Abstract
Adenovirus‐mediated gene therapy is a promising approach for the treatment of pancreatic cancer. We previously reported that radiation enhanced adenovirus‐mediated gene expression in pancreatic cancer, suggesting that adenoviral gene therapy might be more effective in radioresistant pancreatic cancer cells. In the present study, we compared the transduction efficiency of adenovirus‐delivered genes in radiosensitive and radioresistant cells, and investigated the underlying mechanisms. We used an adenovirus expressing the hepatocyte growth factor antagonist, NK4 (Ad‐NK4), as a representative gene therapy. We established two radioresistant human pancreatic cancer cell lines using fractionated irradiation. Radiosensitive and radioresistant pancreatic cancer cells were infected with Ad‐NK4, and NK4 levels in the cells were measured. In order to investigate the mechanisms responsible for the differences in the transduction efficiency between these cells, we measured expression of the genes mediating adenovirus infection and endocytosis. The results revealed that NK4 levels in radioresistant cells were significantly lower (P < 0.01) than those in radiosensitive cells, although there were no significant differences in adenovirus uptake between radiosensitive cells and radioresistant cells. Integrin β3 was up‐regulated and the Coxsackie virus and adenovirus receptor was down‐regulated in radioresistant cells, and inhibition of integrin β3 promoted adenovirus gene transfer. These results suggest that inhibition of integrin β3 in radioresistant pancreatic cancer cells could enhance adenovirus‐mediated gene therapy. (Cancer Sci 2009; 100: 1902–1907)
Pancreatic cancer is a leading cause of cancer‐related death in industrial countries.( 1 , 2 ) Most patients with pancreatic cancer have poor outcomes because early diagnosis is difficult and because conventional therapies have limited efficacies.( 3 ) Recent advances in our understanding of the genetics and epigenetics of pancreatic cancer have revealed that alterations in several tumor‐related genes, including K‐ras, p53, MMP, hepatocyte growth factor (HGF), and epidermal growth factor receptor,( 4 , 5 , 6 , 7 , 8 , 9 ) may underlie the aggressiveness of this neoplasm and its resistance to conventional therapies.( 10 ) Gene therapy therefore provides a promising new approach for treating this often fatal disease. Adenoviruses are among the most commonly used vectors for gene therapy because their gene delivery system is well understood.( 11 ) Adenoviruses bind to cells via the Coxsackie virus and adenovirus receptor (CAR),( 12 ) after which they are rapidly internalized via interactions between the penton base capsid protein and the cell integrins αvβ3 and αvβ5.( 13 , 14 ) Adenovirus internalization also requires dynamin 2,( 15 ) a GTPase involved in the formation of clathrin‐coated pits. Many investigators have used adenovirus‐mediated gene transfer to treat pancreatic cancer, and have reported that adenovirus‐mediated gene therapy inhibited the progression of pancreatic cancer both in vivo and in vitro. ( 16 , 17 ) However, clinical trials have revealed that it is difficult to eradicate pancreatic tumors using adenovirus‐mediated gene therapy alone,( 18 , 19 ) and it may be necessary to select suitable cases in order to maximize the antitumor effects of adenovirus‐mediated gene therapies in pancreatic cancer.
A combination of radiotherapy and adenovirus‐mediated gene therapy has recently been reported to be effective for cancer treatment. Shi et al.( 20 ) found that adenovirus‐mediated gene therapy targeting endostatin enhanced the antitumor effect of radiation therapy in colorectal cancer. Similarly, Geoerger et al.,( 21 ) Portella et al.,( 22 ) and Rogulski et al.( 23 ) reported that ONYX‐015 (an E1B‐55 kDa gene‐deleted adenovirus that replicates selectively in and lyses tumor cells with abnormalities in p53 function) combined with radiation therapy was a promising treatment for gliomas and thyroid cancers, and that these combination therapies produced synergistic effects. Accumulating evidence has shown that HGF accelerates the invasion of pancreatic cancer cells.( 6 , 24 , 25 ) We previously reported that gene therapy with an adenovirus vector expressing NK4 (Ad‐NK4), which acts as a HGF antagonist, significantly inhibited the invasion of pancreatic cancer cells.( 26 , 27 , 28 ) More recently, we reported that the combination of radiation and Ad‐NK4 enhanced NK4 expression in pancreatic cancer.( 29 ) However, it remains unknown if pancreatic cancer cells with acquired radioresistance are also suitable for adenovirus gene therapy.
In the present study, we compared the efficiency of transfer and expression of an adenovirus‐mediated target gene in radioresistant and radiosensitive pancreatic cancer cells. We also investigated the expression levels of genes that mediate adenovirus‐cell attachment and internalization and examined the function of these genes using RNA interference.
Materials and Methods
Cells. The human pancreatic cancer cell line CFPAC‐1 (American Type Culture Collection, Rockville, MD, USA) was cultured in DMEM supplemented with streptomycin, penicillin, and 10% fetal bovine serum (FBS) at 37°C in 5% CO2.
Radiation treatment. Cells were irradiated with a dose of 2 or 5 Gy at room temperature with a 137Cs source (Gamma Cell 40; Atomic Energy of Canada, Ontario, Canada) at a delivery rate of 1.0 Gy/min.
