Abstract
The aim of the present study was to investigate the safety and immune responses of personalized peptide vaccination when administered with gemcitabine (GEM) in advanced pancreatic cancer (APC) patients. Thirteen patients with APC were enrolled. Pre‐vaccination with peripheral blood mononuclear cells and plasma was carried out to examine cellular and humoral responses to 25 or 23 peptides in human leukocyte antigen A24++ or A2+++ patients, respectively. Only the reactive peptides (maximum of four) were then administered weekly at three different dose settings: 1, 2 and 3 mg of peptide. GEM was administered at 1000 mg/m2 per week for 3 weeks, followed by 1 week of rest. The combination therapy was well tolerated. Grade 3 toxicities were: anemia (three patients), neutropenia (two patients) and thrombocytopenia (two patients). Of these 13 patients, 11 (85%) showed clinical responses, such as reduction in tumor size and/or level of tumor markers. Augmentation of peptide‐specific cytotoxic T lymphocyte activity against pancreatic cancer cells was observed at each dose level, whereas the increment of peptide‐specific IgG antibodies was dependent on peptide dose. GEM did not inhibit the immune responses induced by personalized peptide vaccinations, and this new type of immunochemotherapy combination is recommended for further clinical study in APC patients. (Cancer Sci 2007; 98: 605–611)
Pancreatic cancer is the fifth most common cancer worldwide. For patients with advanced pancreatic cancer (APC), the treatment options are limited, with gemcitabine (GEM) the current standard therapy.( 1 , 2 ) Many clinical trials have investigated combination chemotherapies, but as none has identified a strategy that offers a significant improvement for the prognosis of APC, new therapeutic approaches are clearly called for.( 3 , 4 , 5 , 6 , 7 ) One of them could be specific immunotherapy as recent advances in tumor immunology have enabled us to use anticancer vaccines against various types of cancers.( 8 ) However, their clinical responses have been very limited.( 9 ) To overcome this, several modalities have been developed, and one promising approach is combined immunochemotherapy. This idea is based on the notion that cancer is an extremely complex and heterogeneous disease that exhibits a high level of robustness against a range of both host‐defense systems, such as loss of major histocompatibility complex (MHC) antigens, and therapeutic reagents, such as acquisition of multidrug‐resistant proteins.( 10 ) Indeed, in our clinical trials against hormone‐refractory prostate cancer patients, personalized peptide vaccination in combination with estramustine phosphate, a mixture of anticancer and antihormone drugs, showed a superior antitumor effect compared to peptide vaccination alone.( 11 ) We have showed that the personalized peptide vaccination of APC patients is well tolerated.( 12 ) In that clinical trial, one patient who had been vaccination with GEM had prolonged survival. Subsequently, in the present report we have conducted a phase I study of personalized peptide vaccinations administered with GEM in APC patients.
Patients and Methods
Patient eligibility. The following inclusion criteria had to be met: locally advanced, metastatic, and/or recurrent pancreatic cancer with measurable disease. Other inclusion criteria were: HLA‐A24‐positive and/or HLA‐A2‐positive status as determined by commercially available serological tests (SRL, Tokyo, Japan); an Eastern Cooperative Oncology Group performance status of 0–1; age between 18 and 75 years; no central nervous system metastases; life expectancy of at least 3 months; and adequate hematological (neutrophil count ≥ 1500/µL; platelet count ≥ 100 000/µL), renal (serum creatinine < 1.5 × the upper limit of normal [ULN] value) and hepatic (alkaline phosphatase < 3 × ULN value and bilirubin < 1.5 × ULN value) functions. Patients had to have recovered from the toxicity of any previous therapy for at least 4 weeks before trial entry, and also be negative for hepatitis B and C antigens. Pregnant patients and those with autoimmune diseases or an active infection were excluded. The study protocol was approved by the Institutional Ethical Review Boards of Kansai Medical University and Yamaguchi University. Written informed consent was obtained from all patients at the time of enrolment.
