Abstract
CD47 is an integrin‐associated penta‐transmembrane protein that possesses an immunoglobulin‐like domain in its extracellular region. We have now investigated the role of CD47 in the regulation of epithelial cell spreading and migration. CD47 is colocalized with E‐cadherin at cell–cell adhesion sites of epithelial cells. A Ca2+ switch experiment showed that CD47 was endocytosed and then relocalized to cell–cell adhesion sites in a similar manner to E‐cadherin. Such polarized localization of CD47 required the multiple spanning region of this protein. Forced expression of CD47 induced cell spreading with marked lamellipodium formation and resulted in both partial disruption of cell–cell adhesion and enhancement of the hepatocyte growth factor‐stimulated scattering of Madin–Darby canine kidney cells. The CD47‐induced cell spreading was blocked by inhibition of Src and mitogen‐activated protein kinase kinase. Thus, these results suggest that CD47 participates in the regulation of cell–cell adhesion and cell migration through reorganization of the actin cytoskeleton in epithelial cells. This function of CD47 is mediated by the activation of Src and mitogen‐activated protein kinase kinase. (Cancer Sci 2006; 97: 889–895)
Abbreviations:
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- FRG
FGD1‐related
- GEF
guanine nucleotide exchange factor
- HGF
hepatocyte growth factor
- Ig
immunoglobulin
- mAb
monoclonal antibody
- MAP
mitogen‐activated protein
- MAPK
mitogen‐activated protein kinase
- MDCK
Madin–Darby canine kidney
- MEK
mitogen‐activated protein kinase kinase
- PBS
phosphate‐buffered saline
- PTX
pertussis toxin
- RT
room temperature
- SHPS‐1
Src homology 2 domain‐containing protein tyrosine phosphatase substrate‐1.
Proper regulation of the formation of cell–cell adhesions is crucial for a variety of biological functions, such as maintenance of the integrity of organized tissues, the control of cell growth and differentiation, and tissue morphogenesis.( 1 ) In contrast, dysregulation of such formation participates, in part, in the promotion of invasion and metastasis of cancer cells. In polarized epithelial cells, cell–cell adhesion is mediated by a number of adhesion molecules, such as claudin at tight junctions, and E‐cadherin and nectin at adherens junctions.( 2 ) These adhesion molecules play central roles in the maintenance of cell adhesion through homophilic binding in trans. These molecules have also been implicated as participants in intercellular signaling, which regulates cell spreading and migration by reorganization of the actin cytoskeleton.( 3 ) In addition to these adhesion molecules, integrins, which are commonly present at the contact sites between cells and the extracellular matrix, are also localized at cell–cell adhesion sites and play multiple roles in epithelial cell functions.( 4 , 5 )
CD47, also known as integrin‐associated protein, was originally identified in association with the integrin αvβ3.( 6 ) It is a member of the Ig superfamily, possessing an Ig‐V‐like extracellular region, five putative transmembrane domains, and a short cytoplasmic tail.( 7 ) The extracellular region of CD47 is responsible for its association with the integrin β3 subunit. Although most CD47‐mediated cellular responses likely involve the activation of integrins, in particular αvβ3 and αIIbβ3,( 7 ) the molecular mechanism of such activation is not fully understood. CD47 is implicated in the regulation of multiple cellular processes including neutrophil migration,( 8 , 9 ) platelet spreading( 10 ) and Langerhans cell migration,( 11 ) all of which are processes that require rearrangement of the actin cytoskeleton. More recently, in N1E‐115 neuroblastoma cells or cultured neurons, it was found that CD47 promotes neurite and filopodium formation.( 12 ) Thus, CD47 regulates multiple cell functions by controlling the actin cytoskeleton. Certain cellular responses triggered by CD47 appear to be mediated by the PTX‐sensitive heterotrimeric G protein Gi.( 7 ) In addition, Rac and Cdc42 has also been implicated to participate in the CD47‐elicited promotion of polarity in B cells( 13 ) and neurite formation.( 12 ) In addition to integrin, the extracellular region of CD47 binds the putative ligands thrombospondin‐1( 7 ) and SHPS‐1.( 14 ) SHPS‐1 is a receptor‐like transmembrane protein that contains three Ig‐like domains in its extracellular region.( 15 ) We have recently shown that binding of SHPS‐1 to CD47 further enhances CD47‐promoted neurite and filopodium formation.( 12 )
CD47 is expressed and localized at cell–cell junctions of intestinal epithelial cells.( 9 ) However, the physiological roles of CD47 in regulation of cell adhesion, spreading and migration of epithelial cells, and the mode of such actions of remain mostly unknown. Therefore, we have now investigated the role of CD47 in the regulation of cell spreading and migration and downstream signals for CD47 in cultured epithelial cells.