Establishment of radioresistant cell lines. We established two radioresistant cell lines. CFPAC‐1 parent cells were irradiated with 2 Gy every 4 days for four doses and with 5 Gy every 20 days for six doses, to establish one radioresistant CFPAC‐1 cell line (R1). The same CFPAC‐1 parent cell line was irradiated with 2 Gy every 4 days for four doses and with 5 Gy every 20 days for seven doses to establish a second radioresistant CFPAC‐1 cell line (R2).
Cell proliferation assay. Cell proliferation was evaluated by propidium iodide (PI) fluorescence intensity, as described previously.( 30 ) Cells were counted using a PDA‐500 cell counter (Sysmex, Kobe, Japan). Cells were plated at 2 × 104 cells/well in 24‐well tissue culture plates (Becton Dickinson Labware, Bedford, MA, USA), cultured overnight and then irradiated. PI (30 µM) and digitonin (600 µM) were added to each well to label all nuclei with PI. Fluorescence intensity, corresponding to total cells, was measured with a CytoFluor II multi‐well plate reader (PerSeptive Viosystems, Framingham, MA, USA) with 530‐nm excitation and 645‐nm emission filters. Cell proliferation was defined as the ratio of fluorescence intensity at a given time‐point relative to that measured at the beginning of the experiment. All experiments were performed in triplicate wells.
Construction of recombinant adenovirus. A recombinant adenovirus vector expressing human NK4 (Ad‐NK4) was constructed as described previously.( 31 ) In brief, Ad‐NK4 was generated by homologous recombination of the pJM17 plasmid( 32 ) and the shuttle plasmid vector pSV2 +( 33 ) containing an expression cassette and the cytomegalovirus early promoter/enhancer followed by human NK4 cDNA( 34 ) and a polyadenylation signal. A control vector expressing the bacterial β‐galactosidase (β‐gal) gene (lacZ) was constructed by the same procedure with pJM17 and pCA17, which contains the lacZ gene. Recombinant Ad‐NK4 and Ad‐lacZ were propagated in HEK293 cells.
Adenovirus infection of cells. Cells (2 × 105) were seeded in six‐well plates and cultured in DMEM supplemented with 10% FBS for 24 h. Cells were infected with Ad‐NK4 or Ad‐lacZ at 10 multiplicities of infection (MOI) 24 h after seeding. The culture medium was replaced with fresh medium 1.5 h after transfection.
Extraction of proteins from cells infected with Ad‐NK4. Cells were infected with Ad‐NK4 as described above. Two days after infection with Ad‐NK4, the cells were lysed in 500‐µL ice‐cold lysis buffer (150 mM NaCl, 20 mM Tris‐HCl [pH 7.5], 10 mM EDTA, 5 µg/mL leupeptin, 1 mM phenylmethyl sulfonyl fluoride, and 0.5%[v/v] Triton X‐100). Cell debris was removed by centrifugation at 14 000 g for 20 min at 4°C and supernatants were collected. Protein concentrations were measured using a NanoDrop ND‐1000 Spectrophotometer (NanoDrop Technologies, Rockland, DE, USA) at absorbances of 280 nm, and were adjusted to 1.0 mg/mL with lysis buffer.
NK4 expression by Ad‐NK4‐infected cancer cells. After infection with Ad‐NK4 or transfection with NK4‐expression plasmid, the medium was changed every 24 h. The NK4 concentration in the medium and in the cells were measured by enzyme‐linked immunosorbent assay (ELISA) using a Human HGF ELISA Kit (Immunis HGF EIA, Institute of Immunology, Tokyo, Japan), according to the manufacturer's protocol.
Assessment of transgene distribution by evaluation of β‐gal expression. At 48 h after Ad‐lacZ infection, cells were rinsed twice with phosphate‐buffered saline (PBS) and fixed with 0.25% glutaraldehyde in PBS for 15 min at 4°C. β‐Gal activity was detected by immersing cells in 5‐bromo‐4‐chloro‐3‐indolyl‐β‐galactopyranoside (X‐gal) staining solution (5 mM K4FeCN, 5 mM K3FeCN, and 2 mM MgCl 2 containing 1 mg/mL X‐gal) for 6 h at 37°C.
Real‐time polymerase chain reaction (PCR) and reverse transcription‐PCR (RT‐PCR) assays. The Ad‐lacZ DNA content of infected cells was determined by real‐time PCR analysis, as described previously,( 35 ) using primers for the β‐gal gene (5′‐CACGGC AGATACACTTGCTG‐3′ and 3′‐ATCGCCATTTGACCACTACC‐5′).( 36 ) The number of copies of viral DNA was calculated from a standard curve of purified adenovirus vector (CMV‐β‐gal) and was further adjusted to the protein concentration of each lysate. Integrin β3, CAR, integrin αv, integrin β5, and dynamin 2 mRNA levels were quantified by real‐time RT‐PCR assay using a QuantiTect SYBR Green RT‐PCR Kit (Qiagen, Valencia, CA, USA) with 100 ng of total RNA and primers specific for integrinβ3 (5′‐GAGGATGACTGTGTCGTCAG‐3′ and 3′‐CTGGCGCG TTCTTCCTCAAA‐5′), CAR (5′‐GGCGCTCCTGCTGTGC‐3′ and 3′‐ CTTCTCTACTAACTTTTTCGGTTTC‐5′), integrin αv (5′‐ACTGGGAGCACAAGGAGAACC‐3′ and 3′‐CTGGTAGAG TAGTGATTCGCC‐5′), integrin β5 (5′‐CCTGTCCATGAAGG ATGACTTG‐3′ and 3′‐GTCTCACCTGTCGAAGTTACTC‐5′), and dynamin 2 (5′‐AGGAGTACTGGTTTGTGCTGACTG‐3′ and 3′‐GTGCATGATGGTCTTTGGCATGAG‐5′).( 37 ) Levels of these mRNAs were normalized to those of 18S rRNA amplified with specific primers (5′‐GTAACCCGTTGAACCCCATT and 3′‐GCGATGATGGCTAACCTACC)( 36 ) and expressed as ratios compared with radiosensitive cells.
