Abstract
The objective of our study was to investigate the clinicopathological features of the currently ill‐defined subtype of primary cutaneous T‐cell lymphoma of unspecified type (CTCLU) with a cytotoxic phenotype and no Epstein–Barr virus (EBV) association. A series of 27 patients with CTCLU (median age 49 years; range 25–87 years; 18 men) was reviewed. Performance status scores above 1 (7%), clinical stages above 2 (15%), B symptoms (26%), extracutaneous involvement (30%), and a fatal course within 1 year of diagnosis (19%) were observed infrequently. The International Prognostic Index was high or high to intermediate in 11%, and the Prognostic Index for Peripheral T‐cell Lymphoma unspecified was above group 2 in 22%. Notably, the rates of spontaneous regression and T‐cell receptor gene rearrangements by polymerase chain reaction analysis were seen in 26 and 17% of our cases, respectively. Histologically, 22 patients had subcutaneous involvement of whom eight showed a lethal clinical course, and five patients without subcutaneous involvement were all survivors. Immunophenotypical and morphological features allowed us to subclassify our cases according to the following four categories: (1) epidermotropic CD8+ T‐cell lymphoma (n = 5); (2) cutaneous γ/δ T‐cell lymphoma (n = 8); (3) cutaneous α/β pleomorphic T‐cell lymphoma (n = 8); and (4) cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified (n = 6). All four of these groups of lymphomas exhibited a relatively favorable clinical course compared to previous reports. However, epidermotropic CD8+ T‐cell lymphoma appeared to be unique with a higher ratio (80%) of spontaneous regression, a lower ratio (40%) of subcutaneous involvement, and a more favorable clinical course than the other three subcategories. (Cancer Sci 2009; 100: 33–41)
A s proposed in 2005 by Willemze et al. of the World Health Organization–European Organization of Research and Treatment of Cancer (WHO‐EORTC),( 1 ) the new classification for primary cutaneous T‐cell lymphomas now lists several distinct entities, including: mycosis fungoides, sezary syndrome, adult T‐cell leukemia/lymphoma, subcutaneous panniculitis‐like T‐cell lymphoma (SPTCL), nasal type natural killer (NK)/T‐cell lymphoma, and primary cutaneous CD30+ lymphoproliferative disorders (LPD). Lymphoma other than these defined subtypes is classified under the nosological term primary cutaneous T‐cell lymphoma, unspecified (CTCLU). However, this classification remains controversial due to the heterogeneity and rarity of CTCLU. In the series documented by Willemze et al., patients with CTCLU accounted for less than 10% of all primary cutaneous T‐cell lymphomas, and were further subclassified into the following provisional entities: primary cutaneous aggressive epidermotropic CD8+ T‐cell lymphoma, primary cutaneous γ/δ T‐cell lymphoma, and primary cutaneous CD4+ small/medium‐sized pleomorphic T‐cell lymphoma.( 1 )
Cytotoxic molecules (CM) are apoptosis‐inducing molecules that are present in azurophilic cytoplasmic granules of T lymphocytes and NK cells. In the past decade, expression of CM has received much attention as an essential marker for defining extranodal T‐cell and NK‐cell lymphomas, including perforin,( 2 ) granzyme B,( 2 , 3 , 4 ) and T‐cell intracellular antigen (TIA)‐1.( 5 ) They are easily detectable on paraffin sections, are used commonly in routine surgical diagnoses, and are of relevance to predict the prognosis in nodal T‐cell lymphomas.
Among the cutaneous lymphomas, the expression of CM was detected in SPTCL,( 6 , 7 , 8 ) nasal‐type lymphoma,( 9 , 10 , 11 , 12 , 13 ) and primary cutaneous CD30+ LPD.( 14 , 15 , 16 , 17 , 18 , 19 ) They were well‐defined disease entities. SPTCL is characterized by primary subcutaneous infiltrates of neoplastic cells positive for T‐cell receptor (TCR)‐α/β; nasal‐type lymphoma is defined by CD56 positivity and constant Epstein–Barr virus (EBV) harboring; and primary cutaneous CD30+ LPD is characterized by proliferation of predominantly large lymphoid cells with strong expression of CD30 and the almost invariable absence of anaplastic lymphoma kinase (ALK) protein. CM expression was also detectable in CTCLU subtypes, primary cutaneous aggressive epidermotropic CD8+ T‐cell lymphoma, and primary cutaneous γ/δ T‐cell lymphoma.( 20 )
Apart from SPTCL, nasal‐type lymphoma, and primary cutaneous CD30+ LPD, CTCLU subtypes often have overlapping clinical presentations. However, we lack a reliable method for the detection of TCR‐γ/δ on paraffin sections. Therefore, their clinical distinctiveness has been documented in only a limited number of reports, and is not well established.
The objective of the present study was to investigate clinical, histological, immunophenotypic, and molecular features of EBV‐negative CTCLU with a cytotoxic phenotype. We reviewed the cases of 27 patients that had been diagnosed as EBV‐negative CTCLU with a cytotoxic phenotype to verify whether they could be subclassified within recently described specific categories according to the WHO‐EORTC classification for cutaneous lymphomas and the classification for cytotoxic lymphomas of the skin by Massone et al.( 21 ) EBV‐positive lymphoma was excluded, because the combination of EBV and cytotoxic phenotype is virtually regarded as being diagnostic of nasal‐type tumor.
Methods
Patient evaluation. As subjects for this study, 74 cases were selected from a patient file of 160 cases of primary cutaneous T‐cell lymphoma, classified according to the 2005 WHO‐EORTC classifications( 1 ) (Table 1). Patients had been diagnosed during the period 1995 to 2007 at the Department of Pathology, Aichi Cancer Center Hospital and Nagoya University Hospital, Nagoya, Japan. The present study was conducted under approval of the institutional review board of Aichi Cancer Center.
Table 1.
Cutaneous T‐cell lymphoma (CTCL) classification
| CTCL subtype | n | CM+ cases (%) |
|---|---|---|
| Total | 160 | 74 (46) |
| MF and MF variants and subtypes | 12 | 0 (0) |
| Lymphomatoid papulosis | 7 | 1 (14) |
| Primary cutaneous ALK− anaplastic large cell lymphoma | 24 | 15 (62) |
| Primary cutaneous ALK+ anaplastic large cell lymphoma | 8 | 7 (88) |
| Extranodal natural killer/T‐cell lymphoma, nasal type | 9 | 9 (100) |
| Adult T‐cell leukemia/lymphoma | 6 | 0 (0) |
| Subcutaneous panniculitis‐like T‐cell lymphoma | 4 | 4 (100) |
| Primary cutaneous peripheral T‐cell lymphoma, unspecified | 90 | 38 (42) |
| With EBV association | 5 | 5 (100) |
| Without EBV association | 85 | 33 (39) |
ALK, anaplastic lymphoma kinase; CM, cytotoxic molecule; EBV, Epstein–Barr virus; MF, Mycosis fungoides.
