary culture. The content of salt in different types of salted foods may vary depending on the food habits and type of food preparation that is particular to each area. The standard composition table consistently provides the same sodium value, without taking into account the difference in the actual sodium concentration in the food item; this results in a poor estimated value. The sodium excretion level in 24‐h urine samples is considered to be one of the best estimates for evaluating the daily salt intake, particularly at the population level.
Figure 2.
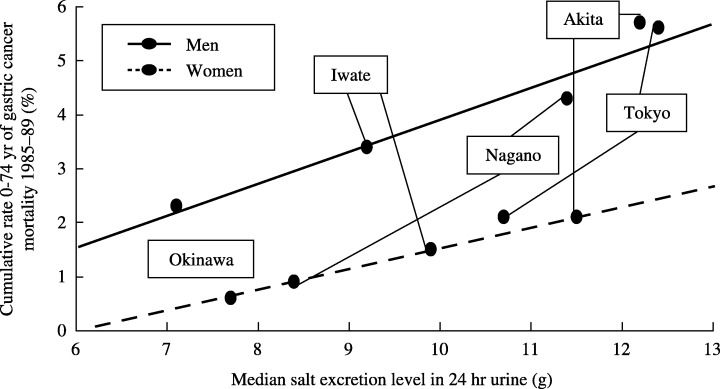
Urinary salt excretion level in 24‐h urine and gastric cancer mortality: an ecological study in five areas in Japan. An almost linear correlation between the urinary salt excretion level in 24‐h urine samples and the cumulative mortality rate of gastric cancer up to 75 years of age (%) were observed.
In summary, the majority of the geographic variation in gastric cancer mortality worldwide and in Japan can be explained at the population level by the daily salt intake level.
Time trend. In Japan, where the incidence of gastric cancer is the highest, both age‐adjusted mortality and incidence rates have been decreasing for several decades (Fig. 3). In the USA (18) and Europe, (19) gastric cancer used to be one of the most common cancers; however, the mortality rates have fallen dramatically during the last 50 years in all developed countries, and gastric cancer became a rare cancer without any specific intervention. This worldwide decline in the incidence of gastric cancer might be attributable to the spread of refrigeration. The use of refrigeration might be inversely correlated with the use of salting, with other methods of food preservation using salt, such as curing and smoking, and with the volume of salt in diets. (4) Furthermore, refrigeration has facilitated the consumption of fresh fruits and vegetables.
Figure 3.
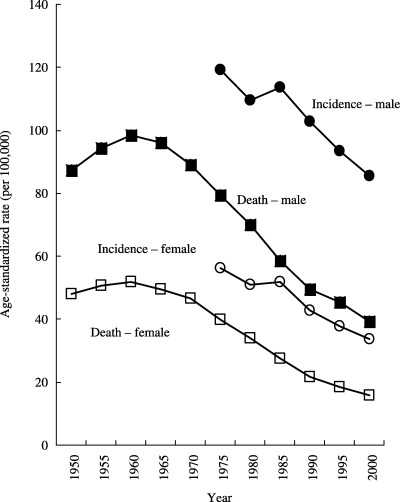
Time trends in the age‐adjusted incidence and mortality of gastric cancer in Japan. Both age‐adjusted mortality and incidence rates have been decreasing for several decades in Japan. Vital statistics and estimates from the population‐based cancer registry.
In summary, the worldwide decline in gastric cancer incidence can be explained by the transition in food preservation methods from salting to refrigeration.
The rate of gastric cancer among Japanese immigrants in the USA and Brazil. Studies on migrants, which offer some clues about the relative importance of genetic and environmental factors in the etiology of cancer, are useful, particularly when the differences in the incidence of cancer and lifestyle are large between the country of origin and the host country. The age‐adjusted (world population) incidence rates (per 100 000) of gastric cancer among Japanese residents in Hawaii, USA, were significantly lower both in men and women when compared with those rates in Japan, while in São Paulo, Brazil, the rates were relatively similar (Fig. 4). (20) These differences in incidence rates among three Japanese populations suggest that lifestyle changes, mainly dietary, were associated with a reduced risk of gastric cancer, depending on the degree to which a Western diet has been adopted and on the individual incidence rates in the host countries (the USA or Brazil).