Inhibition of integrin β3 in cells by RNA interference (RNAi). Cells were transfected with integrin β3‐specific short interfering (si) RNA (B‐Bridge, Mountain View, CA, USA) or control siRNA (Qiagen) using a Nucleofector (Amaxa Biosystems, Cologne, Germany). Cells were then plated at 1 × 106 cells/well in six‐well plates. At 48 h after transfection, the cells were infected with Ad‐NK4 at 10 MOI, as described above. NK4 expression in integrin β3‐specific siRNA‐transfected cells was expressed as a ratio compared with that in control siRNA‐transfected cells.
Statistical analysis. Values are expressed as the mean ± standard deviation (SD). Comparisons between all groups were analyzed using Student's t‐test for comparisons between two groups. The level of statistical significance was set at P < 0.01 or P < 0.05. To confirm the induction results, experiments were repeated at least three times.
Results
Expression of target genes delivered by adenovirus vector in radiosensitive and radioresistant cells. We investigated the radiosensitivity of the parent CFPAC‐1 cells and the two radioresistant cell lines (R1 and R2) by measuring the inhibitory effects of radiation on cell proliferation using a PI assay at 72 h after radiation. As shown in Figure 1(a), we confirmed that parent CFPAC‐1 cells were significantly more sensitive to radiation treatment. We then compared expression of the target NK4 gene in radiosensitive (parent CFPAC‐1) and radioresistant cells (R1 and R2) infected with the Ad‐NK4 adenovirus vector. Parent, R1, and R2 cells (2 × 105 each cell line) were infected with Ad‐NK4 at 10 MOI and NK4 levels were measured 2 days after infection. As shown in Figure 1(b), NK4 expression in both R1 and R2 radioresistant cells was lower than that in the parent radiosensitive cells. Furthermore, NK4 levels in the media were measured on post‐infection days 1, 2, and 3 in order to investigate secreted NK4 levels. NK4 levels in the media peaked on day 2 after transfection (data not shown). As shown in Figure 1(c), NK4 secretion from Ad‐NK4‐treated radioresistant cells was lower than that from Ad‐NK4‐treated radiosensitive cells, similar to the results shown in Figure 1(b). NK4 expression was undetectable in cells that were not infected with Ad‐NK4 (data not shown). These data suggest that radioresistant pancreatic cancer cells expressed lower levels of the adenovirus‐delivered target gene than radiosensitive pancreatic cancer cells.
Figure 1.
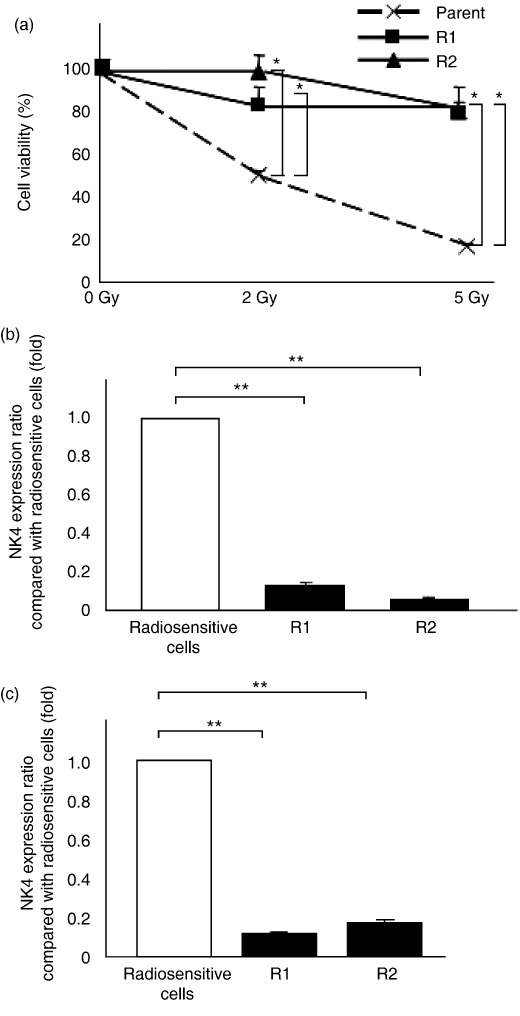
NK4 expression in Ad‐NK4‐treated radioresistant pancreatic cancer cells was much lower than that in Ad‐NK4‐treated radiosensitive pancreatic cancer cells. (a) CFPAC‐1 parent cells, and two established radioresistant pancreatic cancer cells (R1, R2) were plated and irradiated with 2 Gy or 5 Gy. Their survival was determined by propidium iodide assay 72 h after radiation, and defined as the ratio relative to unirradiated cells. Each value represents the mean ± SD of three independent samples. *P < 0.05. (b) Cells were infected with Ad‐NK4 at 10 multiplicities of infection (MOI) and proteins were isolated on post‐infection day 2. NK4 concentrations were determined by enzyme‐linked immunosorbent assay (ELISA) and defined as the ratio relative to radiosensitive cells (CFPAC‐1 parent cells). Each value represents the mean ± SD of three independent samples. **P < 0.01. (c) Cells were infected with Ad‐NK4 at 10 MOI and NK4 levels in the culture media were measured by ELISA on post‐infection day 2 and defined as the ratio relative to radiosensitive cells (CFPAC‐1 parent cells). Each value represents the mean ± SD of three independent samples. **P < 0.01.