Study design. This was an open‐label, dose‐escalation phase I study. All laboratory tests required to assess eligibility for entry had to be completed within 7 days prior to the start of treatment. The peptide (2 mL), which was supplied in vials containing 2 or 4 mg/mL sterile solution, was mixed with an equal volume of incomplete Freund's adjuvant (Montanide ISA‐51VG; Seppic, Paris, France) and emulsified in a 5‐mL sterilized syringe. Then, 0.5, 1.0 or 1.5 mL of each peptide emulsion (maximum of four peptides per vaccination) was injected subcutaneously into the femoral area, once a week for 8 weeks. The dose of administrated vaccine was escalated every three patients by 1, 2 and 3 mg peptide, except in situations of dose‐limiting toxicities (DLT; see below). GEM (1000 mg/m2) was administered as a 30‐min intravenous infusion once a week for 3 weeks, followed by 1 week of rest. That is, one cycle of treatment consisted of eight vaccinations and six GEM administrations over an 8‐week period. The cycle was repeated every 8 weeks as long as the patients agreed to continue and their condition was considered feasible for vaccination. Toxicity was evaluated in patients who received more than four vaccinations, whereas both immunological and clinical evaluations were conducted in those who received more than eight vaccinations (i.e. one cycle). Blood samples for studies of immune responses were obtained at weeks 0, 4 and 8 during cycle 1 and then every 2 months for a total of 2 years. In cases of grade 3–4 neutropenia or grade 2–3 thrombocytopenia, the GEM dose was reduced by 20%. Patients were discontinued from therapy in the event of: grade 3–4 neutropenia complicated by fever (i.e. febrile neutropenia); grade 4 neutropenia lasting longer than 4 days; grade 4 thrombocytopenia; any grade 3–4 non‐haematological toxicity except anorexia, and nausea and vomiting in the absence of appropriate antiemetics; or delay of recovery from treatment‐related toxicity for more than 2 weeks.
DLT and maximum‐tolerated dose. DLT of peptide vaccines administered with GEM were determined during the first treatment cycle and were defined, using the Common Terminology Criteria for Adverse Events version 3.0 scale, as grade 3–4 injection site toxicity or grade 3–4 fever attributable to peptide vaccine. At least three patients were enrolled at each dose level. If DLT were observed after the first cycle in one or two patients, three additional patients were enrolled at the same dose level. If only one or two of six patients experienced DLT, dose escalation continued. Dose escalation did not occur in individual patients. The maximum tolerated dose (MTD) was defined as the dose level that produced DLT in three of six patients or in all of the initial three patients.
Peptides and vaccination. Twenty‐five or 23 peptides were provided for vaccination to HLA‐A24++ or HLA‐A2++ patients, respectively, as reported previously.( 13 ) These peptides were prepared under conditions of Good Manufacturing Practice by the Multiple Peptide System (San Diego, CA, USA), and peptide sequences are shown in a previous manuscript.( 13 ) These peptides have the ability to induce HLA‐A24‐restricted or HLA‐A2‐restricted and tumor‐specific cytotoxic T lymphocyte (CTL) activity in peripheral blood mononuclear cells (PBMC) of cancer patients and are expressed frequently on various tumor cell lines.( 11 , 12 , 13 ) These peptides were dissolved and stored at −80°C. Stock solutions were diluted with saline just before use.
Screening of peptide‐specific CTL precursors. Peripheral blood (30 mL) was obtained before and after the eighth vaccination, and PBMC were isolated by means of Ficoll‐Conray density gradient centrifugation. Peptide‐specific CTL precursors in PBMC were detected using a previously reported culture method.( 13 , 14 ) Briefly, PBMC (1 × 105 cells/well) were incubated with 10 µM of a peptide in 200 µL of culture medium in u‐bottom‐type 96‐well microculture plates (Nunc, Roskilde, Denmark). The culture medium consisted of 45% RPMI‐1640 medium, 45% AIM‐V medium (Gibco BRL, Walkersville, MA, USA), 10% fetal calf serum, 100 IU/mL of interleukin‐2 (IL‐2), and 0.1 µM MEM non‐essential amino acid solution (Gibco BRL). Half of the medium was removed and replaced with new medium containing a corresponding peptide (20 µM) every 3 days. After incubation for 14 days, these cells were harvested and tested for their ability to produce IFN‐γ in response to CIR‐A2402 (kindly provided by Dr M. Takiguchi, Kumamoto University, Japan) or T2 cells that were preloaded with either a corresponding peptide or human immunodeficiency virus (HIV) peptides (RYLRQQLLGI for HLA‐A24 and LLFGYPVYV for HLA‐A2) as a negative control. The level of interferon (IFN)‐γ was determined by enzyme‐linked immunosorbent assay (limit of sensitivity: 10 pg/mL). All assays were carried out in quadruplicate. A two‐tailed Student's t‐test was used for the statistical analyses. A well was considered positive when the level of IFN‐γ production in response to a corresponding peptide was significantly higher (P ≤ 0.05) than that in response to a HIV peptide, and when the mean amount of IFN‐γ production in response to a corresponding peptide was ≥50 ng/mL, compared to that in response to a HIV peptide. Increment of CTL activity was judged as positive if the postvaccination samples, but not the prevaccination samples, showed CTL activity. It was also judged as positive if the level of IFN‐γ produced by the postvaccination sample was greater than two times higher than that produced by the prevaccination sample.