Materials and Methods
Antibodies and reagents
Hybridoma cells producing rat mAb to mouse CD47 (miap 301) and rat P84 mAb to mouse SHPS‐1 were kindly provided by P.‐A. Oldenborg (Umeå University, Umeå, Sweden) and C. F. Lagenaur (University of Pittsburgh, Pittsburgh, PA, USA), respectively. The mAb were purified from serum‐free culture medium of the cells by affinity chromatography with protein G‐sepharose 4 Fast Flow (Amersham Biosciences, Piscataway, NJ, USA). A mouse mAb to the Myc epitope tag (9E10) was also purified from culture supernatants of hybridoma cells. A rat mAb to mouse E‐cadherin (ECCD2) was from Takara Bio (Shiga, Japan). A rabbit polyclonal antibody to mouse ZO‐1 was from Zymed (South San Francisco, CA, USA). A mouse mAb to human CD8 was from eBioscience (San Diego, CA, USA). Alexa488‐conjugated goat antimouse, antirabbit and antirat IgG (Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies. Rhodamine‐conjugated phalloidin was from Invitrogen. PD98059, PP2 and PP3 were from EMD Biosciences (Darmstadt, Germany), and U0126 was from Promega (Madison, WI, USA). Recombinant human HGF was from WAKO Pure Chemicals (Osaka, Japan). Wortmannin was from Sigma (St Louis, MO, USA). PTX was kindly provided by F. Okajima (Gunma University, Gunma, Japan).
Plasmids
Plasmids (pCAGGS vectors) encoding mouse wild‐type CD47, the CD47 deletion mutant CD47ΔCterm (lacking amino acids 290–321), the chimeric protein CD47EX‐TM (the extracellular Ig region of mouse CD47 fused to the transmembrane region and proximal portion of the cytoplasmic tail of SHPS‐1), and the chimeric protein CD8‐CD47MMS (the extracellular Ig region of human CD8 fused to the multiple membrane‐spanning domains and short cytoplasmic tail of mouse CD47) were generated and preparated as described previously.( 12 )
Cell culture and transfection
Madin–Darby canine kidney cells and MTD‐1A cells were kindly provided by Y. Takai (Osaka University, Osaka, Japan) and by T. Sasaki (Tokushima University, Tokushima, Japan), respectively. All cells were cultured in DMEM supplemented with 10% FBS at 37°C under a humidified atmosphere of 5% CO2 in air. For the generation of MDCK cells expressing mouse CD47 (MDCK‐CD47), MDCK cells were transfected with 1 µg of pTracer‐CMV containing wild‐type mouse CD47 cDNA using 3 µL of Lipofectamine 2000. The cells were selected with 100 µg/mL of Zeozin (Invitrogen). Several cell lines expressing mouse CD47 were identified by immunostaining with a mAb to CD47. Among them, clone #9 was used for the following experiments.
Immunofluorescence microscopy
Immunofluorescence microscopy was carried out as described previously.( 16 ) Briefly, the cells were fixed with 4% formaldehyde in PBS for 10 min at RT, permeabilized with 0.2% Triton X‐100 in PBS and blocked with 10% FBS in PBS for 30 min at RT. The cells were treated with the first antibody in 10% FBS/PBS for 1 h. The cells were then washed with PBS three times, followed by incubation with the second antibody in 10% FBS/PBS for 1 h. After the cells were washed with PBS three times, they were examined with a confocal laser scanning microscope (LSM Pascal; Zeiss, Oberkochen, Germany) as described previously.( 12 )
Ca2+ switch assay
The Ca2+ switch experiments using MTD‐1A cells were performed as described previously.( 17 , 18 ) Briefly, the cells (5 × 104) were seeded in 35‐mm dishes. After 48 h, the cells were washed with PBS and cultured at a normal Ca2+ concentration (2 mM) in DMEM without serum for 4 h. The cells were then cultured at a low Ca2+ concentration (2 µM in DMEM with 5 mM ethylene glycol bis‐(β‐aminoethylether)‐N,N,N′,N′‐tetraacetic acid) for 2 h. After culturing, cells were washed with PBS and cultured at normal Ca2+ in DMEM without serum for the indicated periods of time. The cells were then fixed and immunostained.