Figure 4.
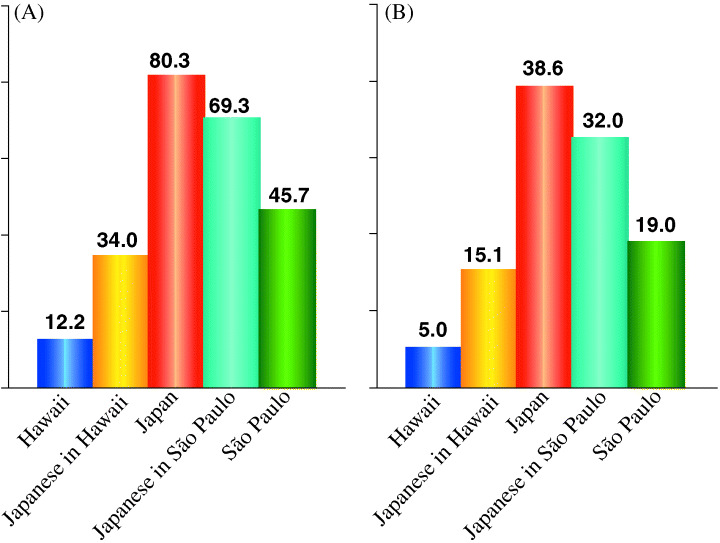
Gastric cancer rates among Japanese residents in Hawaii and São Paulo ca 1980. (A) Males; (B) Females. The age‐adjusted incidence rates of gastric cancer among Japanese residents in Hawaii, USA, were significantly lower both in men and women compared with the rates in Japan, while in São Paulo, Brazil, the rates were relatively similar.
According to cross‐sectional studies of randomly selected Japanese Brazilians in the city of São Paulo (1989) and of Japanese living in five prefectures across Japan (1989–1991), the dietary habits of Japanese Brazilians shifted toward the pattern seen in Western countries. (21) However, the subjects still consumed traditional and salted Japanese food: 15% of Japanese Brazilian men aged 40–49 years consumed miso soup almost every day and 4% consumed pickled vegetables. (22) The degree to which a Western diet has been adopted was not striking when compared with that seen in Japanese Americans: only 2% of Japanese American men aged 45–69 years in Hawaii consumed miso soup almost every day in the 1960s. (23) Further, in São Paulo, the salt excretion levels in the 24‐h urine samples of Japan‐born male residents aged 40–59 years were 14 g/day for 21 participants who were originally from Iwate, Akita, and Nagasaki prefectures, and 8.7 g/day for 12 participants from the Okinawa prefecture. (24) These levels were almost comparable with those seen in each prefecture in Japan.
In summary, the substantial decrease in the incidence of gastric cancer among Japanese immigrants in the USA and the minimal decrease among Japanese immigrants in Brazil can be explained by the extent to which migrants continued to maintain a Japanese dietary habit, which is expected to be high in salt.
Evidence from Case–Control Studies
Many, but not all, the results of case–control studies have shown a positive association between gastric cancer and the intake of high‐salt foods such as salted fish, cured meat, and salted vegetables, or the use of table salt. (5) Several studies have quantitatively estimated the total salt intake and found a strong positive association with the risk of gastric cancer, while several studies have evaluated the association with the intake of salted food such as salted fish and vegetables. In the evaluation carried out by the World Cancer Research Fund and the American Institute for Cancer Research in 1997, (4) the results of 16 case–control studies reported the association between salt or salted food and the risk of gastric cancer. Eight of these studies estimated the overall dietary salt or sodium intake; of these, four showed strong statistically significant increases in risk (odds ratio [OR] = 2.1–5.0 for the highest intake level). However, the remaining four studies showed no substantial association. Six of the studies have specifically examined the use of table salt, with three studies showing statistically significant increased risks (OR = 1.6–6.2 for the highest intake) and two showing non‐significant OR. Recently, several case–control studies have also shown that salted food was associated with the risk of gastric cancer. 25 , 26 , 27 , 28
In summary, most case–control studies have shown that patients with gastric cancer tended to have consumed more salt and salted food as a result of their past dietary habits.