β‐Gal expression induced by Ad‐lacZ infection in radiosensitive and radioresistant cells. To investigate the expression of a different gene delivered by an adenovirus vector, we used Ad‐lacZ instead of Ad‐NK4 and examined the activity of β‐gal in the transfected cells. CFPAC‐1, R1, and R2 cells were infected with Ad‐lacZ at 10 MOI. At 48 h after infection, cells were stained for β‐gal. As shown in Figure 2(A), many radiosensitive parent cells showed the characteristic blue staining indicative of β‐gal activity, but only a small number of radioresistant cells (both R1 and R2) were positive for β‐gal. There were significantly fewer β‐gal‐positive cells in five independent fields of radioresistant cells, compared with radiosensitive cells (Fig. 2B, P < 0.01). These data are consistent with the results described above (Fig. 1).
Figure 2.
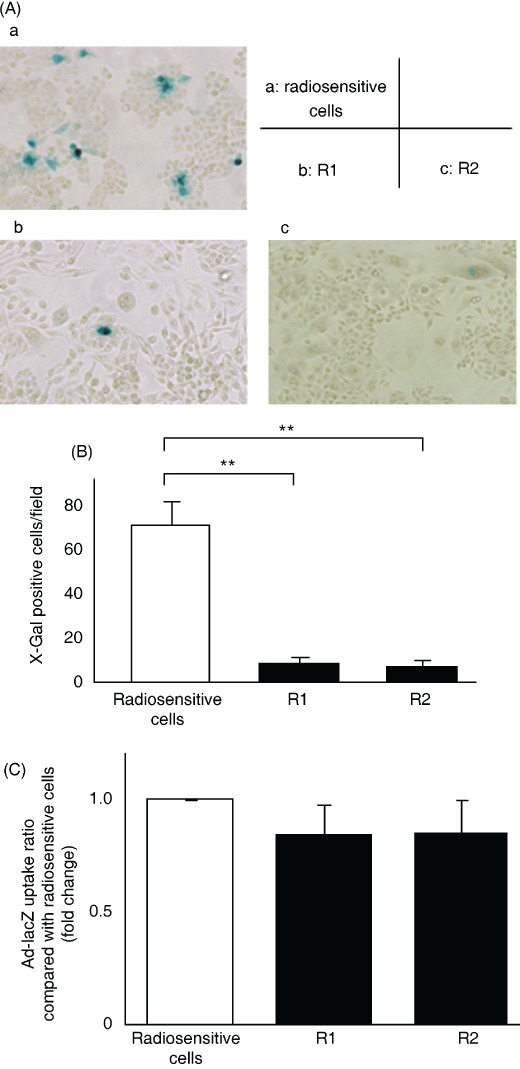
β‐Galactosidase (β‐gal) expression in Ad‐lacZ‐treated radioresistant cells was lower than that in Ad‐lacZ‐treated radiosensitive cells, but there were no differences in adenovirus uptake between radioresistant and radiosensitive pancreatic cells. Cells were infected with Ad‐lacZ at 10 multiplicities of infection (MOI) and β‐gal activity was assessed by X‐gal staining at 48 h after infection. (A) Photomicrographs of X‐gal‐stained radiosensitive or radioresistant cells, ×100. a, CFPAC‐1 parent cells; b, R1 cells; c, R2 cells. (B) Number of β‐gal‐positive cells. Each value represents the mean ± SD of five independent fields. **P < 0.01. (C) Cells were infected with Ad‐lacZ at 10 MOI and DNA was extracted at 24 h after infection. Viral DNA content was quantified by real‐time PCR and defined as the ratio compared with radiosensitive cells. Each value represents the mean ± SD of triplicate measurements.
Adenovirus uptake in radiosensitive and radioresistant cells. We investigated adenovirus uptake in the different pancreatic cancer cells by quantifying viral DNA content in cells as previously reported.( 29 , 35 , 37 ) CFPAC‐1, R1, and R2 cells were infected with Ad‐lacZ at 10 MOI. At 24 h after infection, the viral DNA content was quantified by real‐time PCR. As shown in Figure 2(C), there were no significant differences between the viral DNA contents of these three cell lines. These data suggest that there were no differences in adenovirus uptake between radiosensitive and radioresistant cells.