Screening of peptide‐specific IgG. The levels of antipeptide IgG were measured using the Luminex system (Austin, TX, USA), as reported previously.( 13 , 15 ) In brief, plasma was incubated with 25 µL of peptide‐coupled color‐coded beads for 2 h at room temperature on a plate shaker. After incubation, the mixture was washed with a vacuum manifold apparatus and incubated with 100 µL of biotinylated goat antihuman IgG (γ‐chain specific) for 1 h at room temperature. The plate was then washed, followed by the addition of 100 µL of streptavidin‐phycoerythrin (PE), into wells, and was incubated for 30 min at room temperature on a plate shaker. The bound beads were washed three times followed by the addition of 100 µL of Tween–phosphate‐buffered saline into each well. Each sample (50 µL) was then analysed using the Luminex system. Values of postvaccination plasma that were >50 fluorescence intensity units (FIU) compared to those of prevaccination plasma were considered to be elevated.
Cytotoxicity assay. Cytotoxic activity was measured using a standard 6‐h 51Cr‐release assay, as reported previously.( 10 ) In brief, YPK‐1 (HLA‐A24+A2− pancreatic carcinoma), Panc‐1 (HLA‐A24−A2+ pancreatic adenocarcinoma), and phytohemagglutinin‐blastoid T cells (HLA‐A24+ or HLA‐A2+) were used as target cells. Plasma levels of peptide‐specific IgG were measured using the Luminex system, as reported previously.( 8 ) FIU of IgG antibodies reactive to the corresponding peptide were used as the indication score, and the limit of sensitivity of this assay was set at 5 FIU.( 13 ) According to the results of both peptide‐specific IgG level measurements and CTL precursor assays, up to four positive peptides were selected for each patient and used as the personalized peptide vaccine. The screening of peptide‐specific CTL precursors and IgG level was also carried out using the same method after eight vaccinations to evaluate the in vivo cellular and humoral responses to the peptides.
Adverse events and clinical responses. Adverse events were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. The clinical response was evaluated based on clinical observations and radiological findings. All known sites of disease were evaluated on a monthly basis by computer tomography (CT) scan or magnetic resonance imaging (MRI) examination before and after each cycle. The tumor size was estimated via direct measurement of the region of abnormal enhancement observed by CT scan or MRI examination. Patients were assigned a response category according to the Response Evaluation Criteria in Solid Tumors (RECIST). Survival time was estimated from the date of the initial vaccination.
Results
Patient demographics and vaccinated peptides. Fifteen patients were enrolled into this trial, and 13 patients received more than eight vaccinations (one cycle); the remaining two patients received one and three vaccinations, respectively, due to rapid disease progression. Therefore, 13 patients were assessable for toxicity, immunological and clinical evaluations. Patients’ characteristics are listed in Table 1. Ten (77%), three (23%) and three (23%) patients had metastatic disease, recurrent disease and locally advanced pancreatic cancer, respectively. Nine patients had received prior chemotherapy, seven of them with GEM.
Table 1.