Evidence from Cohort Studies
Prospective data are scarce and only two studies were evaluated in a 1997 report. (4) One reported no association with the intake of table salt or soy sauce, while salted fish intake was associated with an increased risk of gastric cancer in white American men, largely of Scandinavian and German descent (relative risk = 1.9 for the highest intake level). Recently, three studies have also reported this association. In a study that examined 13 000 Japanese men and women and 116 gastric cancer deaths with a 10‐year follow up, a higher consumption of pickled food and traditional soups showed an increased risk; however, this was not statistically significant. (29) The Netherlands Cohort Study examined 120 852 men and women and 282 incident gastric cancer cases that were documented during 6.3 years of follow up. (30) The salt intake of the cases was measured by calculating the mean daily sodium intake (dietary salt) from 150 food items and by using specific questions related to the consumption of salt. The findings suggested that the intake of dietary salt and several types of cured meat were weakly positively associated with the risk of gastric cancer.
Tsugane et al. conducted a population‐based prospective study in four areas in Japan (Iwate, Akita, Nagano, and Okinawa) with a total of 18 684 men and 20 381 women aged between 40 and 59 years and documented 358 men and 128 women with histologically confirmed gastric cancer during 12 years of follow up. (31) The daily salt intake was calculated by multiplying the consumption frequency of each of the 44 food items by the salt content of their standard portions/units, as specified in the Standard Tables of Food Composition (Science and Technology Agency, 1982) and totaling the value for all foods. (32) The validity among subsamples (94 men and 107 women) was assessed using actual nutrient intake data, which was calculated based on a 28‐day dietary record (7 consecutive days in four seasons; 14 days in Okinawa). The Spearman rank correlation between the two indices for sodium intake was 0.49 in men and 0.54 in women. (32) For those with the 2‐day 24‐h urine excretion level (32 men and 57 women, except from Okinawa), the correlation was 0.38 in men and only 0.12 in women. (32) The scatter‐grams showing the correlation between the two indices for men in each area are shown in Figure 5. This figure also shows that, although the validity is relatively higher across the wide range of salt intake in the four areas combined, it was modest within each area when the range of salt intake was narrow. This finding suggests that the estimated salt intake level obtained using a questionnaire is not sensitive enough to detect small differences, but that it is useful to detect relatively large differences, which had been observed in the cohort across the populations. Although multiple 24‐h urine collection may be an ideal method with which to estimate habitual salt intake, this is not feasible for a large‐scale cohort study.
Figure 5.
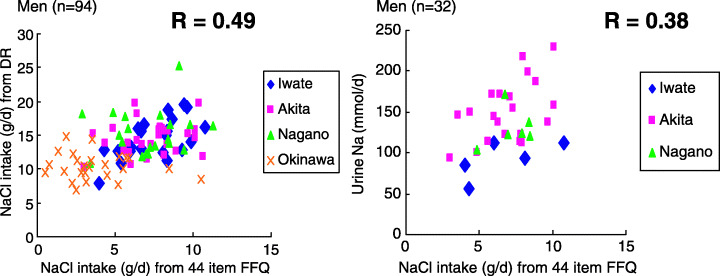
The validity of a 44‐item food frequency questionnaire (FFQ) with 28‐day dietary record (DR) and two 24‐h urinary excretions for sodium by PHC area. Japan Public Health Center‐based prospective study FFQ Validation Study, Cohort I. The Spearman rank correlation between the estimate from the FFQ and the estimate from the DR in men was 0.49 and between the estimate from the FFQ and the level in the urine excretion in men was 0.38. The scatter‐gram between the two indices shows that although the validity is relatively higher across the wide range of salt intake in the four areas combined, it was modest within each area where the range of salt intake was narrow.