Expression of CAR and integrin β3 in radiosensitive and radioresistant pancreatic cancer cells. To compare the expression of genes mediating adenovirus attachment or internalization in radiosensitive and radioresistant pancreatic cancer cells, we quantified CAR, dynamin 2, integrin αv, integrin β3, and integrin β5 mRNA levels in CFPAC‐1, R1, and R2 cells using real‐time RT‐PCR. We found that CAR mRNA expression was significantly lower in radioresistant cells (R1, P = 0.006; R2, P = 0.006) than in radiosensitive cells (Fig. 3A). We also found that radioresistant cells expressed much higher levels of integrin β3 mRNA than radiosensitive cells (R1, 66.05 ± 9.80‐fold, P = 0.0001; R2, 119.56 ± 34.94‐fold, P = 0.007) (Fig. 3A). There were no significant differences in mRNA levels for dynamin 2, integrin αv (data not shown), and integrin β5 (Suppl. Fig. 1).
Figure 3.
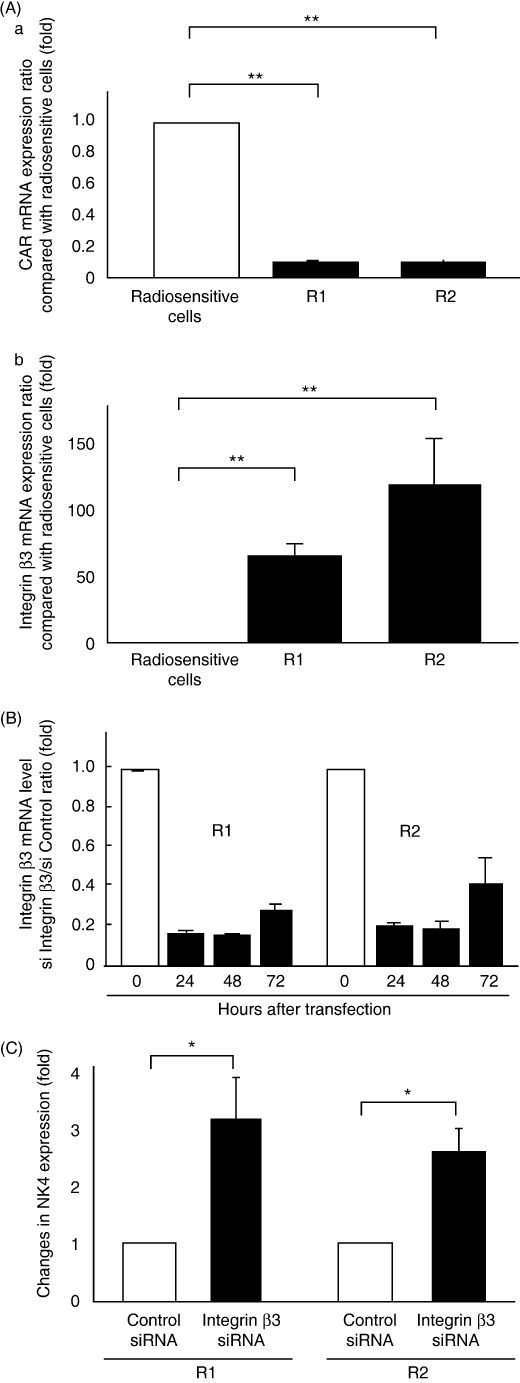
Lower expression of Coxsackie virus and adenovirus receptor (CAR) and much higher expression of integrin β3 in radioresistant cells were associated with decreased adenovirus gene expression (A) (a) CAR and (b) integrin β3 mRNA expression in cells. CAR and integrin β3 mRNA were quantified by real‐time RT‐PCR from total RNA from radiosensitive cells (CFPAC‐1 parent cells) or radioresistant cells (R1, R2) and defined as the ratio relative to radiosensitive cells. (B and C) Inhibition of integrin β3 mRNA expression by siRNA‐recovered NK4 expression in radioresistant cells. (B) Integrin β3 mRNA was quantified by real‐time RT‐PCR from total cellular RNA from integrin β3‐specific siRNA‐transfected cells or control siRNA‐transfected cells at 24, 48, and 72 h after transfection and was expressed as fold‐decrease compared with control siRNA cells. Each value represents the mean ± SD of triplicate measurements. (C) Radioresistant cells were transfected with integrin β3‐specific siRNA or control siRNA and infected with Ad‐NK4 at 10 multiplicities of infection at 48 h after transfection. NK4 levels in the culture media were measured by enzyme‐linked immunosorbent assay and expressed as fold‐increase compared with control siRNA cells. Each value represents the mean ± SD of three independent samples. *P < 0.05.
We used siRNA to inhibit integrin β3 expression in radioresistant cells to determine whether the level of integrin β3 expression affected virus‐mediated gene transfer. We found that integrin β3 mRNA expression was significantly inhibited in R1 and R2 cells transfected with integrin β3‐specific siRNA compared with cells transfected with control siRNA (Fig. 3B). The four cell lines (R1 transfected with control siRNA, R1 transfected with integrin β3‐specific siRNA, R2 transfected with control siRNA, and R2 transfected with integrin β3‐specific siRNA) were infected with Ad‐NK4 at 48 h after transfection of the indicated siRNAs. NK4 expression in cells transfected with integrin β3‐specific siRNA was significantly higher than in cells transfected with control siRNA (R1, 3.09 ± 0.71‐fold; R2, 2.55 ± 0.41‐fold) (Fig. 3C). These data suggest that up‐regulation of integrin β3 in radioresistant pancreatic cancer cells prevents adenovirus‐mediated gene expression. Integrin β5 mRNA expression was not changed in R1 and R2 cells transfected with integrin β3‐specific siRNA compared with cells transfected with control siRNA (Suppl. Fig. 2).