All specimens for the initial diagnosis were obtained from skin biopsies. The 33 EBV‐negative CTCLU with a cytotoxic phenotype cases enrolled in this study were classified through a combination of morphological examination and immunostaining. Six cases were excluded from our analysis because of a lack of clinical data. We therefore reviewed 27 EBV‐negative CTCLU cases in this study. Five EBV+ cytotoxic lymphoma cases shared an aggressive clinical course, appeared to be within the boundaries of nasal‐type tumor (data not shown), and were excluded from the present analysis. All cases were either primary cutaneous, or presented with skin lesions as the first manifestation of the disease. Primary skin involvement was defined as the presence of cutaneous lymphoma without nodal or visceral involvement after complete staging procedures. Our CTCLU cases were further subclassified according to the EORTC criteria as modified by Massone et al.:( 21 ) (1) epidermotropic CD8+ T‐cell lymphoma; (2) cutaneous γ/δ T‐cell lymphoma; (3) cutaneous α/β pleomorphic T‐cell lymphoma; and (4) cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified. Because the specimens were embedded in paraffin, we could not carry out immunohistology for the γ/δ chain. Therefore, as in the previous report of Massone et al.( 21 ) the subtype we designated as cutaneous γ/δ T‐cell lymphoma consisted entirely of cases negative for TCR‐β with either CD4− phenotype, and should have been regarded as putative γ/δ phenotype. Also, our cutaneous α/β pleomorphic T‐cell lymphoma consisted of cases positive for TCR‐β with a CD8− phenotype, our epidermotropic CD8+ T‐cell lymphoma consisted of cases positive for TCR‐β with a CD8+ phenotype, and the subtype we designated as cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified did not fit into any of the above three subtypes. Their immunophenotypic features related with the disease definition are summarized briefly in Table 2. Each case was reviewed by two pathologists (M.H. and S.N.).
Table 2.
Epstein–Barr virus (EBV)− cutaneous T‐cell lymphoma (CTCL) subclassification
| EBV− CTCLU subtype | Immunophenotype | |||
|---|---|---|---|---|
| CD4 | CD8 | TCR‐α/β | CM | |
| Epidermotropic CD8+ T‐cell lymphoma | + or – | + | + | + |
| Cutaneous γ/δ T‐cell lymphoma | – | – or + | – | + |
| Cutaneous α/β pleomorphic T‐cell lymphoma | + or – | – | + | + |
| Cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified | + | +οr – | – | + |
CM, cytotoxic molecule; TCR, T‐cell receptor.
Histology. Biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin–eosin for histopathological evaluation.
Immunohistology. Detailed immunophenotypic analysis was carried out on formalin‐fixed, paraffin‐embedded tissue sections. Antibody binding was visualized using an avidin–biotin peroxidase complex. The monoclonal antibodies used in these studies were specific for the following antigens: CD2, CD4, CD5, CD7, CD25, CD56, and perforin( 2 ) (Novocastra Laboratories, Newcastle, UK); CD3, CD8, CD45RO, CD20, CD30, ALK1, and MIB1 (Dako, Santa Fe, CA, USA); CCR4 and CXCR3 (BD Biosciences‐Pharmingen, San Diego, CA, USA); βF1 (TCR‐β; Santa Cruz); FoxP3 (Abcam, Cambridge, UK); granzyme B( 2 , 3 , 4 ) (Monosan, Uden, the Netherlands); and TIA‐1( 5 ) (Immunotech, Marseille, France). Antibody binding was considered positive when more than 30% of the tumor cells were stained, including the reactions with perforin,( 2 ) granzyme B,( 2 , 3 , 4 ) and TIA‐1.( 5 ) This criterion was adopted to avoid overestimation; primary cutaneous T‐cell lymphomas were often accompanied by varying numbers of bystander cells that exhibited CM expression.
Molecular biology. Polymerase chain reaction (PCR) was used in the analysis of TCR‐γ gene rearrangement. PCR was carried out according to standard procedures as described previously.( 22 )
Response to treatment. Briefly, complete remission was defined as the disappearance of all clinical evidence of the disease and the normalization of all laboratory values and radiological findings. Partial remission was defined as more than 50% reduction of the disease. No response was defined as less than 50% reduction in tumor size or evidence of a stable or progressive disease. All deaths due to disease progression or related to treatment toxicity were considered treatment failures, and these cases were included in the no response group.
Prognostic model. To assess the prognosis of primary cutaneous cytotoxic T‐cell lymphomas, we used two prognostic models: the International Prognostic Index (IPI) and the Prognostic Index for Peripheral T‐cell Lymphoma, Unspecified (PIT).( 23 )
Statistical analysis. Correlations between two groups were determined by χ2 analysis, Fisher's exact test, Student's t‐test, and the Mann–Whitney U‐test. Patient survival rates were determined by the Kaplan–Meier method and comparisons were made by the log‐rank test. Survival time was measured in months from the time of diagnosis until the date of death or the last follow up. Univariate and multivariate analyses were carried out using the Cox proportional hazard regression model. All data were analyzed with the SAS system (SAS Institute, Cary, NC, USA).
Results
Expression of CM and other findings in EBV− CTCLU. Immunohistological analysis indicated tumor cell expression of at least one of TIA‐1, granzyme B, or perforin in all 27 cases. These patients showed no EBER labeling, and are summarized in terms of their clinicopathological features at presentation (Table 3).
Table 3.
Clinicopathological characteristics of cytotoxic molecule‐positive, Epstein–Barr virus‐negative cutaneous T‐cell lymphoma, unspecified
| Characteristic | n | % |
|---|---|---|
| Total | 27 | |
| Sex (male/female) | 18/9 | |
| Age (years), median (range) | 49 (25–87) | |
| Performance status score 2–4 | 2 | 7 |
| Clinical stages higher than 2 | 4 | 15 |
| B symptoms | 7 | 26 |
| Case limited to the skin | 19 | 70 |
| Lymph node involvement | 7 | 26 |
| Bone marrow involvement | 1 | 4 |
| LDH higher than normal | 8 | 30 |
| International Prognostic Index, HI/H | 3 | 11 |
| Prognostic Index for Peripheral T‐cell Lymphoma group 3–4 | 6 | 22 |
| Gross appearance | ||
| Nodule | 18 | 67 |
| Multiple nodules | 16 | 59 |
| Spontaneous regression | 7 | 26 |
| Fatal course within 1 year of diagnosis | 5 | 19 |
| Death | 8 | 30 |
| Histology | ||
| Subcutaneous involvement | 22 | 81 |
| Immunophenotype | ||
| CD2 | 25 | 93 |
| cyCD3 | 23/26 † | 88 |
| CD4 | 14 | 52 |
| CD5 | 14/26 † | 54 |
| CD7 | 9 | 33 |
| CD8 | 9 | 33 |
| CD20 | 0 | 0 |
| CD25 | 17/25 † | 68 |
| CD30 | 17 | 63 |
| CD45RO | 21 | 78 |
| CD56 | 5 | 19 |
| TCR‐β | 13 | 48 |
| EBER | 0 | 0 |
| CCR4 | 0 | 0 |
| CXCR3 | 27 | 100 |
| FOXP3 | 0/25 † | 0 |
| T‐cell intracellular antigen‐1 | 22 | 81 |
| Granzyme B | 25 | 93 |
| Perforin | 19/25 † | 76 |
| Polymerase chain reaction | ||
| TCR gene rearrangements | 4/24 † | 17 |
EBER, Epstein‐Barr virus encoded small RNA; HI/H, high to intermediate risk/high risk; LDH, lectate dehydrogenase; TCR, T‐cell receptor.