The quintile category of salt intake was dose‐dependently associated with the risk of gastric cancer in men, after adjusting for potential confounding factors (for trend, P < 0.001), while a trend was not clear in women (for trend, P = 0.48; Fig. 6). The weak association observed between salt intake and gastric cancer in women may be due to the relatively low validity of the estimated salt intake; the Spearman rank correlation with a 2‐day urinary excretion level was 0.12 for women, while it was 0.38 in men. The stratification by study area attenuated the clear associations observed, as expected from the findings of the above‐mentioned validation study (Fig. 5). Although this association was less clear for miso soup, pickled vegetables, and dried fish, the consumption frequency category for highly salted food such as salted fish roe and salted fish preserves was strongly associated with the risk of gastric cancer in both sexes (Fig. 7). The observed associations were attenuated after further adjusting for salt intake or further stratification by study area; they were still evident for salted fish roe and salted fish preserves. The salt content of these items is >5%; that of other salted foods varied widely. It is <5% in most food items. The observed findings imply that either the intake of highly salted food increased the risk of gastric cancer or that it was merely an indication of the preference for salted food or salt intake in general. An alternative explanation for the strong association between highly salted food and gastric cancer may be due to chemical carcinogens, which can be formed by reacting nitrate or nitrite during the process of preservation and of digestion in the stomach.
Figure 6.
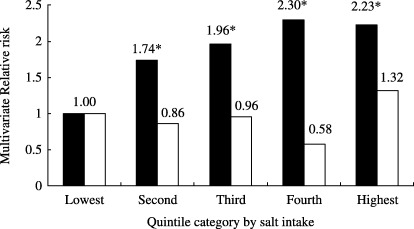
Salt intake# and risk of gastric cancer. JPHC Study Cohort I, 1990–2001. The quintile category of salt intake was dose‐dependently associated with the risk of gastric cancer in men (▪, for trend, P < 0.001), while a trend was not clear in women (□, P = 0.48). #, based on 44‐item food frequency questionnaire use in Japan Public Health Center‐based prospective study baseline survey in 1990; *statistically significant at P = 0.05.
Figure 7.
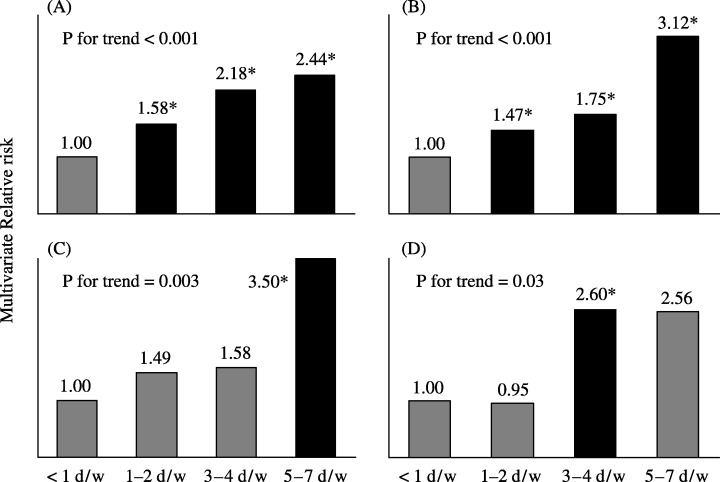
Highly salted food intake and risk of gastric cancer. Japan Public Health Center‐based prospective study Cohort I, 1990–2001. The frequency category of highly salted food such as salted fish roe and salted fish preserves was strongly associated with the risk of gastric cancer in both sexes. (A) Men, salted fish roe; (B) men, salted fish preserves; (C) women, salted fish row; (D) women, salted fish preserves. *statistically significant at P < 0.05.
From our dietary pattern analysis using the same data set, the traditional dietary pattern, which is closely correlated with the intake of salted food such as salted fish roe, salted fish preserves, pickled vegetables, dried fish, and miso soup, was significantly associated with the increased risk of gastric cancer in both sexes (relative risk for the highest quartile = 2.88 for men and 2.40 for women; for trend, P < 0.0001 and 0.007, respectively). (33)
In summary, several cohort studies, including those reported from Japan, have shown that a higher intake of salt and salted food was associated with a subsequent risk of gastric cancer.