Scattering assay
Madin–Darby canine kidney and MDCK‐CD47 cells (1 × 104) plated in 35‐mm dishes were serum deprived for 24 h. They were then cultured with medium containing HGF (10 ng/mL). The cells were imaged with a phase‐contrast light microscope (model DMIRBE; Leica, Wetzlar, Germany) equipped with a cooled charge‐coupled device camera (model Pengunin 600 CL; Pixera, Los Gatos, CA, USA) at the indicated time points after addition of HGF.
Assays for cell spreading and formation of lamellipodia and filopodia
To determine the cell spreading and formation of lamellipodia and filopodia, MDCK and MDCK‐CD47 cells (1 × 105 cells) were plated in 35‐mm dishes and, after 1 h, were fixed and immunostained with rat mAb to mouse CD47 or with rhodamine‐conjugated phalloidin. Alternatively, MDCK and MDCK‐CD47 cells (1 × 104 cells) were cultured for 12 h in the absence or presence of inhibitors for Src or MEK. The cells were then fixed and stained with rhodamine‐conjugated phalloidin or with the mAb to E‐cadherin.
Results
Localization of CD47 and SHPS‐1 in cultured epithelial cells
We first examined the localization of CD47 and its ligand SHPS‐1 in mouse breast cancer‐derived epithelial cells, MTD‐1A. The immunoreactivity of CD47 was detected at cell–cell adhesion sites and colocalized with cortical actin as well as E‐cadherin and ZO‐1, which are known to be localized at cell–cell adhesion sites of epithelial cells (Fig. 1A).( 2 , 19 ) However, the low but detectable immunoreactivity of SHPS‐1, a ligand for CD47, was observed diffusely in the cytoplasm of cells and was not localized at cell–cell adhesion sites (Fig. 1A).
Figure 1.
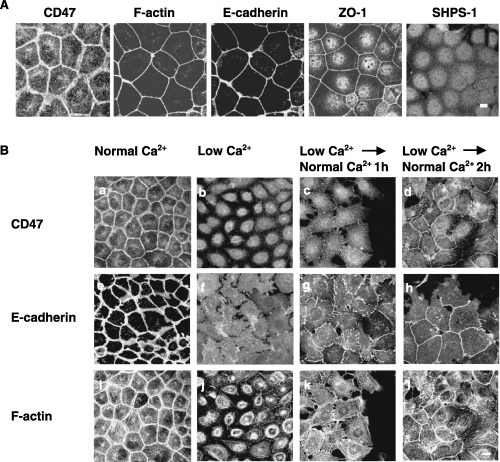
Localization of CD47 and Src homology 2 domain‐containing protein tyrosine phosphatase substrate‐1 (SHPS‐1) in MTD‐1A cells. (A) MTD‐1A cells were immunostained with a monoclonal antibody (mAb) to mouse CD47, a mAb to mouse E‐cadherin, a rabbit polyclonal antibody to mouse ZO‐1, or a mAb to mouse SHPS‐1. Alexa488‐conjugated goat antimouse, antirabbit or antirat immunoglobulin (Ig) G was used as a secondary antibody. F‐actin was visualized by staining with rhodamine‐conjugated phalloidin. Scale bar = 10 µm. (B) MTD‐1A cells were treated with low or high Ca2+ conditions as indicated. The cells were then fixed, followed by immunostaining with (a–d) a mAb to mouse CD47, (e–h) a mAb to mouse E‐cadherin, or (i–l) rhodamine‐conjugated phalloidin (F‐actin). (a,e,i) Normal Ca2+, MTD‐1A cells were cultured in medium containing 2 mM Ca2+. (b,f,j) Low Ca2+, cells were cultured in medium containing 2 µM Ca2+ for 2 h. Low Ca2+ Normal Ca2+, cells were precultured with 2 µM Ca2+ for 2 h and subsequently cultured in medium containing 2 mM Ca2+ (c,g,k) for 1 h or (d,h,l) for 2 h. Scale bar = 10 µm. All results shown are representative of three separate experiments.