The denominator represents the number of cases examined.
Nineteen (70%) presented clinically with disease limited to the skin, and were characterized by the presence of localized or disseminated eruptive papules, nodules, and tumors; lesions often showed central ulceration and necrosis. Extracutaneous sites of involvement at diagnosis were the lymph nodes (n = 7) and bone marrow (n = 1). Four patients (15%) were associated with stages higher than 2, two (7%) had performance status scores higher than 1, seven (26%) had B symptoms, eight (30%) had high lectate dehydrogenase levels, three (11%) had high IPI scores, and six (22%) were PIT higher than group 2. Notably, seven patients (26%) showed partial or complete spontaneous resolution in the clinical course.
Treatment consisted of chemotherapeutic regimens containing anthracycline for 12 patients, five and two of whom underwent radiation therapy and autologous stem cell transplantation, respectively. Five cases received only radiation therapy. Two cases received only corticosteroid therapy. One case received surgical excision therapy alone. Seven cases received no therapy, all of whom had low IPI scores and PIT, showed spontaneous regression, and were alive after the last follow‐up examination.
Among the patients with follow‐up information, eight died of disease dissemination between 1 and 47 months after the initial diagnosis, and five pursued an aggressive fatal course within 1 year. At the last follow‐up examination, seven patients survived with no evidence of skin disease, and 12 patients survived with skin disease.
We found a statistically significant reduction in survival between PIT group 1–2 (n = 21) and group 3–4 (n = 6) (P = 0.02) (Fig. 1). Four of each group had a lethal clinical course within 5 years, with death rates of 19% in PIT group 1–2 and 67% in PIT group 3–4. Also, we found a statistically significant reduction in survival between low and high IPI scores (P = 0.015).
Figure 1.
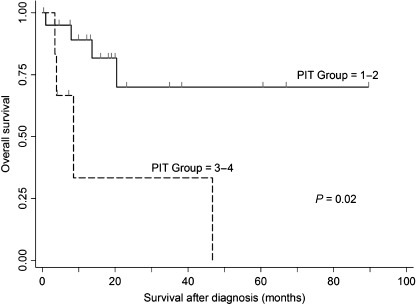
Overall survival rates of patients with Prognostic Index for Peripheral T‐cell Lymphoma, Unspecified (PIT) group 1–2 (n = 21) versus group 3–4 (n = 6) (P = 0.02).
Histologically, tumor cells were small to medium or medium to large with pleomorphic or blastic nuclei, and constantly expressed varying degrees of pan T‐cell markers. Their cytopathological features varied in appearance from patient to patient, and were occasionally characterized by a reniform or horseshoe‐like nuclear indentation. A small to moderate number of reactive cells such as eosinophils and small lymphocytes were admixed among tumor cells. Four (17%) of 24 evaluable patients showed TCR rearrangements by PCR analysis. There were no significant differences in CD4, CD8, CD30, CD56, or TCR‐β expression, or TCR rearrangement, but there was a higher rate of spontaneous regression in patients with CD8‐positive lymphomas. TIA‐1, granzyme B, and perforin were positive in 81, 93, and 76% of tumor cells, respectively. There were no prognostic differences between cases that were only TIA‐1+ and those expressing granzyme B or perforin. All of our cases were also positive for CXCR3 with no CCR4 or FoxP3 expression.
Histological patterns of involvement were evaluated in all 27 patients. Only epidermotropic or dermal involvement was present in five cases; all five showed multiple nodules without B symptoms, PS scores above 1, or high IPI scores. Three patients (60%) in this group experienced spontaneous regression. All five survived through the last follow‐up examination. The other 22 cases had subcutaneous involvement without rimming of fat spaces, seven (32%) of which had B symptoms, high LDH levels, and extracutaneous involvement. Four (18%) had spontaneous resolution, eight (36%) died of disease dissemination, and five (23%) pursued an aggressive fatal course within 1 year. There was no statistically significant difference in clinicopathological findings between these two groups with and without subcutaneous involvement.
In accordance with Massone et al.,( 21 ) we subdivided the present CTCLU cases into the following four categories: (1) epidermotropic CD8+ T‐cell lymphoma (n = 5) (Fig. 2); (2) cutaneous γ/δ T‐cell lymphoma (n = 8) (Fig. 3); (3) cutaneous α/β pleomorphic T‐cell lymphoma (n = 8) (Fig. 4); and (4) cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified (n = 6). These three categories of patients are summarized in terms of their clinicopathological features at presentation (Table 4).
Figure 2.
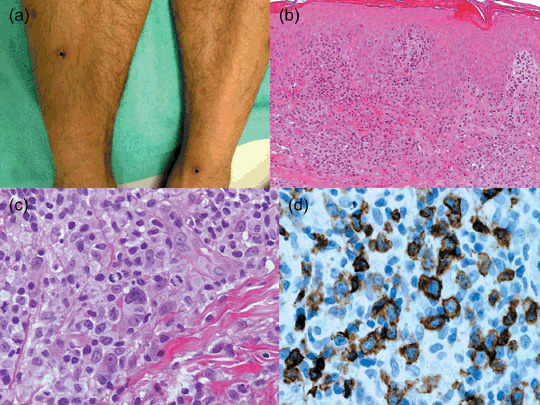
Cutaneous epidermotropic CD8+ T‐cell lymphoma. (a) Red patches, plaques, and indurated nodules on the legs. Some of them have ulceration with thick crusts and some are in spontaneous regression. (b) Hyperplastic epidermis with many intraepidermal atypical lymphocytes. (c) Neoplastic cells include medium to large pleomorphic or blastic nuclei in mitosis. (d) Staining for CD8 shows strong positivity of neoplastic cells.
Figure 3.