Discussion
Previous studies have suggested that radiation could improve the efficiency of gene therapy in many cancers. Zhang et al.( 37 ) reported that radiation improved gene transfer efficiency in human colon, breast, and brain cancer cells, and we also reported similar results for human pancreatic cancer cells.( 29 ) However, whether pancreatic cancer cells with acquired radioresistance are also suitable for adenovirus‐mediated gene therapy remains unknown.
In the present study, we found that adenovirus‐mediated gene expression in radioresistant cells was lower than that in radiosensitive cells, suggesting that some improvements to enhance adenovirus gene transfer are required when gene therapy is performed following radiation therapy.( 38 ) Furthermore, radioresistant cells expressed lower levels of CAR, which mediates adenovirus‐binding,( 39 ) and much higher levels of integrin β3, which mediates adenovirus‐endocytosis,( 39 ) than radiosensitive pancreatic cancer cells. These data suggest that the radiosensitivity of pancreatic cancer might be associated with the adenovirus–endocytosis pathway, as well as with adenovirus‐binding to the cell surface. Further understanding of this pathway might be helpful for selecting patients for adenovirus gene therapy, or improving the efficiency of adenovirus‐mediated gene therapy.
Adenovirus attaches to cells via CAR, and internalizes through integrin αvβ3 and integrin αvβ5.( 39 ) In the present study, we found that the level of CAR expression was lower in radioresistant cells than in radiosensitive cells. However, we found no difference in adenovirus uptake between radioresistant and radiosensitive cells. The present data also demonstrated that the level of integrin β3 was much higher in radioresistant cells than in radiosensitive cells. These data suggest that up‐regulation of integrin β3 might compensate for the decrease of adenovirus uptake induced by down‐regulation of CAR. The present data also revealed that Ad‐NK4‐treated radioresistant cells expressed much lower levels of NK4 than Ad‐NK4‐treated radiosensitive cells although there was no difference in adenovirus uptake. Following internalization of adenovirus into cells, to penetrate the barrier of the host cell membrane, the adenovirus then disrupts cell endosomes,( 40 ) allowing partially uncoated virions to be released into the cytoplasm where they transit to nuclear pore complexes.( 14 , 41 ) Recent studies have shed some light on the mechanisms whereby the adenovirus penetrates the host cell plasma membrane. Wang et al.,( 14 ) Wickham et al.,( 42 ) and Majhen et al.( 43 ) showed that only integrin αvβ5 selectively facilitated adenovirus‐mediated membrane permeabilization and endosome rupture, although both integrin αvβ3 and αvβ5 promoted adenovirus internalization into cells. Our data revealed that integrin β3 was expressed at 66–120‐fold higher levels in radioresistant cells than in radiosensitive cells, while there were no significant differences in integrin αv and β5 expression. We also found that adenovirus gene transfer efficiency in radioresistant cells recovered following inhibition of integrin β3 expression. Therefore, there is a possibility that overexpression of integrin β3 in radioresistant cells interferes with the formation of αvβ5 complexes, leading to inhibition of integrin αvβ5‐induced adenovirus escape from endosomes and decrease of NK4 expression in radioresistant cells.
In clinics, combinations of adenovirus gene therapy and conventional therapies targeting CAR( 44 ) and integrin( 45 , 46 ) could be promising new strategies for the treatment of pancreatic cancer, although inhibition of integrin β3 may induce side effects, such as cardiovascular diseases, bleeding disorders,( 47 , 48 ) and osteoporosis.( 49 ) Further studies regarding the inhibition of integrin β3 in vivo are required.
RNAi has been heralded as a great therapeutic intervention for gene medicine against a wide range of human diseases. To deliver the siRNA in vivo, some carriers, such as liposome( 50 , 51 ) and atelocollagen,( 52 , 53 ) making complexes with siRNA have been studied. Therefore, to inhibit integrin β3 in situ, systemic or local administration of siRNA complexed with such carriers may be useful. Such additional therapies to inhibit integrin β3 may improve adenovirus gene expression in radioresistant pancreatic cancer.
Although our results partially explain the mechanisms responsible for the low efficiency of adenovirus gene transfer in radioresistant pancreatic cancer cells, the detailed mechanisms controlling adenovirus gene delivery systems in pancreatic cancer cells remain unclear. Further investigations into the underlying mechanisms are therefore required not only to enhance the gene therapy in radioresistant cases, but also to provide the information for selection of individual cases suitable for adenovirus gene therapy or to establish new therapeutic viruses or drug delivery systems.
Supporting information
Fig. S1. Integrin β5 mRNA expression in cells. The levels of integrin β5 mRNA in radiosensitive cells (CFPAC‐1 parent cells) or radioresistant cells (R1, R2) were quantified by real‐time RT‐PCR and defined as the ratio relative to radiosensitive cells. Each value represents the mean ± SD of triplicate measurements.