Some junctional proteins, such as E‐cadherin, were shown to be endocytosed in response to a reduction of Ca2+ in the medium and, subsequently, to be recruited to cell–cell adhesion sites when the concentration of Ca2+ in the medium was returned to normal levels.( 17 , 18 ) Indeed, the reduction of Ca2+ in the medium induced the disruption of cell–cell adhesions between MTD‐1A cells, followed by the disappearance of E‐cadherin and cortical actin from cell–cell adhesion sites 2 h after treatment (Fig. 1Be,f,i,j). Similar to such changes, CD47 also disappeared from the cell–cell adhesion sites in response to the reduction in medium Ca2+ (Fig. 1Ba,b). In contrast, the increase in medium Ca2+ induced the relocalization of E‐cadherin and cortical actin to the cell–cell adhesion sites (Fig. 1Bg,h,k,l). CD47 was also relocalized to the cell–cell adhesion sites in response to the reincrease in medium Ca2+, although such a change was delayed slightly, compared with that apparent with E‐cadherin (Fig. 1Bc,d). Thus, both CD47 and E‐cadherin were endocytosed and relocalized to cell–cell adhesion sites by a Ca2+ switch in a similar manner.
Determination of the regions of CD47 required for junctional localization, and induction of lamellipodium formation by CD47 in MDCK cells
We next determined which regions of CD47 are required for localization of CD47 at the cell–cell adhesion sites. For this purpose, we used a plasmid containing a cDNA that encodes a mouse CD47 mutant lacking the short cytoplasmic tail at the COOH terminus (CD47ΔCterm). In addition, we also used plasmids containing two cDNAs encoding chimeric proteins: one encoding the extracellular Ig region of mouse CD47 fused to the transmembrane region and proximal portion of the cytoplasmic tail of mouse SHPS‐1 (CD47EX‐TM), and the other encoding the extracellular Ig region of human CD8 fused to the multiple membrane‐spanning domains and short cytoplasmic tail of mouse CD47 (CD8‐CD47MMS). Expression of either wild‐type mouse CD47 (CD47WT) or CD47ΔCterm in MDCK cells showed marked localization of these exogenously expressed proteins at cell–cell adhesion sites (Fig. 2). Expression of CD8‐CD47MMS also showed marked localization of this chimeric protein at cell–cell junctions (Fig. 2). In contrast, CD47EX‐TM failed to be localized to cell–cell adhesion sites and its expression was observed diffusely in the cytoplasm (Fig. 2).
Figure 2.
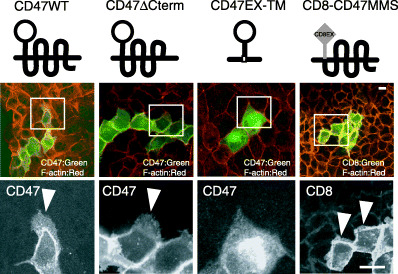
Determination of the regions of CD47 required for junctional localization and induction of lamellipodium formation by CD47 in Madin–Darby canine kidney (MDCK) cells. Schematic representation of the CD47 mutants studied. MDCK cells were transfected with a vector for CD47WT, CD47ΔCterm, CD47EX‐TM or CD8‐CD47MMS, as indicated. At 48 h after transfection, the cells were fixed and stained with rhodamine‐conjugated phalloidin (red); those transfected with the CD47 vector were also immunostained with a monoclonal antibody (mAb) to CD47 or a mAb to human CD8 (green). The stained cells were analyzed with a conforcal laser scanning microscope. Magnified images of areas indicated in the upper panels are shown in the lower panels. Arrow heads indicate lamellipodia. Scale bar = 10 µm. Results are representative of three separate experiments.
Forced expression of CD47WT or that of CD47ΔCterm also induced marked lamellipodium formation in MDCK cells (Fig. 2). Expression of CD8‐CD47MMS also showed lamellipodium formation, although its effect was less than that of CD47WT or CD47ΔCterm (Fig. 2). However, expression of CD47EX‐TM failed to induce lamellipodium formation (Fig. 2). Thus, these data suggest that the forced expression of CD47 induces lamellipodium formation. The multiple membrane‐spanning domains of CD47 are required for both localization of CD47 at cell–cell adhesion sites and lamellipodium formation by this protein.