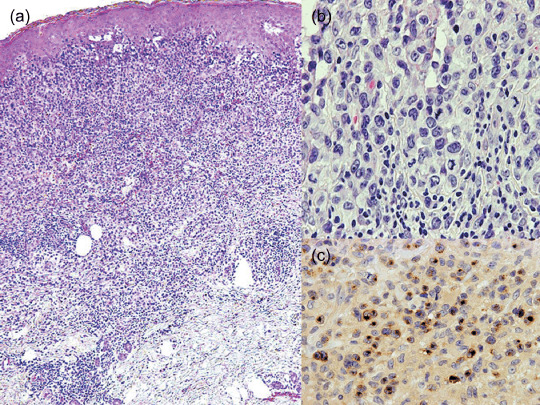
Cutaneous γ/δ T‐cell lymphoma. This patient had several lesions with different clinicopathological aspects. (a) Coexistent epidermotropic, dermal, and subcutaneous patterns of involvement in a single biopsy. (Top) Epidermal hyperplasia and a band‐like lymphocytic infiltrate in the papillary dermis with epidermotropism. (Middle) The infiltrate extends into the reticular dermis. (Bottom) There is an infiltration of subcutaneous tissue without lobular panniculitis. (b) Neoplastic cells reveal medium to large pleomorphic nuclei in mitosis and reactive lymphocytes. (c) Neoplastic cells show strong granular staining for granzyme B.
Figure 4.
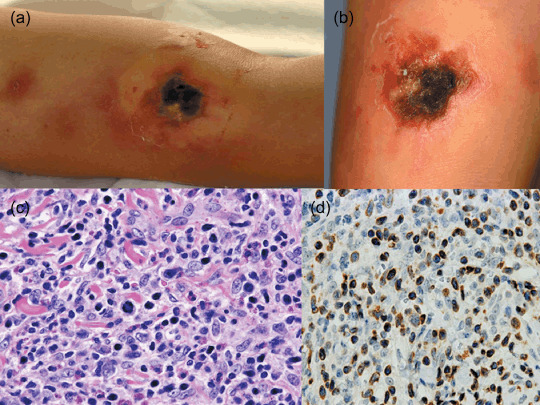
Cutaneous α/β pleomorphic T‐cell lymphoma. (a) Multiple dark red or brown indurations and an ulcerated induration with black necrotic tissue on the arm. (b) Another ulcerated lesion with hemorrhagic crust and necrotic tissue. (c) Neoplastic cells reveal medium to large pleomorphic nuclei in mitosis and a mixture of reactive lymphocytes, histiocytes, and eosinophils. (d) Neoplastic cells show strong granular staining for perforin.
Table 4.
Clinicopathological characteristics of cytotoxic molecule‐positive, Epstein–Barr virus‐negative cutaneous T‐cell lymphoma, unspecified subtypes
| Characteristic | CD8+ | γ/δ | α/β pleomorphic | P * | P ** | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Total | 5 | 8 | 8 | |||||
| Sex (male/female) | 5/0 | 4/4 | 7/1 | 0.06 | 0.11 | |||
| Age (years), median (range) | 58 (28–79) | 58 (36–76) | 49 (29–81) | 0.77 | 0.96 | |||
| Performance status score 2–4 | 0 | 0 | 0 | 0 | 2 | 25 | 0.17 | |
| Clinical stage 3–4 | 0 | 0 | 2 | 25 | 1 | 13 | 0.22 | 0.52 |
| B symptoms | 0 | 0 | 3 | 38 | 4 | 50 | 0.12 | 0.61 |
| Case limited to the skin | 4 | 80 | 6 | 75 | 4 | 50 | 0.84 | 0.30 |
| Lymph node involvement | 1 | 20 | 1 | 13 | 4 | 50 | 0.72 | 0.11 |
| Bone marrow involvement | 0 | 0 | 1 | 13 | 0 | 0 | 0.41 | 0.30 |
| LDH higher than normal | 1 | 20 | 2 | 25 | 3 | 38 | 0.84 | 0.59 |
| International Prognostic Index, HI/H | 0 | 0 | 1 | 13 | 1 | 13 | 0.41 | |
| Prognostic Index for Peripheral T‐cell Lymphoma group 3–4 | 1 | 20 | 1 | 13 | 3 | 38 | 0.72 | 0.25 |
| Gross appearance | ||||||||
| Nodule | 4 | 80 | 5 | 63 | 5 | 63 | 0.51 | |
| Multiple nodules | 4 | 80 | 4 | 50 | 5 | 63 | 0.28 | 0.61 |
| Spontaneous regression | 4 | 80 | 1 | 13 | 1 | 13 | 0.01 | |
| Fatal course within 1 year of diagnosis | 0 | 0 | 2 | 25 | 1 | 13 | 0.22 | 0.52 |
| Death | 1 | 20 | 2 | 25 | 3 | 38 | 0.84 | 0.59 |
| Histology | ||||||||
| Subcutaneous involvement | 2 | 40 | 7 | 88 | 8 | 100 | 0.07 | 0.30 |
| Immunophenotype | ||||||||
| CD2 | 5 | 100 | 7 | 88 | 7 | 88 | 0.41 | |
| cyCD3 | 4/4 † | 100 | 6 | 75 | 8 | 100 | 0.27 | 0.13 |
| CD4 | 3 | 60 | 0 | 0 | 5 | 63 | 0.01 | 0.007 |
| CD5 | 4 | 80 | 4 | 50 | 4 | 50 | 0.28 | |
| CD7 | 2 | 40 | 2 | 25 | 4 | 50 | 0.28 | 0.30 |
| CD8 | 5 | 100 | 3 | 38 | 0 | 0 | 0.02 | 0.05 |
| CD25 | 3/4 | 75 | 4 | 50 | 7 | 88 | 0.41 | 0.11 |
| CD30 | 2 | 40 | 4 | 50 | 5 | 63 | 0.72 | 0.61 |
| CD45RO | 5 | 100 | 5 | 63 | 7 | 88 | 0.12 | 0.25 |
| CD56 | 0 | 0 | 4 | 50 | 1 | 13 | 0.06 | 0.11 |
| TCR‐β | 5 | 100 | 0 | 0 | 8 | 100 | 0.0001 | 0.0001 |
| T‐cell intracellular antigen‐1 | 5 | 100 | 6 | 75 | 5 | 63 | 0.27 | 0.31 |
| Granzyme B | 5 | 100 | 8 | 100 | 8 | 100 | ||
| Perforin | 3/4 † | 75 | 6 | 75 | 7 | 88 | 0.79 | |
| Polymerase chain reaction | ||||||||
| TCR gene rearrangements | 0 | 0 | 1/6 | 17 | 2/7 | 29 | 0.34 | 0.61 |
CD8+, epidermotoropic CD8+ T‐cell lymphoma; γ/δ, cutaneous γ/δ T‐cell lymphoma; α/β pleomorphic; cutaneous α/β pleomorphic T‐cell lymphoma; HI/H, high to intermediate risk/high risk; LDH, lactate dehydrogenase; TCR, T‐cell receptor.