Patient characteristics
| No. | HLA | Age (years) | Sex | PS | Primary site | Site of metastasis | Previous treatment | |
|---|---|---|---|---|---|---|---|---|
| Surgery | Chemotherapy/ chemoradiotherapy | |||||||
| 1 | A24 | 65 | M | 1 | Body | Liver | None | GEM |
| 2 | A2 | 57 | M | 0 | Body | Liver | None | GEM/40Gy |
| 3 | A2 | 75 | M | 1 | Pbt | Liver | PD | None |
| 4 | A24 | 72 | M | 1 | Body | Liver | None | None |
| 5 | A24 | 51 | F | 1 | Tail | Liver | DP | GEM |
| Peritoneum | ||||||||
| 6 | A24 | 69 | M | 0 | Pbt | None | None | GEM |
| 7 | A24 | 70 | F | 1 | Pbt | None | None | GEM |
| 8 | A24 | 72 | F | 0 | Pbt | Liver | DP | None |
| 9 | A24 | 59 | M | 1 | Head | Liver | None | None |
| Lung | ||||||||
| 10 | A2 | 41 | F | 1 | Tail | Liver | None | S‐1/CDDP |
| Lung | ||||||||
| Bone | ||||||||
| 11 | A2 | 68 | M | 0 | Body | Liver | None | S‐1/CDDP |
| 12 | A24 | 53 | M | 1 | Head | None | None | GEM/40Gy |
| 13 | A24 | 62 | M | 0 | Head | Liver | None | GEM/40Gy |
D, pancreatoduodenectomy; DP, distal pancreatectomy; GEM, gemcitabine; GEM/40 Gy, chemoradiotherapy; HLA, human leukocyte antigen; Pbt, body and tail of pancreas; PS, performance status by ECOG score.
For the selection of peptides for vaccination, prevaccination PBMC and plasma were provided to investigate their reactivity to each of the 25 or 23 peptides in the HLA‐A24 patients (n = 9) or HLA‐A2 patients (n = 4), respectively. A summary of the peptides administered is shown in Table 2. Two peptides, in which the highest and the second highest levels of IgG had been detected, were administered as the first and second vaccinations, as plasma levels of IgG were measured within 2 days after entry into the study. The remaining two peptides were administered (total four peptides) from the third to eighth vaccinations, based on the results of screening of CTL activity for the following criteria: a peptide recognized by both PBMC and plasma; a peptide recognized by PBMC; and a peptide recognized by plasma. This delayed choice of two peptides was primarily due to the fact that 18 days are required for the accomplishment of the CTL assay after entry into the study. Interestingly, the vaccinated peptide profiles of all 13 patients were entirely different.
Table 2.
Immunological responses and clinical outcomes
| No. | Vaccinated peptides | CTL response† | IgG‡ | No. vaccination | Clinical response | TTP (days) | OS (days) | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post 8th | Pre | Post 8th | ||||||
| 1 | SART2‐899 | NT | NT | 60 | 35 | 8 | PD | 40 | 108 |
| SART3‐109 | NT | 503 | 408 | ||||||
| MRP3‐1293 | NT | NT | 45 | 25 | |||||
| EGFR‐124 | NT | NT | 7 | 5 | |||||
| NT | |||||||||
| 2 | UBE‐43 | 0 | 89 | 475 | 551 | 31 | SD | 118 | 270 |
| EIF‐51 | 2733 | 560 | 236 | 203 | |||||
| CypB‐129 | 0 | 23 | 329 | 271 | |||||
| EGFR‐479 | 0 | 0 | 81 | 57 | |||||
| 3 | Lck‐422 | 0 | 0 | 92 | 78 | 8 | PR | 119 | 205 |
| MAP‐294 | 0 | 0 | 35 | 35 | |||||
| EGFR‐479 | 0 | 51 | 92 | 74 | |||||
| EZH2‐569 | 0 | 183 | 63 | 0 | |||||
| 4 | SART3‐109 | 1283 | 0 | 330 | 326 | 16 | SD | 113 | 196 |
| HER2/neu‐553 | 963 | 0 | 36 | 41 | |||||
| CypB‐91 | 0 | 0 | 38 | 25 | |||||
| PSCA‐76 | 0 | 2067 | 23 | 25 | |||||
| 5 | SART3‐109 | 0 | 0 | 363 | 675 | 13 | PD | 154 | 232 |
| MRP3‐503 | 0 | 0 | 94 | 808 | |||||
| SART2‐93 | 0 | 0 | 11 | 15 | |||||