Effects of forced expression of CD47 on cell–cell adhesion, spreading and migration of MDCK cells
We next generated MDCK cells (MDCK‐CD47) that expressed wild‐type mouse CD47 stably. Expression of mouse CD47 protein in MDCK‐CD47 cells was confirmed by immunostaining (Fig. 3B). Parental MDCK cells formed cell–cell adhesions with adjacent cells, and both E‐cadherin and cortical actin filaments were localized to these adhesion sites (Fig. 3A). In contrast, MDCK‐CD47 cells spread markedly compared with parental MDCK cells, their cell–cell adhesions were partly disrupted and E‐cadherin staining was markedly diminished in the subconfluent culture (Fig. 3A,B). HGF is known to stimulate the migration of MDCK cells.( 20 ) Indeed, HGF induced the migration of parental MDCK cells in a time‐dependent manner (Fig. 3C). Forced expression of CD47 further promoted the HGF‐stimulated migration of MDCK cells (Fig. 3C). Thus, these data suggest that CD47 regulates cell migration positively in cooperation with stimulation by growth factor through reorganization of the actin cytoskeleton.
Figure 3.
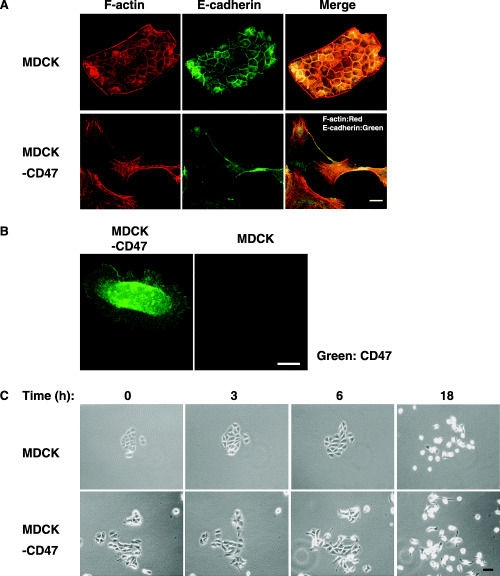
Effects of forced expression of CD47 on the formation of cell–cell adhesions, spreading and migration of Madin–Darby canine kidney (MDCK). (A) MDCK (upper panels) or MDCK‐CD47 (lower panels) cells were fixed and immunostained using a monoclonal antibody (mAb) to E‐cadherin (green), followed by staining with rhodamine‐conjugated phalloidin (red). Scale bar = 10 µm. (B) MDCK (right panel) or MDCK‐CD47 (left panel) cells were plated onto dishes. After 1 h of culture, cells were fixed and immunostained with a mAb to mouse CD47 (green). Scale bar = 10 µm. (C) MDCK (upper panels) or MDCK‐CD47 (lower panels) cells (1 × 104) were serum deprived for 12 h and subsequently cultured with medium containing 10 ng hepatocyte growth factor (HGF). The cells are shown at the time points indicated after addition of HGF. Scale bar = 50 µm. All results are representative of three separate experiments.
Participation of Src and MEK in the CD47‐induced cell spreading of MDCK cells
We next tried to find the downstream signaling molecules responsible for CD47‐induced cell spreading in MDCK cells. A PTX‐sensitive Gi is thought to mediate CD47‐dependent cellular responses such as cell spreading and platelet activation.( 7 ) In addition, the spreading of platelets induced by CD47 is sensitive to the phosphoinositide 3‐kinase inhibitor wortmannin.( 21 ) However, neither PTX nor wortmannin inhibited CD47‐induced cell spreading or the formation of lamellipodia in MDCK‐CD47 cells (data not shown). In contrast, treatment of MDCK‐CD47 with PP2, an inhibitor of the Src family of kinases,( 22 ) markedly inhibited cell spreading and partly recovered the localization of E‐cadherin and cortical actin filaments at cell–cell adhesion sites (Fig. 4g). PP3, an inactive analog of PP2, did not affect the morphology of MDCK‐CD47 cells (Fig. 4h). Furthermore, neither PP2 nor PP3 affected the morphology of parental MDCK cells (Fig. 4b,c). We next investigated the effects of two different MEK inhibitors: PD98059( 23 ) and U0126.( 24 ) Treatment of MDCK‐CD47 cells with either PD98059 or U0126 prevented cell spreading and formation of lamellipodia and partly recovered the localization of E‐cadherin and cortical actin filaments at cell–cell adhesion sites (Fig. 4i,j). These data thus suggest that activation of a Src family kinase or the MEK/MAPK pathway contributes to cell spreading and formation of lamellipodia induced by forced expression of CD47 in MDCK cells.