Epidermotoropic CD8+ T‐cell lymphoma versus cutaneous γ/δ T‐cell lymphoma.
Cutaneous γ/δ T‐cell lymphoma versus cutaneous α/β pleomorphic T‐cell lymphoma.
The denominator represents the number of cases examined.
Epidermotropic CD8+ T‐cell lymphoma (n = 5). All five patients were men, and had PS scores of 0 or 1, no B symptoms, stage 1 or 2, and low IPI scores. Four presented with disease limited to the skin, and were accompanied by spontaneous regression. Interestingly, this regression ratio (80%) was significantly higher than those observed in the other categories (P = 0.01). These cases survived through the last follow‐up examination. Only one patient initially had lymph node involvement, and died of disease dissemination 20 months after diagnosis. Immunophenotypically, all were positive for CD8 and TCR‐β, without CD56 expression or TCR rearrangements by PCR analysis.
Cutaneous γ/δ T‐cell lymphoma (n = 8). Four patients were men, and six presented with disease limited to the skin. Two had extracutaneous involvement of lymph nodes (n = 1) and BM (n = 1) at diagnosis. No patients were associated with PS scores above 1, two had stages higher than 2, three had B symptoms, two had high LDH levels, and one had a high IPI score and PIT higher than group 2. Only one case (13%) showed spontaneous regression. Two died of disease dissemination within 1 year of initial diagnosis. All were negative for TCR‐β with expression of CD5, CD30, and CD56 in just half of the cases. Only one of six evaluable patients (17%) showed TCR rearrangement by PCR analysis.
Cutaneous α/β pleomorphic T‐cell lymphoma (n = 8). Seven patients were men and four presented with disease limited to the skin. Four had extracutaneous involvement of the lymph nodes at diagnosis. Two were associated with a PS scores above 1, one with a stage higher than 2, four with B symptoms, three with high LDH levels, one with a high IPI score, and three with PIT higher than group 2. Only one case (13%) showed spontaneous regression. Three patients (38%) died of disease dissemination. Immunophenotypically, all were positive for TCR‐β without CD8 positivity. Two of seven evaluable patients (29%) showed TCR rearrangement by PCR analysis.
Cutaneous medium/large pleomorphic T‐cell lymphoma, not otherwise specified (n = 6). The clinicopathological data are summarized in Table 5. Two patients (33%) were men, and only one had extracutaneous involvement of the lymph nodes at diagnosis. None were associated with PS scores above 1, or had B symptoms. Only one case (17%) showed spontaneous regression. Two died of disease dissemination within 1 year of initial diagnosis. Immunophenotypically, all were positive for CD4 and CD30 without CD56 or TCR‐β expression. Only one patient (17%) showed TCR rearrangement by PCR analysis.
Table 5.
Clinicopathological data on cutaneous cytotoxic medium/large pleomorphic T‐cell lymphoma, not otherwise specified
| Case no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex/Age (years) | M/33 | F/55 | M/87 | F/43 | F/83 | F/25 |
| Case limited to the skin | + | + | + | + | + | – (LN involvement) |
| Performance status score 2–4 | – | – | – | – | – | – |
| Clinical stage | 2 | 2 | 1 | 1 | 1 | 4 |
| B symptoms | – | – | – | – | – | – |
| LDH higher than normal | – | – | – | – | + | + |
| International Prognostic Index | L | L | L | L | LI | HI |
| Prognostic Index for Peripheral T‐cell Lymphoma group | 1 | 1 | 2 | 1 | 3 | 2 |
| Gross appearence | ||||||
| Spontaneous regression | – | – | + | – | – | – |
| Clinical Outcome | AW | AW | AW | AW | DOD | DOD |
| (months) | 10 | 19 | 23 | 13 | 4 | 8 |
| Histology | ||||||
| Subcutaneous involvement | + | + | + | – | + | + |
| Immunophenotype | ||||||
| CD2 | + | + | + | + | + | + |
| CD3 | + | – | + | + | + | + |
| CD4 | + | + | + | + | + | + |
| CD5 | – | – | + | – | + | – |
| CD7 | – | – | + | – | – | – |
| CD8 | – | – | + | – | – | – |
| CD30 | + | + | + | + | + | + |
| CD56 | – | – | – | – | – | – |
| TCR‐β | – | – | – | – | – | – |
| EBER | – | – | – | – | – | – |
| T‐cell intracellular antigen‐1 | + | + | + | + | + | + |
| Granzyme B | – | + | + | + | + | + |
| Perforin | – | nd | + | + | + | – |
| Polymerase chain reaction | ||||||
| TCR gene rearrangements | + | – | – | – | – | – |
AW, alive and well; DOD, died of disease; EBER, Epstein‐Barr virus encoded small RNA; HI, high to intermediate risk; L, low risk; LI, low to intermediate risk; LN, lymph node; nd, not done; TCR, T‐cell receptor.
Survival analysis of CTCLU. Based on the above findings, the CTCLU patients were divided into the following three clinical subgroups: subgroup A, seven cases that survived with partial or complete spontaneous resolution; subgroup B, 12 cases that survived without partial or complete spontaneous resolution; and subgroup C, eight cases that had a fatal clinical course without any signs of spontaneous resolution after the initial diagnosis (Table 6). Subgroup A appeared to be unique compared to the other two groups. All seven patients of subgroup A were men, presented with disease limited to the skin, and did not exhibit aggressive clinical parameters, except one case with high LDH level and PIT higher than group 2. Five cases (71%) were positive for CD8. No evaluable patients showed CD56 expression or TCR rearrangement by PCR analysis. Four cases of subgroup A patients (57%) were diagnosed as epidermotropic CD8+ T‐cell lymphoma.
Table 6.