| HER2/neu‐553 | 109 | 120 | 95 | 104 | |||||
| 6 | SART3‐109 | 0 | 52 | 513 | 1866 | 63+ | PR | 503+ | 503+ |
| HER2/neu‐553 | 0 | 0 | 24 | 30 | |||||
| EZH2‐735 | 858 | 0 | 14 | 25 | |||||
| CypB‐91 | 0 | 1078 | 18 | 20 | |||||
| 7 | SART3‐109 | 206 | 791 | 449 | 22 696 | 17 | SD | 130 | 152 |
| EZH2‐291 | 0 | 348 | 109 | 23 930 | |||||
| CypB‐91 | 307 | 0 | 35 | 38 | |||||
| MRP3‐1293 | 0 | 0 | 81 | 87 | |||||
| 8 | SART3‐109 | 0 | 0 | 318 | 268 | 44+ | SD | 458+ | 458+ |
| SART2‐93 | 0 | 0 | 9 | 3 | |||||
| PTHrP‐102 | 0 | 196 | 113 | 99 | |||||
| MRP3‐503 | 0 | 62 | 66 | 39 | |||||
| 9 | SART2‐93 | 0 | 0 | 2 | 3 | 13 | SD | 99 | 238 |
| SART3‐109 | 0 | 816 | 171 | 227 | |||||
| CypB‐91 | 0 | 0 | 16 | 9 | |||||
| Lck‐486 | 0 | 262 | 15 | 18 | |||||
| 10 | PSCA‐21 | 0 | 0 | 196 | 498 | 8 | PD | 61 | 80 |
| EZH2‐569 | 801 | 0 | 159 | 4813 | |||||
| EIF‐51 | 0 | 0 | 100 | 156 | |||||
| MAP‐294 | 32 | 0 | 70 | 362 | |||||
| 11 | SART3‐302 | 0 | 0 | 53 | 52 | 37 | SD | 272 | 365 |
| UBE‐85 | 0 | 0 | 45 | 48 | |||||
| MAP‐432 | 157 | 350 | 7 | 3 | |||||
| EZH2‐569 | 0 | 0 | 40 | 114 | |||||
| 12 | SART3‐109 | 0 | 299 | 233 | 617 | 19 | SD | 186 | 276+ |
| Lck‐486 | 0 | 0 | 42 | 29 | |||||
| HER2/neu‐553 | 47 | 744 | 41 | 32 | |||||
| PTHrP‐102 | 0 | 0 | 36 | 30 | |||||
| 13 | SART2‐161 | 0 | 0 | 7 | 0 | 16 | PD | 68 | 187 |
| SART3‐109 | 0 | 0 | 424 | 519 | |||||
| Lck‐486 | 0 | 0 | 143 | 140 | |||||
| HER2/neu‐553 | 153 | 57 | 116 | 96 | |||||
Values indicate interferon (IFN)‐γ production of peripheral blood mononuclear cells (PBMC) reactive to the corresponding peptide (pg/mL). A two‐tailed Student's t‐test was used for the statistical analysis. A well was considered positive when the level of IFN‐γ production in response to a corresponding peptide was significantly higher (P < 0.05) than that in response to an HIV peptide, and also when the mean amount of IFN‐γ production in response to a corresponding peptide was >50 ng/mL, compared to an HIV peptide. Increment of cytotoxic T lymphocyte (CTL) activity was judged as positive if the postvaccination, but not prevaccination, samples showed CTL activity. It was also judged as positive if the level of IFN‐γ produced by the postvaccination sample was greater than two times higher than that by the prevaccination sample. The values showing the increment are shown in italics.
Plasma levels of peptide‐specific IgG were measured using the Luminex system as reported previously.( 13 , 15 ) Values indicate fluorescence intensity units (FIU) of IgG antibodies reactive to the corresponding peptide. The values of postvaccination plasma that were >50 FIU compared with those of prevaccination plasma were considered to be elevated. The values showing positive responses are shown in italics. NT, not tested; OS, overall survival; PD, progression disease; Post 8th, evaluation after the 8th vaccination; PR, partial response; SD, stable disease; TTP, time to progression.
For the patients who entered the second cycle, the post (eight vaccinations) samples were also provided for the screening, and all four peptides were chosen based on the same criteria described above for cycle 1 for the remaining two peptides, and these four peptides were administered at least eight times as the second cycle. The same process was repeated for patients who entered the third cycle.