Figure 4.
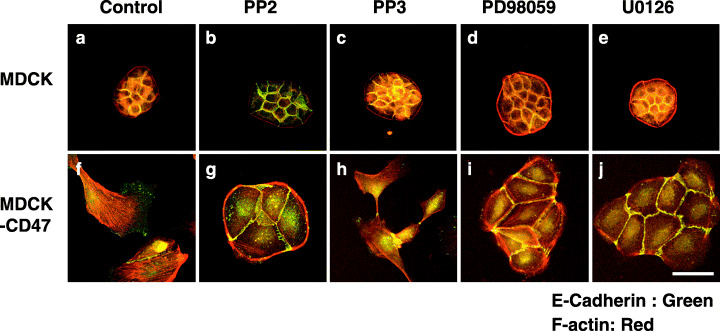
Participation of Src and mitogen‐activated protein kinase kinase (MEK) in CD47‐induced cell spreading in Madin–Darby canine kidney (MDCK) cells. MDCK (upper panels) or MDCK‐CD47 (lower panels) cells (1 × 104) were cultured 12 h in (a,f) the absence or (b,g) the presence of 20 µM PP2, (c,h) 20 µM PP3, (d,i) 50 µM PD98059 or (e,j) 2 µM U0126. The cells were fixed and immunostained with a monoclonal antibody to E‐cadherin (green), followed by staining with rhodamine‐conjugated phalloidin (red). Scale bar = 10 µm.
Discussion
We have shown that CD47 is localized to the cell–cell adhesion sites of cultured epithelial cells, together with E‐cadherin and ZO‐1. In addition, CD47 was endocytosed in response to low levels of Ca2+ in the medium, whereas it was relocalized to cell–cell adhesion sites by high levels of Ca2+ in the medium. Such changes in CD47 localization were well correlated with those of E‐cadherin. In addition to E‐cadherin, other adhesion molecules (such as claudin‐1 and occludin), E‐cadherin‐associated proteins (such as α‐catenin) and HGF receptor c‐Met were shown to be endocytosed or relocalized by a medium‐Ca2+ switch, whereas neither nectin‐1, an IgG‐like transmembrane protein, nor ZO‐1 were endocytosed in response to low levels of Ca2+ in the medium.( 18 , 25 ) Thus, CD47 is a new junctional membrane protein that is colocalized with and translocated similarly to E‐cadherin. However, E‐cadherin is known to be linked to the actin cytoskeleton through peripheral membrane proteins, including α‐catenin and β‐catenin, which bind the cytoplasmic tail of E‐cadherin.( 1 , 2 ) Similarly, claudin and occludin are also associated with the actin cytoskeleton through the peripheral membrane proteins ZO‐1, ZO‐2 and ZO‐3.( 26 ) Complex formation of E‐cadherin with α‐catenin or β‐catenin, or that of claudin or occludin with the ZO proteins is required for localization at cell–cell adhesion sites.( 1 , 27 ) We found that localization of CD47 at cell–cell adhesion sites requires the multiple membrane‐spanning region but not the extracellular region of this protein. The precise molecular mechanism for localization of CD47 at cell–cell adhesion sites remains unknown; however, it is possible that the multiple‐spanning region of CD47 might bind an as yet unidentified protein, which couples CD47 to the actin cytoskeleton.
The biological significance of CD47 localization at cell–cell adhesion sites may be to regulate cell–cell adhesion and of cell spreading. We found that forced expression of CD47 did indeed induce formation of lamellipodia at cell edges and a marked spreading of cells in MDCK cells. The multiple‐spanning region of CD47 is required for lamellipodium formation. However, expression of CD8‐CD47MMS exhibited a small effect on lamellipodium formation, suggesting that the extracellular region of CD47 also, in part, participates in the formation of lamellipodia. In addition, forced expression of CD47 induced a partial disruption of cell–cell adhesions and promotion of the HGF‐stimulated scattering of MDCK cells. Thus, these data suggest that CD47 regulates cell migration positively in cooperation with stimulation by growth factor through reorganization of the actin cytoskeleton.