Salt, Salted Food Intake, and Helicobacter pylori Infection
Infection with H. pylori is an established risk factor, but not a sufficient cause for the development of gastric cancer. 7 , 34 It is important to elucidate the role that salt and salted food intake plays in the causal link between H. pylori infection and gastric cancer.
In a cross‐sectional study of 634 men, aged 40–49 years and randomly selected from five areas in Japan, Tsugane et al. tested the association between lifestyle factors and H. pylori infection; 474 of the 628 men evaluated were positive for the IgG antibody against the bacterium. (35) A frequent intake of pickled vegetables was associated with the prevalence of H. pylori (OR against men who consume <1 day/week = 1.19 for 1–2 days/week, 1.92 for 3–4 days/week, 1.90 for 5–7 days/week; for trend, P = 0.02), and the daily consumption of miso soup was also associated with an increased risk (OR against non‐daily consumption = 1.60, 95% confidence interval = 1.03–2.49; Fig. 8). Occupation, number of siblings, education, smoking, alcohol consumption, and other dietary habits were not significantly associated with the prevalence of infection in this population. Although there are limitations in a cross‐sectional study such as this, the consumption of salted food appears to increase the risk of H. pylori infection. Mucosal damage induced by salt and salted food may increase the possibility of persistent infection with H. pylori. (36) While the salted food intake may increase the risk of H. pylori infection, it has been shown to act synergistically to promote the development of gastric adenocarcinoma in Mongolian gerbils treated with N‐methyl‐N‐nitrosourea (MNU). (37) The synergistic enhancing effect of H. pylori infection and salted food intake was also reported in a case–control study in Korea. (28) An infection with H. pylori, however, is by itself unlikely to increase the intake of salted food. Therefore, H. pylori infection cannot be confounded in the causal link between salt and salted food intake and gastric cancer (Fig. 9). Restricting salt and salted food intake can, at least, reduce the risk of gastric cancer.
Figure 8.
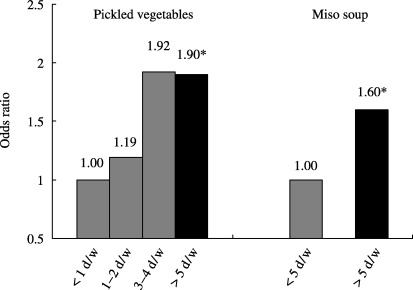
Salted food intake and Helicobacter pylori seropositivity: cross‐sectional analysis in an ecological study in five areas in Japan. The frequent intake of pickled vegetables was associated with the prevalence of H. pylori (for trend, P = 0.02), and the daily consumption of miso soup was also associated with an increased risk (odds ratio against non‐daily consumer = 1.60, 95% confidence interval = 1.03–2.49). *Statistically significant at P < 0.05.
Figure 9.
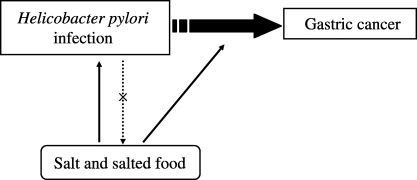
Salt and salted food intake, Helicobacter pylori infection and gastric cancer. While salted food intake may increase the risk of H. pylori infection, it may act synergistically to promote the development of gastric cancer. H. pylori infection cannot be confounded in the causal link between salt and salted food intake and gastric cancer, because the infection is by itself unlikely to increase the intake of salted food.
Gastric Cancer Prevention by Restriction of Salt and Salted Food
Although the eradication of H. pylori might be a promising strategy for gastric cancer prevention, a majority of middle‐aged and elderly Japanese people are already infected with it. (33) The worldwide decrease in the age‐adjusted incidence rate of gastric cancer has not been related to the program of intentional eradication of H. pylori infection, but can be related to a reduction in salt and salted food intake, and to an increase in fresh fruit and vegetable intake with the popularization of refrigerators. Based on sufficient evidence from descriptive and analytic epidemiologic studies, including those with a Japanese population, on the association between salt and salted food intake and risk of gastric cancer, dietary modification to reduce the intake of salt and salted food is a practical strategy with which to prevent gastric cancer in high‐risk areas in Japan.