Toxicity and treatment cycles. A total of 31 vaccination cycles plus GEM were administered to 13 patients; the median number of cycles was two (range: one to six). There was no grade 4 toxicity. One patient in level 1 had grade 3 thrombocytopenia or anemia, whereas 1 patient in level 2 had grade 3 neutropenia, anemia or thrombocytopenia. One patient in level 3 had grade 3 neutropenia or anemia. All 13 patients had grade 1 or 2 dermatological reactions at the vaccination site. No grade ≥3 non‐hematological toxicities were observed. No DLT leading to MTD was observed at any level. These results indicate that this protocol was generally well tolerated.
Immune responses. Peptide‐specific cellular and humoral immune activities were measured at 8‐ and 4‐week intervals in the 13 evaluable patients. The peptides used for vaccination and the corresponding immune responses are described in Table 2.
Augmentation of peptide‐specific CTL responses in postvaccination PBMC (eighth vaccination). At least one of the vaccinated peptides increased the level of INF‐γ production in 2/2, 2/3 or 5/7 patients at levels 1 (patients 2 and 3), 2 (patients 4–6) or 3 (patients 7–13), respectively. The same results were obtained by means of the 51Cr‐relase assay, except for patient 5 whose postvaccination PBMC showed CTL activity by means of 51Cr‐relase assay when stimulated with the HER2/neu‐553 peptide; representative results are shown in Fig. 1.
Figure 1.
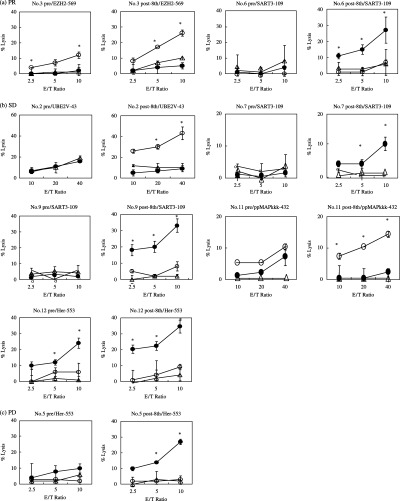
Cytotoxicity before and after the peptide vaccination. Pre‐ and postvaccination peripheral blood mononuclear cells (PBMC) were incubated for 21–25 days with interleukin‐2 (IL‐2) and the corresponding peptide in culture followed by measurement of their cytotoxicity against YPK‐1 (‐•‐) (HLA‐A24 + A2– pancreatic carcinoma), Panc‐1 (□‐○‐□) (HLA‐A24‐A2 + pancreatic adenocarcinoma) and phytohemagglutinin (PHA)‐blastoid T cells (‐□‐) (HLA‐A24+ or HLA‐A2+) by a 6‐h 51Cr‐release assay at effector/target (E/T) ratios of 2.5/1, 5/1 and 10/1 (patient no. 3, 5–7, 9 and 12) or 10/1, 20/1 and 40/1 (patient no. 2 and 11). The assay was carried out in triplicate, and the mean and standard deviations are shown. *A two‐tailed Student's t‐test (P < 0.05) was used for the statistical analysis.
Increased levels of peptide‐specific IgG titers in postvaccination plasma (eighth vaccination). At least one of the vaccinated peptides was observed in 1/3, 2/3 and 6/7 patients at levels 1, 2 and 3, respectively (Table 2). Kinetic studies of peptide‐specific IgG titers at pre‐ and post‐vaccination (fourth, eighth, 12th and 16th) plasma were available for seven patients (patients 2, 4, 6, 8, 11, 12 and 13), and the representative results are given in Fig. 2. Levels of peptide‐specific IgG depended on the number of vaccinations in all but one patient (patient 2) who received 1 mg/peptide, and very high levels of IgG were found in plasma after the 16th vaccination in four of seven patients (patients 4, 6, 12, 13).
Figure 2.