Characteristics of cytotoxic molecule‐positive, Epstein–Barr virus‐negative cutaneous T‐cell lymphoma, unspecified (CTCLU) clinical subgroups
| Characteristic | Subgroup A | Subgroup B | Subgroup C | P * | P ** | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Total | 7 | 12 | 8 | |||||
| Sex (male/female) | 7/0 | 6/6 | 5/3 | 0.02 | 0.58 | |||
| Age (years), median (range) | 58 (40–87) | 52 (29–81) | 43 (25–83) | 0.40 | 0.51 | |||
| Performance status score 2–4 | 0 | 0 | 0 | 0 | 2 | 25 | ||
| Clinical stage 3–4 | 0 | 0 | 1 | 8 | 3 | 38 | 0.43 | 0.11 |
| B symptoms | 0 | 0 | 3 | 25 | 4 | 50 | 0.15 | 0.25 |
| Case limited to the skin | 7 | 100 | 9 | 75 | 3 | 38 | 0.15 | 0.09 |
| Lymph node involvement | 0 | 0 | 3 | 25 | 4 | 50 | 0.15 | 0.25 |
| LDH higher than normal | 1 | 14 | 2 | 17 | 5 | 63 | 0.89 | 0.035 |
| International Prognostic Index, HI/H | 0 | 0 | 0 | 0 | 3 | 38 | ||
| Prognostic Index for Peripheral T‐cell Lymphoma group 3–4 | 1 | 14 | 1 | 8 | 4 | 50 | 0.68 | 0.035 |
| Gross appearance | ||||||||
| Nodule | 5 | 71 | 9 | 75 | 4 | 50 | 0.86 | 0.25 |
| Multiple nodules | 4 | 57 | 9 | 75 | 3 | 38 | 0.42 | 0.09 |
| Histology | ||||||||
| Subcutaneous involvement | 4 | 57 | 10 | 83 | 8 | 100 | 0.21 | 0.22 |
| Immunophenotype | ||||||||
| CD2 | 7 | 100 | 11 | 92 | 7 | 88 | 0.43 | 0.76 |
| cyCD3 | 7 | 100 | 10 | 83 | 6/7 | 86 | 0.25 | 0.89 |
| CD4 | 4 | 57 | 5 | 42 | 5 | 63 | 0.51 | 0.36 |
| CD5 | 5 | 71 | 4 | 33 | 5 | 63 | 0.11 | 0.21 |
| CD7 | 3 | 43 | 2 | 17 | 4 | 50 | 0.25 | 0.25 |
| CD8 | 5 | 71 | 2 | 17 | 2 | 25 | 0.02 | 0.65 |
| CD25 | 6 | 86 | 6 | 55 | 5/7 | 71 | 0.17 | 0.47 |
| CD30 | 4 | 57 | 8 | 67 | 5 | 63 | 0.68 | 0.85 |
| CD45RO | 6 | 86 | 8 | 67 | 7 | 88 | 0.36 | 0.29 |
| CD56 | 0 | 0 | 2 | 17 | 3 | 38 | 0.25 | 0.29 |
| TCR‐β | 5 | 71 | 4 | 33 | 4 | 50 | 0.11 | 0.46 |
| T‐cell intracellular antigen‐1 | 7 | 100 | 8 | 67 | 7 | 88 | 0.17 | 0.29 |
| Granzyme B | 7 | 100 | 11 | 92 | 7 | 88 | 0.43 | 0.76 |
| Perforin | 6 | 86 | 7/11 † | 64 | 6/7 † | 86 | 0.44 | 0.44 |
| Polymerase chain reaction | ||||||||
| TCR gene rearrangements | 0 | 0 | 3/10 † | 30 | 1/7 | 14 | 0.11 | 0.45 |
| CTCLU subtypes | ||||||||
| CD8+ | 4 | 57 | 0 | 0 | 1 | 13 | ||
| γ/δ | 1 | 14 | 5 | 42 | 2 | 25 | ||
| α/β pleomorphic | 1 | 14 | 4 | 33 | 3 | 38 | ||
| Not otherwise specified | 1 | 14 | 3 | 25 | 2 | 25 | ||
CTCLU, primary cutaneous T‐cell lymphoma, unspecified; HI/H, high to intermediate risk/high risk; subgroup A, alive with partial or complete spontaneous resolution; subgroup B, alive without partial or complete spontaneous resolution; subgroup C, fatal clinical coarse without any sign of spontaneous resolution after the initial diagnosis.
Subgroup A versus subgroup B;
**subgroup B versus subgroup C;
† the denominator represents the number of cases examined.
Patients in subgroup C exhibited significantly aggressive clinical parameters compared to patients in subgroup B, although there were no differences in the immunophenotypic findings between these two clinical subgroups. Histologically, both subgroups B and C were characterized by a relatively high incidence of subcutaneous involvement (83 and 100%, respectively) compared with subgroup A (57%).
Discussion
The lymphoma classifications established by WHO‐EORTC are largely defined by a combination of clinical, immunophenotypic, and genetic features.( 1 ) However, diagnosis is sometimes difficult due to clinicopathological similarities among disease entities. As a result, there is a need for refining the main disease classes with subclassifications. The subclassification of most lymphoid malignancies is based on lineage; however, studies on cytotoxic EBV− CTCLU are limited, because it is difficult to recruit large numbers of these patients due to their rarity and overlapping clinicopathological features. Most studies involving small series and case reports have indicated heterogeneity in clinical behavior, except that cutaneous γ/δ T‐cell lymphoma is consistently regarded as an aggressive disease.( 20 , 24 , 25 , 26 )
In the present study, we shed some light on the clinical features and biological behaviors of cytotoxic EBV− CTCLU patients (n = 27). Poor clinical parameters, such as PS scores above 1 (7%), clinical stages higher than 2 (15%), B symptoms (26%), extracutaneous involvement (30%), high LDH levels (30%), high IPI scores (11%), and PIT above group 2 (22%), were observed infrequently in our series. We demonstrated that these clinical parameters are useful in predicting the prognosis of CTCLU patients based on a statistically significant survival difference in patients with low and high IPI scores or PIT (P < 0.05). However, we found no statistically significant difference in clinicopathological findings between two groups with and without subcutaneous involvement, although all members of the latter group had survived at the last follow‐up examination. All cases were positive for CXCR3, but not CCR4 or FoxP3, which was contrasted with a CCR4‐dominant expression pattern of non‐cytotoxic T‐cell lymphoma, such as mycosis fungoides and adult T‐cell leukemia/lymphoma.( 27 ) In our series, CTCLU occupied 56% of all cutaneous T‐cell and NK‐cell lymphomas, which was higher than that (<10%) of a previous report( 1 ) and was considered to be biased by the inclusion of consultation cases.
The clinicopathological features of each category in our series were generally consistent with the report documented by Massone et al. ( 21 ) Indeed, our epidermotropic CD8+ T‐cell lymphoma cases were exclusively male and none had detectable TCR gene rearrangements by PCR analysis. However, we found that these patients were characterized by a high ratio (80%) of spontaneous regression and low ratio of subcutaneous involvement (40%); this result was not reported in the previous study. Our other CTCLU subtypes were also associated with a relatively favorable clinical course compared to those studied by Massone et al., despite a few differences in either clinical stage or extracutaneous involvement.( 21 ) In general, cutaneous CD8+ epidermotropic T‐cell lymphoma( 28 , 29 , 30 , 31 , 32 ) and cutaneous γ/δ T‐cell lymphoma( 20 , 24 , 25 , 26 ) were previously characterized by widespread dissemination, aggressive clinical behavior, and a very poor clinical outcome. Many of our cases were limited to the skin without extracutaneous involvement, and this might have influenced the favorable clinical outcome observed in the present study. Petrella et al. recently studied four interesting cases of CD8+ cytotoxic lymphoid proliferation of the ear with an indolent clinical course.( 33 ) They were characterized by no epidermotropism, but one experienced spontaneous regression and three exhibited TCR gene rearrangements. Our cases were not affected in the ears during the entire clinical course, and were somewhat distinct from those cases. These issues may be clarified in future larger series.