We have shown that treatment of MDCK‐CD47 cells with inhibitors of Src family kinases or of MEK markedly inhibits cell spreading. It also recovered the formation of cell–cell adhesions in MDCK‐CD47 cells. In contrast, treatment of MDCK‐CD47 cells with these inhibitors induced enlargement of cell size. Inhibitors may not completely block the effect of CD47 on cell spreading. The mechanism by which CD47 activates a Src family kinase and thereby promotes cell spreading and formation of lamellipodia is currently unknown. Integrin may participate, at least in part, in the CD47‐induced activation of a Src family kinase, because Src is recruited to the β3 subunit of integrin and is thereby activated in response to engagement of integrin.( 28 ) In addition, it is shown that Rac and/or Cdc42 participate in the CD47‐induced promotion of migration in B cells,( 13 ) and in CD47‐induced neurite formation.( 12 ) Furthermore, the GEF activity of FRG for Cdc42 is increased by tyrosine phosphorylation of this protein by Src.( 29 ) The activity of Vav2, another GEF for Rac, is also increased by tyrosine phosphorylation.( 30 , 31 ) Thus, it is possible that forced expression of CD47 induces activation of Src and subsequent activation of GEF such as FRG or Vav2. The mechanism by which CD47 induces activation of the MEK/MAPK pathway is also unknown at present. However, it was shown recently that CD47‐induced neurite formation in cortical neurons is blocked by inhibitors of MEK and that expression of CD47 does indeed induce activation of MAPK.( 32 )
Overall, the present study has shown that CD47 participates in the regulation of cell–cell adhesion and cell migration through reorganization of the actin cytoskeleton in epithelial cells. This function of CD47 is mediated by the activation of Src and MEK/MAPK. CD47 is known to be markedly upregulated in cancers such as ovarian carcinoma.( 7 ) Thus, the present results suggest that overexpression of CD47 participates in the promotion of growth and metastasis of these cancer cells.
Acknowledgments
We thank P.‐A. Oldenborg, C. F. Lagenaur, Y. Takai, T. Sasaki and F. Okajima for reagents and cell lines, as well as Y. Niwayama for technical assistance. This work was supported by a Grant‐in‐Aid for Scientific Research on Priority Areas Cancer, a Grant‐in‐Aid for Scientific Research (B), a Grant‐in‐Aid for Young Scientists, and a grant of the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan as well as by a grant from the Public Trust Haraguchi Memorial Cancer Research Fund.
References
- 1. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991; 251: 1451–5. [DOI] [PubMed] [Google Scholar]
- 2. Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 2003; 116: 17–27. [DOI] [PubMed] [Google Scholar]
- 3. Perez‐Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell 2003; 112: 535–48. [DOI] [PubMed] [Google Scholar]
- 4. Larjava H, Peltonen J, Akiyama SK et al. Novel function for β1 integrins in keratinocyte cell–cell interactions. J Cell Biol 1990; 110: 803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoenenberger CA, Zuk A, Zinkl GM, Kendall D, Matlin KS. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci 1994; 107: 527–41. [DOI] [PubMed] [Google Scholar]
- 6. Brown EJ, Hooper L, Ho T, Gresham H. Integrin‐associated protein: a 50‐kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol 1990; 111: 2785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown EJ, Frazier WA. Integrin‐associated protein (CD47) and its ligands. Trends Cell Biol 2001; 11: 130–5. [DOI] [PubMed] [Google Scholar]
- 8. Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin‐associated protein (CD47). Proc Natl Acad Sci USA 1995; 92: 3978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parkos CA, Colgan SP, Liang TW et al. CD47 mediates post‐adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol 1996; 132: 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung J, Gao AG, Frazier WA. Thrombospondin acts via integrin‐associated protein to activate the platelet integrin αIIbβ3. J Biol Chem 1997; 272: 14740–6. [DOI] [PubMed] [Google Scholar]