Fig. S2. The levels of integrin β5 mRNA in integrin β3‐specific siRNA‐transfected cells or control siRNA‐transfected cells at 48 h after transfection was quantified by real‐time RT‐PCR and was expressed as fold‐change compared with control siRNA cells. Each value represents the mean ± SD of triplicate measurements.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgement
This study was supported in part by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Gunzburg WH, Salmons B. Novel clinical strategies for the treatment of pancreatic carcinoma. Trends Mol Med 2001; 7: 30–7. [DOI] [PubMed] [Google Scholar]
- 2. Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Eng J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 3. Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13 560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995; 82: 111–5. [DOI] [PubMed] [Google Scholar]
- 4. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nature Rev 2002; 2: 897–909. [DOI] [PubMed] [Google Scholar]
- 5. Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell 2002; 2: 25–8. [DOI] [PubMed] [Google Scholar]
- 6. Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 1995; 55: 1129–38. [PubMed] [Google Scholar]
- 7. Bloomston M, Zervos EE, Rosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol 2002; 9: 668–74. [DOI] [PubMed] [Google Scholar]
- 8. Sato N, Goggins M. The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg 2006; 13: 286–95. [DOI] [PubMed] [Google Scholar]
- 9. Jimeno A, Hidalgo M. Molecular biomarkers: their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Mol Cancer Ther 2006; 5: 787–96. [DOI] [PubMed] [Google Scholar]
- 10. MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol 2004; 5: 541–9. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol 2006; 133: 9–29. [DOI] [PubMed] [Google Scholar]
- 12. Bergelson JM, Cunningham JA, Droguett G et al . Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science (New York, NY) 1997; 275: 1320–3. [DOI] [PubMed] [Google Scholar]
- 13. Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993; 73: 309–19. [DOI] [PubMed] [Google Scholar]
- 14. Wang K, Guan T, Cheresh DA, Nemerow GR. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin beta5. J Virol 2000; 74: 2731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang K, Huang S, Kapoor‐Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol 1998; 72: 3455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogura Y, Mizumoto K, Nagai E et al . Peritumoral injection of adenovirus vector expressing NK4 combined with gemcitabine treatment suppresses growth and metastasis of human pancreatic cancer cells implanted orthotopically in nude mice and prolongs survival. Cancer Gene Ther 2006; 13: 520–9. [DOI] [PubMed] [Google Scholar]
- 17. Murakami M, Nagai E, Mizumoto K et al . Suppression of metastasis of human pancreatic cancer to the liver by transportal injection of recombinant adenoviral NK4 in nude mice. Int J Cancer 2005; 117: 160–5. [DOI] [PubMed] [Google Scholar]
- 18. Mulvihill S, Warren R, Venook A et al . Safety and feasibility of injection with an E1B‐55 kDa gene‐deleted, replication‐selective adenovirus (ONYX‐015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther 2001; 8: 308–15. [DOI] [PubMed] [Google Scholar]
- 19. Sangro B, Mazzolini G, Ruiz J et al . Phase I trial of intratumoral injection of an adenovirus encoding interleukin‐12 for advanced digestive tumors. J Clin Oncol 2004; 22: 1389–97. [DOI] [PubMed] [Google Scholar]
- 20. Shi W, Teschendorf C, Muzyczka N, Siemann DW. Gene therapy delivery of endostatin enhances the treatment efficacy of radiation. Radiother Oncol 2003; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 21. Geoerger B, Grill J, Opolon P et al . Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX‐015) in human malignant glioma xenografts. Br J Cancer 2003; 89: 577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Portella G, Pacelli R, Libertini S et al . ONYX‐015 enhances radiation‐induced death of human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab 2003; 88: 5027–32. [DOI] [PubMed] [Google Scholar]
- 23. Rogulski KR, Freytag SO, Zhang K et al . In vivo antitumor activity of ONYX‐015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res 2000; 60: 1193–6. [PubMed] [Google Scholar]
- 24. Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M. Coexpression of the c‐met proto‐oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 1994; 54: 5775–8. [PubMed] [Google Scholar]
- 25. Paciucci R, Vila MR, Adell T et al . Activation of the urokinase plasminogen activator/urokinase plasminogen activator receptor system and redistribution of E‐cadherin are associated with hepatocyte growth factor‐induced motility of pancreas tumor cells overexpressing Met. Am J Pathol 1998; 153: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M, Nakamura T. NK4, a four‐kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br J Cancer 2001; 84: 864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maehara N, Nagai E, Mizumoto K et al . Gene transduction of NK4, HGF antagonist, inhibits in vitro invasion and in vivo growth of human pancreatic cancer. Clin Exp Metastasis 2002; 19: 417–26. [DOI] [PubMed] [Google Scholar]
- 28. Saimura M, Nagai E, Mizumoto K et al . Intraperitoneal injection of adenovirus‐mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC‐1 in nude mice. Cancer Gene Ther 2002; 9: 799–806. [DOI] [PubMed] [Google Scholar]