T‐cell receptor gene rearrangements provide a useful tool for establishing the diagnosis of T‐cell lymphoma. In the present study, we found a low incidence (17%) of TCR gene rearrangements. Our results were somewhat consistent with those of Massone et al. ( 21 ) Reasons for the discrepancies may include the presence of massive necrosis, the use of archive material dating back several years or even decades, and the localized disease of many of our patients. To date, we have found no clinicopathological or immunophenotypic differences between CTCLU with and without these monoclonal rearrangements. Juarez et al. also reported that detection of a monoclonal T‐cell population by PCR in lymph nodes of patients with cutaneous T‐cell lymphoma did not enhance the prediction of clinical outcome or the probability of survival beyond what could be determined from clinical examination and histological lymph node scores.( 34 ) These results implied that cutaneous cytotoxic lymphoma would impose diagnostic and therapeutic problems on pathologists and clinicians alike.
We also subdivided CTCLU cases into three clinical subgroups. Subgroup A was exclusively male, highly positive for TCR‐β and CD8, negative for CD56 and TCR gene rearrangements, had few poorer clinical parameters, and appeared to be distinct from the other subgroups. However, subgroup A was still heterogeneous in terms of CTCLU subtypes, and its relationship with CD30+ cutaneous LPD remains to be elucidated. However, IPI scores and PIT were useful markers for distinguishing between subgroups B and C without any differences in the immunophenotypic findings.
In our previous report, we found that nodal peripheral T‐cell lymphoma, unspecified (PTCL‐U) with a cytotoxic phenotype is a systemic disease with an aggressive clinical course, and in approximately 50% of patients it is associated with EBV.( 35 ) However, in that report, there was no prognostic difference between EBV+ and EBV− groups. In addition, cytotoxic T‐cell lymphomas that appeared in other anatomical sites (e.g. hepatosplenic T‐cell lymphoma( 36 , 37 ) and enteropathy‐type T‐cell lymphoma( 38 , 39 )) shared aggressive clinical behavior despite an absence of any association with EBV. In contrast, cutaneous cytotoxic lymphoma cases that were unassociated with EBV appeared to be unique in their relatively favorable clinical course and spontaneous regression in some patients; thus these should be differentiated from EBV+ cases.
Clinical staging that distinguishes between ‘primary’ and ‘secondary’ cutaneous involvement is mandatory in order to establish an appropriate therapeutic strategy. Our results also suggest that EBV− CTCLU should be considered separately from the others, and may provide support for the WHO‐EORTC classification of cutaneous lymphomas.( 1 ) Our CTCLU patients appeared to be different in their relatively localized disease and favorable prognosis compared to those of Massone et al. ( 21 ) and Toro et al. ( 20 ) This study was the first to shed light on the high frequency of spontaneous regression in epidermotropic CD8+ T‐cell lymphoma, despite the fact that this disease was originally regarded as an aggressive entity.( 28 , 29 , 30 , 31 , 32 ) These cases should be carefully differentiated from secondary involvement of cytotoxic lymphomas that developed originally in other anatomical sites, including nodal sites. We showed that morphological and phenotypic characteristics allow clear identification of most CTCLU cases; this will contribute to further understanding of their biological behavior and promote the development of novel therapeutic approaches for this enigmatic disease. The drawback of the present study was the limited number of patients enrolled. Further investigations are necessary for more definitive classification of clinical behaviors in each of the disease categories.
References
- 1. Willemze R, Jaffe ES, Burg G et al . WHO‐EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768–85. [DOI] [PubMed] [Google Scholar]
- 2. Yamashita Y, Yatabe Y, Tsuzuki T et al . Perforin and granzyme expression in cytotoxic T‐cell lymphomas. Mod Pathol 1998; 11: 313–23. [PubMed] [Google Scholar]
- 3. De Bruin PC, Kummer JA, Van der Valk P et al . Granzyme B expressing peripheral T‐cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa‐associated lymphoid tissue localization. Blood 1994; 84: 3785–91. [PubMed] [Google Scholar]
- 4. Bladergroen BA, Strik MC, Wolbink AM. The granzyme B inhibitor proteinase inhibitor 9 (PI9) is expressed by human mast cells. Eur J Immunol 2005; 35: 1175–83. [DOI] [PubMed] [Google Scholar]
- 5. Felgar RE, Macon WR, Kinney MC et al . TIA‐1 expression in lymphoid neoplasm: identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol 1997; 150: 1893–900. [PMC free article] [PubMed] [Google Scholar]
- 6. Ghobrial IM, Weenig RH, Pittlekow MR et al . Clinical outcome of patients with subcutaneous panniculitis‐like T‐cell lymphoma. Leuk Lymphoma 2005; 46: 703–8. [DOI] [PubMed] [Google Scholar]
- 7. Hoque SR, Child FJ, Whittaker SJ et al . Subcutaneous panniculitis‐like T‐cell lymphoma: a clinicopathological, immunophenotypic and molecular analysis of six patients. Br J Dermatol 2003; 148: 516–25. [DOI] [PubMed] [Google Scholar]
- 8. Ma L, Bandarchi B, Glusac EJ et al . Fatal subcutaneous panniculitis‐like T‐cell lymphoma with interface change and dermal mucin, a dead ringer for lupus erythematosus. J Cutan Pathol 2005; 32: 360–5. [DOI] [PubMed] [Google Scholar]
- 9. Berger TG, Voll RE, Simon MJr et al . Blastic CD56+ natural killer‐cell lymphoma with primary cutaneous manifestation. Acta Derm Venereol 2004; 84: 53–6. [DOI] [PubMed] [Google Scholar]
- 10. Chattopadhyay A, Slater DN, Hancock BW et al . Cutaneous CD56 positive natural killer and cytotoxic T‐cell lymphomas. Int J Oncol 2005; 26: 1559–62. [DOI] [PubMed] [Google Scholar]
- 11. Jang KA, Choi JH, Sung KJ et al . Primary CD56+ nasal‐type T/natural killer‐cell subcutaneous panniculitic lymphoma: presentation as haemophagocytic syndrome. Br J Dermatol 1999; 141: 706–9. [DOI] [PubMed] [Google Scholar]