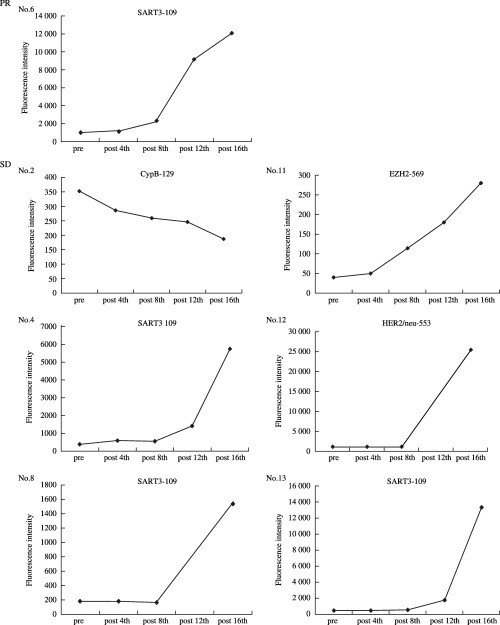
Serial changes of IgG levels specific to the peptides. Pre‐and postvaccination plasma were diluted at 100:1 and the levels of peptide‐specific IgG were measured using the Luminex system, as reported previously. (8 ) Representative results are given of IgG levels obtained from one partial response (PR) and six stable disease (SD) cases in response to the vaccinated peptide.
Clinical responses and correlation to the immune responses. Although the assessment of clinical response was not an objective of this small phase I study, all 13 patients were nevertheless evaluated in order to provide clinical information for future trials. All clinical responses were confirmed by an independent review, and were as follows: two partial responses (PR) (15%), seven stable disease (SD) (54%) and four progressive disease (PD). The disease control rate was 69%. Before treatment, the levels of CA 19‐9 were elevated (>37 IU/L) in 10 of 13 patients. In these same 10 patients after treatment, the levels decreased ≥50% and <50% compared with those before treatment in two (20%) and five (50%) patients, respectively. Eleven of 13 (85%) patients for whom the efficacy of this treatment could be assessed showed clinical responses such as reduction in tumor size and/or tumor marks. After a median follow‐up of 6.7 months (range: 3.1–13 months), the median time to progression (TTP) was 17 weeks (range: 8–59 weeks) and the median survival time (MST) was 7.6 months (range: 3.1–13 months). Three patients are still alive.
From a point of correlation between clinical responses and cellular immune responses, it is of note that augmentation of CTL activity detected by IFN‐γ assay to two, one or two, and no peptides among four peptides were seen in two patients with PR, seven with SD, and four with PD, respectively. In contrast, no definite correlation was seen between clinical responses and humoral responses.
Discussion
We previously reported increased cellular and humoral responses to at least one of the peptides used for personalized vaccination monotherapy, under a biweekly protocol in the postvaccination (sixth) PBMC or plasma from four of eight or four of ten APC patients.( 12 ) One might consider that personalized peptide vaccination combined with GEM would induce lower levels of immune response than the peptide vaccination on its own. Our protocol of combined peptide vaccination and GEM produced the same degree of myelosuppression as GEM monotherapy. Although the doses and intervals of this protocol were different from those of the previous one, it suppressed neither peptide‐specific cellular nor humoral responses compared with vaccination alone.( 12 ) The precise mechanisms of no apparent immunosuppression by GEM are presently unclear. GEM by itself might have a stimulatory effect on the activated or memory T cells.( 16 ) Alternatively, GEM might not impede the function of memory or activated T cells, but suppress naive or inactivated T cells. This assumption, however, is not based on any direct evidence, but is based on the clinical observation showing that the non‐personalized peptide vaccination stimulates naive or resting T cells, while at the same time suppressing memory or activated T cells, whereas personalized peptide vaccination stimulates the latter types of T cells.( 11 , 12 , 13 , 17 , 18 ) Further studies with more patient samples along with basic studies are needed to clarify this issue.
Dose dependency was observed in the induction of humoral response, but not cellular response, suggesting that administration of ≥2 mg/peptide is needed for the induction of IgG reactive to peptides. We previously showed that increased humoral responses to at least one of the vaccinated peptides were observed in the postvaccination (sixth) plasma from seven of 11 patients with advanced brain tumors under weekly administration of 2 mg/peptide.( 13 ) This study showed that increased humoral responses to at least one of the vaccinated peptides were observed in the postvaccination (eighth) plasma from seven of 10 APC patients under weekly administration of 2 mg or 3 mg/peptide. These results suggest that this protocol suppressed neither peptide‐specific cellular nor humoral responses induced by the personalized peptide vaccination. Collectively, increases in cellular or humoral responses specific to at least one of the vaccinated peptides were observed in the postvaccination (eighth) samples of all 12 of 13 patients, regardless of the administration of a full dose of GEM.