- 29. Egami T, Ohuchida K, Mizumoto K et al . Radiation enhances adenoviral gene therapy in pancreatic cancer via activation of cytomegalovirus promoter and increased adenovirus uptake. Clin Cancer Res 2008; 14: 1859–67. [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Mizumoto K, Sato N et al . Quantitative determination of apoptotic death in cultured human pancreatic cancer cells by propidium iodide and digitonin. Cancer Lett 1999; 142: 129–37. [DOI] [PubMed] [Google Scholar]
- 31. Maemondo M, Narumi K, Saijo Y et al . Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther 2002; 5: 177–85. [DOI] [PubMed] [Google Scholar]
- 32. McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology 1988; 163: 614–7. [DOI] [PubMed] [Google Scholar]
- 33. Korst RJ, Bewig B, Crystal RG. In vitro and in vivo transfer and expression of human surfactant SP‐A‐ and SP‐B‐associated protein cDNAs mediated by replication‐deficient, recombinant adenoviral vectors. Hum Gene Ther 1995; 6: 277–87. [DOI] [PubMed] [Google Scholar]
- 34. Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 1997; 420: 1–6. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Li S, Li J, Ensminger WD, Lawrence TS. Ionizing radiation increases adenovirus uptake and improves transgene expression in intrahepatic colon cancer xenografts. Mol Ther 2003; 8: 21–8. [DOI] [PubMed] [Google Scholar]
- 36. Ohuchida K, Mizumoto K, Ohhashi S et al . S100A11, a putative tumor suppressor gene, is overexpressed in pancreatic carcinogenesis. Clin Cancer Res 2006; 12: 5417–22. [DOI] [PubMed] [Google Scholar]
- 37. Qian J, Yang J, Dragovic AF, Abu‐Isa E, Lawrence TS, Zhang M. Ionizing radiation‐induced adenovirus infection is mediated by Dynamin 2. Cancer Res 2005; 65: 5493–7. [DOI] [PubMed] [Google Scholar]
- 38. Hingorani M, White CL, Merron A et al . Inhibition of repair of radiation‐induced DNA damage enhances gene expression from replication‐defective adenoviral vectors. Cancer Res 2008; 68: 9771–8. [DOI] [PubMed] [Google Scholar]
- 39. Medina‐Kauwe LK. Endocytosis of adenovirus and adenovirus capsid proteins. Adv Drug Deliv Rev 2003; 55: 1485–96. [DOI] [PubMed] [Google Scholar]
- 40. Greber UF, Webster P, Weber J, Helenius A. The role of the adenovirus protease on virus entry into cells. EMBO J 1996; 15: 1766–77. [PMC free article] [PubMed] [Google Scholar]
- 41. Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology 1970; 40: 478–85. [DOI] [PubMed] [Google Scholar]
- 42. Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol 1994; 127: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Majhen D, Nemet J, Richardson J et al . Differential role of alpha (v) beta (3) and alpha (v) beta (5) integrins in internalization and transduction efficacies of wild type and RGD4C fiber‐modified adenoviruses. Virus Res 2009; 139: 64–73. [DOI] [PubMed] [Google Scholar]
- 44. Lacher MD, Tiirikainen MI, Saunier EF et al . Transforming growth factor‐beta receptor inhibition enhances adenoviral infectability of carcinoma cells via up‐regulation of Coxsackie and Adenovirus Receptor in conjunction with reversal of epithelial‐mesenchymal transition. Cancer Res 2006; 66: 1648–57. [DOI] [PubMed] [Google Scholar]
- 45. Davison E, Kirby I, Whitehouse J, Hart I, Marshall JF, Santis G. Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by alpha (v) beta1 integrin and can be modulated by changes in beta1 integrin function. J Gene Med 2001; 3: 550–9. [DOI] [PubMed] [Google Scholar]
- 46. Ambriovic‐Ristov A, Gabrilovac J, Cimbora‐Zovko T, Osmak M. Increased adenoviral transduction efficacy in human laryngeal carcinoma cells resistant to cisplatin is associated with increased expression of integrin alphavbeta3 and coxsackie adenovirus receptor. Int J Cancer 2004; 110: 660–7. [DOI] [PubMed] [Google Scholar]
- 47. Belluci S, Caen J. Molecular basis of Glanzmann's thrombasthenia and current strategies in treatment. Blood Rev 2002; 16: 193–202. [DOI] [PubMed] [Google Scholar]
- 48. Switala‐Jelen K, Dabrowska A, Opolski A et al . The biological functions of β3 integrins. Folia Biol 2004; 50: 143–52. [PubMed] [Google Scholar]
- 49. Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2003; 2: 703–16. [DOI] [PubMed] [Google Scholar]
- 50. Yano J, Hirabayashi K, Nakagawa S et al . Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin Cancer Res 2004; 10: 7721–6. [DOI] [PubMed] [Google Scholar]
- 51. Nogawa M, Yuasa T, Kimura S et al . Intravesical administration of small interfering RNA targeting PLK‐1 successfully prevents the growth of bladder cancer. J Clin Invest 2005; 115: 978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res 2004; 64: 3365–70. [DOI] [PubMed] [Google Scholar]
- 53. Takeshita F, Minakuchi Y, Nagahara S et al . Efficient delivery of small interfering RNA to bone‐metastatic tumors by using atelocollagen in vivo . Proc Natl Acad Sci USA 2005; 102: 12177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Integrin β5 mRNA expression in cells. The levels of integrin β5 mRNA in radiosensitive cells (CFPAC‐1 parent cells) or radioresistant cells (R1, R2) were quantified by real‐time RT‐PCR and defined as the ratio relative to radiosensitive cells. Each value represents the mean ± SD of triplicate measurements.
Fig. S2. The levels of integrin β5 mRNA in integrin β3‐specific siRNA‐transfected cells or control siRNA‐transfected cells at 48 h after transfection was quantified by real‐time RT‐PCR and was expressed as fold‐change compared with control siRNA cells. Each value represents the mean ± SD of triplicate measurements.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