- 12. Slater DN. New CD56 positive and cytotoxic T‐cell cutaneous lymphomas from the World Health Organisation. Br J Dermatol 2003; 148: 385–7. [DOI] [PubMed] [Google Scholar]
- 13. Warnnissorn N, Kanitsap N, Kulkantrakorn K et al . Natural killer cell malignancy associated with Epstein–Barr virus and hemophagocytic syndrome. J Med Assoc Thai 2007; 90: 982–7. [PubMed] [Google Scholar]
- 14. Beljaards RC, Kaudewitz P, Berti E et al . Primary cutaneous CD30+ large cell lymphoma: definition of a new type of cutaneous lymphoma with a favorable prognosis. A European Multicenter Study of 47 patients. Cancer 1993; 71: 2097–104. [DOI] [PubMed] [Google Scholar]
- 15. Kadin ME, Carpenter C. Systemic and primary cutaneous anaplastic large cell lymphomas. Semin Hemato 2003; 40: 244–56. [DOI] [PubMed] [Google Scholar]
- 16. Liu HL, Hoppe RT, Kohler S et al . CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol 2003; 49: 1049–58. [DOI] [PubMed] [Google Scholar]
- 17. Paulli M, Berti E, Rosso R et al . CD30/Ki‐1‐positive lymphoproliferative disorders of the skin – clinicopathologic correlation and statistical analysis of 86 cases: a multicentric study from the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Project Group. J Clin Oncol 1995; 13: 1343–54. [DOI] [PubMed] [Google Scholar]
- 18. Stein H, Foss HD, Durkop H et al . CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000; 96: 3681–95. [PubMed] [Google Scholar]
- 19. Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30+ lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol 1993; 28: 973–80. [DOI] [PubMed] [Google Scholar]
- 20. Toro JR, Liewehr DJ, Pabby N et al . Gamma‐delta T‐cell phenotype is associated with significantly decreased survival in cutaneous T‐cell lymphoma. Blood 2003; 101: 3407–12. [DOI] [PubMed] [Google Scholar]
- 21. Massone C, Chott A, Metze D et al . Subcutaneous, blastic natural killer (NK), NK/T‐cell, other cytotoxic lymphomas of the skin: a morphologic, immunophenotypic, and molecular study of 50 patients. Am J Surg Pathol 2004; 28: 719–35. [DOI] [PubMed] [Google Scholar]
- 22. McCarthy KP, Sloane JP, Kabarowski JHS et al . A simplified method of detection of clonal rearrangements of the T‐cell receptor‐γ chain gene. Diagn Mol Pathol 1992; 1: 173–9. [PubMed] [Google Scholar]
- 23. Gallamini A, Stelitano C, Calvi R et al . Peripheral T‐cell lymphoma unspecified (PTCL‐U): a new prognostic model from a retrospective multicentric clinical study. Blood 2004; 103: 2474–9. [DOI] [PubMed] [Google Scholar]
- 24. Avinoach I, Halevy S, Argov S et al . Gamma/delta T‐cell lymphoma involving the subcutaneous tissue and associated with a hemophagocytic syndrome. Am J Dermatopathol 1994; 16: 426–33. [DOI] [PubMed] [Google Scholar]
- 25. Toro JR, Beaty M, Sorbara L et al . Gamma delta T‐cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol 2000; 136: 1024–32. [DOI] [PubMed] [Google Scholar]
- 26. Ralfkiaer E, Wolf‐Sneedorff A, Thomsen K et al . T‐cell receptor gamma delta‐positive peripheral T‐cell lymphomas presenting in the skin: a clinical, histological and immunophenotypic study. Exp Dermatol 1992; 1: 31–6. [DOI] [PubMed] [Google Scholar]
- 27. Ishida T, Inagaki H, Utsunomiya A et al . CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T‐cell and NK‐cell lymphomas with special reference to clinicopathological significance for peripheral T‐cell lymphoma, unspecified. Clin Cancer Res 2004; 10: 5494–500. [DOI] [PubMed] [Google Scholar]
- 28. Berti E, Tomasini D, Vermeer MH et al . Primary cutaneous CD8‐positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol 1999; 155: 483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fika Z, Karkos PD, Badran K et al . Primary cutaneous aggressive epidermotropic CD8 positive cytotoxic T‐cell lymphoma of the ear. J Laryngol Otol 2007; 121: 503–5. [DOI] [PubMed] [Google Scholar]
- 30. Kim SK, Kim YC, Kang HY et al . Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T‐cell lymphoma with atypical presentation. J Dermatol 2006; 33: 632–4. [DOI] [PubMed] [Google Scholar]
- 31. Marzano AV, Ghislanzoni M, Gianelli U et al . Fatal CD8+ epidermotropic cytotoxic primary cutaneous T‐cell lymphoma with multiorgan involvement. Dermatol 2005; 211: 281–5. [DOI] [PubMed] [Google Scholar]
- 32. Poszepczynska‐Guigne E, Jagou M, Wechsler J et al . [Cutaneous CD8+ epidermotropic cytotoxic T‐cell lymphoma with aggressive course.] Ann Dermatol Venereol 2006; 133: 253–6. [DOI] [PubMed] [Google Scholar]
- 33. Petrella T, Maubec E, Cornillet‐Lefebvre P et al . Indolent CD8‐positive lymphoid proliferation of the ear: a distinct primary cutaneous T‐cell lymphoma? Am J Surg Pathol 2007; 31: 1887–92. [DOI] [PubMed] [Google Scholar]
- 34. Juarez T, Isenhath SN, Polissar NL et al . Analysis of T‐cell receptor gene rearrangement for predicting clinical outcome in patients with cutaneous T‐cell lymphoma: a comparison of Southern blot and polymerase chain reaction methods. Arch Dermatol 2005; 141: 1107–13. [DOI] [PubMed] [Google Scholar]
- 35. Asano N, Suzuki R, Kagami Y et al . Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T‐cell lymphoma, unspecified. Am J Surg Pathol 2005; 29: 1284–93. [DOI] [PubMed] [Google Scholar]
- 36. Belhadj K, Reyes F, Farcet JP et al . Hepatosplenic γ/δ T‐cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 2003; 102: 4261–9. [DOI] [PubMed] [Google Scholar]
- 37. Cooke CB, Krenacs L, Stetler‐Stevenson M et al . Hepatosplenic T‐cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma delta T‐cell origin. Blood 1996; 88: 4265–74. [PubMed] [Google Scholar]
- 38. Daum S, Foss HD, Anagnostopoulos I et al . Expression of cytotoxic molecules in intestinal T‐cell lymphomas. The German Study Group on Intestinal Non‐Hodgkin Lymphoma. J Pathol 1997; 182: 311–17. [DOI] [PubMed] [Google Scholar]
- 39. De Bruin PC, Kummer JA, Van der Valk P et al . Granzyme B expressing peripheral T‐cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa‐associated lymphoid tissue localization. Blood 1994; 84: 3785–91. [PubMed] [Google Scholar]