Acknowledgments
This study was supported by Grants‐in‐aid for Cancer Research, for the Third‐Term Comprehensive Ten‐Year Strategy for Cancer Control and for Risk Analysis Research on Food and Pharmaceuticals from the Ministry of Health, Labor and Welfare of Japan, and for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology.
The author wishes to thank Dr Manami Inoue for preparing some of figures.
References
- 1. Stewart BW, Kleihues P, eds. World Cancer Report. Lyon: IARC Press, 2003. [Google Scholar]
- 2. Ministry of Health, Labor and Welfare . Journal of Health and Welfare Statistics 2003. Tokyo: Health and Welfare Statistics Association, 2003. [Google Scholar]
- 3. The Research Group for Population‐based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1998: estimates based on data from 12 population‐based cancer registries. Jpn J Clin Oncol 2003; 33: 241–5. [DOI] [PubMed] [Google Scholar]
- 4. World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington: American Institute for Cancer Research, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Kono S, Hirohata T. Nutrition and stomach cancer. Cancer Causes Control 1996; 7: 41–55. [DOI] [PubMed] [Google Scholar]
- 6. International Agency for Research on Cancer. Tobacco Smoking and Tobacco Smoke. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, 83.Lyon: International Agency for Research on Cancer, 2004. [Google Scholar]
- 7. International Agency for Research on Cancer. Schistosomes, Liver Flukes, and Helicobacter pylori. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, 61.Lyon: International Agency for Research on Cancer, 1994. [PMC free article] [PubMed] [Google Scholar]
- 8. Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T. Effects in rats of salt on experimental gastric cancers induced by N‐methyl‐N‐nitro‐N‐nitrosoguanidine or 4‐nitroquinoline‐1‐oxide. J Natl Cancer Inst 1975; 55: 101–6. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi M, Hasegawa R. Enhancing effects of dietary salt on both initiation and promotion stages of rat gastric carcinogenesis. Princess Takamatsu Symp 1985; 16: 169–82. [PubMed] [Google Scholar]
- 10. World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916. Geneva: World Health Organization, 2003. [PubMed] [Google Scholar]
- 11. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, eds. Cancer Incidence in Five Continents, Vol. VIII. IARC. Scientific Publications, no. 155. Lyon: IARC Press, 2002. [Google Scholar]
- 12. Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol 1996; 25: 494–504. [DOI] [PubMed] [Google Scholar]
- 13. Kneller RW, Guo WD, Hsing AW, Chen JS, Blot WJ, Li JY, Forman D, Fraumeni JF Jr. Risk factors for stomach cancer in sixty‐five Chinese counties. Cancer Epidemiol Biomarkers Prev 1992; 1: 113–18. [PubMed] [Google Scholar]
- 14. Honjo S, Kono S, Yamaguchi M. Salt and geographic variation in stomach cancer mortality in Japan. Cancer Causes Control 1994; 5: 285–6. [DOI] [PubMed] [Google Scholar]
- 15. Tsugane S, Gey F, Ichinowatari Y, Miyajima Y, Ishibashi T, Matsushima S, Hirota Y, Inami T, Yamaguchi M, Karita K, Kabuto M, Takashima Y, Todoriki H, Tsuda M, Akabane M, Furuichi Y, Hamada G, Watanabe S. Cross‐sectional epidemiologic study for assessing cancer risks at the population level, I. Study design and participation rate. J Epidemiol 1992; 2: 75–81. [Google Scholar]
- 16. Tsugane S, Gey F, Ichinowatari Y, Miyajima Y, Ishibashi T, Matsushima S, Hirota Y, Inami T, Yamaguchi M, Karita K, Kabuto M, Takashima Y, Todoriki H, Tsuda M, Akabane M, Furuichi Y, Hamada G, Watanabe S. Cross‐sectional epidemiologic study for assessing cancer risks at the population level, II. Baseline data and correlation analysis. J Epidemiol 1992; 2: 83–9. [Google Scholar]
- 17. Tsugane S, Akabane M, Inami T, Matsushima S, Ishibashi T, Ichinowatari Y, Miyajima Y, Watanabe S. Urinary salt excretion and stomach cancer mortality among four Japanese populations. Cancer Causes Control 1991; 2: 165–8. [DOI] [PubMed] [Google Scholar]