Augmentation of CTL activity to two, one or two, and no peptides among four peptides was seen in two patients with PR, seven with SD, and three with PD. Thus, there might be a good correlation between clinical responses and peptide‐induced CTL activity against tumor cells. The same results were obtained in advanced brain tumors under the personalized peptide vaccination.( 13 ) Further studies with more patients are needed to confirm this issue. With regard to overall survival, we previously demonstrated that an increase in peptide‐specific IgG in the plasma of advanced cancer patients is a good laboratory marker for the prediction of improved overall survival of vaccinated patients, although the specific biological roles played by peptide‐specific IgG are presently unclear.( 17 ) However, we also found that the increased levels of peptide‐specific IgG did not correlate with clinical responses evaluated by tumor reduction.( 13 , 17 ) Those previous results are most likely consistent with the results shown in this trial. Further studies are required to confirm this issue as this phase I trial had very small numbers of APC patients and limited observation periods.
The present study involved two patients with newly diagnosed pancreatic cancer, three patients with recurrent disease (one patient had received adjuvant chemotherapy for 1 year and had disease progression after the chemotherapy using GEM) and seven patients who had already been treated with chemotherapy (six with PD after the previous chemotherapy). In spite of the fact that seven patients (54%) had previously failed chemotherapy, including GEM, in our study the disease control rate was 69% and the MST was 7.6 months. Therefore, it appears that the combination of peptide vaccine and GEM provided an additive and synergistic effect, leading to an enhancement of antitumor activity. The vast majority of patients (12/13) received this treatment as outpatients, and their performance status remained good throughout the treatment periods.
In conclusion, this protocol of personalized peptide vaccine with GEM was well tolerated. It is noteworthy that both cellular and humoral responses to the immunizing peptides were observed in the vast majority of patients, regardless of whether they had received a full dose of GEM. The survival benefit in comparison with GEM alone needs to be confirmed in future clinical studies.
References
- 1. Rothenberg ML, Moore MJ, Cripps MC et al. A phase II trial of gemcitabine in patients with 5‐FU‐refractory pancreas cancer. Ann Oncol 1996; 7: 347–53. [DOI] [PubMed] [Google Scholar]
- 2. Burris HA III, Moore MJ, Andersen J et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 3. Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB III. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002; 20: 3270–5. [DOI] [PubMed] [Google Scholar]
- 4. Rocha Lima CM, Green MR, Rotche R et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004; 22: 3776–83. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Van De Velde H, Karasek P et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004; 22: 1430–8. [DOI] [PubMed] [Google Scholar]
- 6. Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA, Marimastat Pancreatic Cancer Study Group. Marimastat as first‐line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 2001; 19: 3447–55. [DOI] [PubMed] [Google Scholar]
- 7. Moore MJ, Hamm J, Dancey J et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12‐9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21: 3296–302. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 1999; 10: 281–7. [DOI] [PubMed] [Google Scholar]
- 9. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitano H. Cancer as a robust system: implications for anticancer therapy. Nat Rev 2004; 4: 227–35. [DOI] [PubMed] [Google Scholar]
- 11. Noguchi M, Itoh K, Yao A et al. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA‐A24+ HRPC patients. Prostate 2005; 63: 1–12. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto K, Mine T, Katagiri K et al. Immunological evaluation of personalized peptide vaccination for patients with pancreatic cancer. Oncol Rep 2005; 13: 874–83. [PubMed] [Google Scholar]
- 13. Yajima N, Yamanaka R, Mine T et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11: 5900–11. [DOI] [PubMed] [Google Scholar]
- 14. Hida N, Maeda Y, Katagiri K et al. A new culture protocol to detect peptide‐specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother 2002; 51: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometric assay to measure anti‐peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Invest 2004; 64: 1–11. [DOI] [PubMed] [Google Scholar]
- 16. Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo‐immunotherapy. Cancer Res 2002; 62: 2353–8. [PubMed] [Google Scholar]
- 17. Mine T, Sato Y, Noguchi M et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre‐existing, peptide‐specific cellular responses. Clin Cancer Res 2002; 10: 929–37. [DOI] [PubMed] [Google Scholar]
- 18. Mochizuki K, Sato Y, Tsuda N et al. Immunological evaluation of vaccination with pre‐designated peptides frequently selected as vaccine candidates in an individualized peptide vaccination regimen. Int J Oncol 2004; 25: 121–31. [PubMed] [Google Scholar]