- 11. Yu X, Fukunaga A, Nagai H et al. Engagement of CD47 inhibits the contact hypersensitivity response via the suppression of motility and B7 expression by Langerhans cells. J Invest Dermatol 2006; 126: 797–807. [DOI] [PubMed] [Google Scholar]
- 12. Miyashita M, Ohnishi H, Okazawa H et al. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell 2004; 15: 3950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida H, Tomiyama Y, Ishikawa J et al. Integrin‐associated protein/CD47 regulates motile activity in human B‐cell lines through CDC42. Blood 2000; 96: 234–41. [PubMed] [Google Scholar]
- 14. Jiang P, Lagenaur CF, Narayanan V. Integrin‐associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999; 274: 559–62. [DOI] [PubMed] [Google Scholar]
- 15. Fujioka Y, Matozaki T, Noguchi T et al. A novel membrane glycoprotein, SHPS‐1, that binds the SH2‐domain‐containing protein tyrosine phosphatase SHP‐2 in response to mitogens and cell adhesion. Mol Cell Biol 1996; 16: 6887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kodama A, Matozaki T, Fukuhara A, Kikyo M, Ichihashi M, Takai Y. Involvement of an SHP‐2‐Rho small G protein pathway in hepatocyte growth factor/scatter factor‐induced cell scattering. Mol Biol Cell 2000; 11: 2565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol 1991; 113: 881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamei T, Matozaki T, Sakisaka T et al. Coendocytosis of cadherin and c‐Met coupled to disruption of cell–cell adhesion in MDCK cells − regulation by Rho, Rac and Rab small G proteins. Oncogene 1999; 18: 6776–84. [DOI] [PubMed] [Google Scholar]
- 19. Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO‐1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 1986; 103: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gherardi E, Stoker M. Hepatocyte growth factor − scatter factor: mitogen, motogen, and met. Cancer Cells 1991; 3: 227–32. [PubMed] [Google Scholar]
- 21. Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates αvβ3 function through integrin‐associated protein. J Cell Biol 1996; 135: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanke JH, Gardner JP, Dow RL et al. Discovery of a novel, potent, and Src family‐selective tyrosine kinase inhibitor. Study of Lck‐ and FynT‐dependent T cell activation. J Biol Chem 1996; 271: 695–701. [DOI] [PubMed] [Google Scholar]
- 23. Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen‐activated protein kinase cascade. Proc Natl Acad Sci USA 1995; 92: 7686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Favata MF, Horiuchi KY, Manos EJ et al. Identification of a novel inhibitor of mitogen‐activated protein kinase kinase. J Biol Chem 1998; 273: 18623–32. [DOI] [PubMed] [Google Scholar]
- 25. Okamoto R, Irie K, Yamada A, Katata T, Fukuhara A, Takai Y. Recruitment of E‐cadherin associated with alpha‐ and beta‐catenins and p120ctn to the nectin‐based cell–cell adhesion sites by the action of 12‐O‐tetradecanoylphorbol‐13‐acetate in MDCK cells. Genes Cells 2005; 10: 435–45. [DOI] [PubMed] [Google Scholar]
- 26. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2: 285–93. [DOI] [PubMed] [Google Scholar]
- 27. Mitic LL, Schneeberger EE, Fanning AS, Anderson JM. Connexin–occludin chimeras containing the ZO‐binding domain of occludin localize at MDCK tight junctions and NRK cell contacts. J Cell Biol 1999; 146: 683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arias‐Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci USA 2003; 100: 13298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyamoto Y, Yamauchi J, Itoh H. Src kinase regulates the activation of a novel FGD‐1‐related Cdc42 guanine nucleotide exchange factor in the signaling pathway from the endothelin A receptor to JNK. J Biol Chem 2003; 278: 29890–900. [DOI] [PubMed] [Google Scholar]
- 30. Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation‐dependent and constitutive activation of Rho proteins by wild‐type and oncogenic Vav‐2. EMBO J 1998; 17: 6608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol 2000; 20: 1461–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Numakawa T, Ishimoto T, Suzuki S et al. Neuronal roles of the integrin‐associated protein (IAP/CD47) in developing cortical neurons. J Biol Chem 2004; 279: 43245–53. [DOI] [PubMed] [Google Scholar]