- 18. National Cancer Institute. Cancer Rates and Risks. Cancer Mortality in the United States, 1950–91. Changing Pattern for Major Cancers. Available from URL: http://seer.cancer.gov/publications/raterisk/index.html
- 19. La Vecchia C, Franceschi S, Levi F. Epidemiological research on cancer with a focus on Europe. Eur J Cancer Prev 2003; 12: 5–14. [DOI] [PubMed] [Google Scholar]
- 20. Tsugane S, De Souza JM, Costa ML Jr, Mirra AP, Gotlieb SL, Laurenti R, Watanabe S. Cancer incidence rates among Japanese immigrants in the city of Sao Paulo, Brazil, 1969–78. Cancer Causes Control 1990; 1: 189–93. [DOI] [PubMed] [Google Scholar]
- 21. Tsugane S, Hamada GS, De Souza JM, Gotlieb SLD, Takashima Y, Todoriki H, Kabuto M, Karita K, Yamaguchi M, Watanabe S, Laurenti R. Lifestyle and health related factors among randomly selected Japanese residents in the city of São Paulo, Brazil, and their comparisons with Japanese in Japan. J Epidemiol 1994; 4: 37–46. [Google Scholar]
- 22. Tsugane S, Hamada GS, Karita K, Tsubono Y, Laurenti R. Cancer patterns and lifestyle among Japanese immigrants and their descendants in the city of Sao Paulo, Brazil. In: Tajima K, Sonoda S, eds. Ethnoepidemiology of Cancer. Gann Monograph on Cancer Research 44, Tokyo: Japan Scientific Societies Press, 1996;. 43–50. [Google Scholar]
- 23. Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 1989; 49: 1857–60. [PubMed] [Google Scholar]
- 24. Tsugane S. Lifestyle and health among Japanese immigrants in Sao Paulo. In: Yanagida T (ed). Japanese Immigrants in America. Tokyo: Dobunsya, 1995; 119–52. [Google Scholar]
- 25. Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a case‐control study in Fujian Province, China. World J Gastroenterol 1998; 4: 516–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward MH, Lopez‐Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am J Epidemiol 1999; 149: 925–32. [DOI] [PubMed] [Google Scholar]
- 27. Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case‐control study. Int J Cancer 2002; 97: 531–5. [DOI] [PubMed] [Google Scholar]
- 28. Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol 2003; 13: 162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer 2002; 87: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Den Brandt PA, Botterweck AA, Goldbohm RA. Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands). Cancer Causes Control 2003; 14: 427–38. [DOI] [PubMed] [Google Scholar]
- 31. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S, for the JPHC Study Group. Salt and salted food intake and subsequent risk of gastric cancer among middle‐aged Japanese men and women. Br J Cancer 2004; 90: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsubono Y, Kobayashi M, Sasaki S, Tsugane S. Validity and reproducibility of a self‐administered food frequency questionnaire used in the baseline survey of the JPHC Study Cohort I. J Epidemiol 2003; 13: S125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim MK, Sasaki S, Sasazuki S, Tsugane S, for the Japan Public Health Center‐based Prospective Study Group. Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int J Cancer 2004; 110: 435–42. [DOI] [PubMed] [Google Scholar]
- 34. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case‐control studies nested within prospective cohorts. Gut 2001; 49: 347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K. Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res 1994; 85: 474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High‐salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res 1999; 59: 4823–8. [PubMed] [Google Scholar]
- 37. Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high‐salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res 2002; 93: 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


