Abstract
We previously reported that 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG) was effective in suppressing the growth of solid tumors due to hemorrhagic necrosis in vivo. In the present study, we investigated the antiangiogenic effect of SQMG. In vivo assessment of antitumor assays showed that some tumor cell lines, but not others, were sensitive to SQMG. Microscopic study suggested that in SQMG‐sensitive tumors, but not SQMG‐resistant tumors, angiogenesis was reduced. We next investigated gene expression relating to angiogenesis in tumor tissues by quantitative real‐time polymerase chain reaction. Consequently, although vascular endothelial growth factor gene expression was not detected with significant differences among the cases, significant downregulation of Tie2 gene expression was observed in all SQMG‐sensitive tumors as compared with controls, but not in SQMG‐resistant tumors. These data suggested that the antitumor effects of SQMG could be attributed to antiangiogenic effects, possibly via the downregulation of Tie2 gene expression in SQMG‐sensitive tumors. (Cancer Sci 2008; 99: 1063–1070)
Angiogenesis, the formation of new blood vessels, is a fundamental process required for normal embryonic development and for the development of pathological conditions such as cancer.( 1 , 2 ) Its importance in solid tumor growth and metastasis has been widely recognized by multiple studies.( 2 ) Therefore, antiangiogenic treatments may be a promising target for the treatment of cancer. For example, it was reported that agents such as angiostatin, endostatin, and anti‐vascular endothelial growth factor (VEGF) antibodies that inhibited VEGF receptor tyrosine kinase were developed, resulting in effective inhibition of solid tumor growth in vivo.( 3 , 4 , 5 , 6 , 7 , 8 , 9 ) However, it was reported that the receptor tyrosine kinase Tie2 could play a critical role in tumor‐induced angiogenesis.( 10 , 11 ) It was also demonstrated that the suppression of Tie2 signaling caused by using specific blocking agents such as soluble dominant‐negative receptors,( 11 , 12 , 13 , 14 ) an antisense oligonucleotide,( 15 ) RNA aptamers and RNA interference,( 16 , 17 ) and a short synthetic peptide( 18 ) resulted in antitumor effects by influencing antiangiogenesis. Thus, therapeutic antiangiogenesis for cancer treatment using multiple strategies was reported and its importance has been widely recognized as a promising treatment for cancer chemotherapy. However, there are few reports about chemotherapeutic compounds for cancer targeting Tie2.
We previously reported that the growth of human adenocarcinoma tumors treated with 3¢‐sulfoquinovosyl‐1¢‐monoacylglycerol (SQMG) was inhibited, and these tumors showed extensive hemorrhagic necrosis by pathohistological examination.( 19 , 20 ) However the mechanism by which hemorrhagic necrosis occurs via the antiangiogenesis activity of SQMG remains undefined. Recently, Sakimoto et al. reported that combined treatment with a‐SQMG (C18:0) and radiation synergistically inhibited the growth of human tumors transplanted into nude mice, accompanied by a significant reduction in the vascularity of the tumors.( 21 ) Here, we demonstrate that the antitumor effects of SQMG could be attributed to inhibition of tumor antiangiogenesis, which seems to be involved in downregulation of the Tie2 gene. Thus, SQMG is a promising candidate as an antitumor drug targeting angiogenesis.
Materials and Methods
Synthesis of SQMG. The chemical structure of the synthesized compound SQMG containing fatty acid 18:1 (oleic acid C18:1) is shown in Figure 1. The procedure for synthesis of SQMG was described previously.( 20 )
Figure 1.
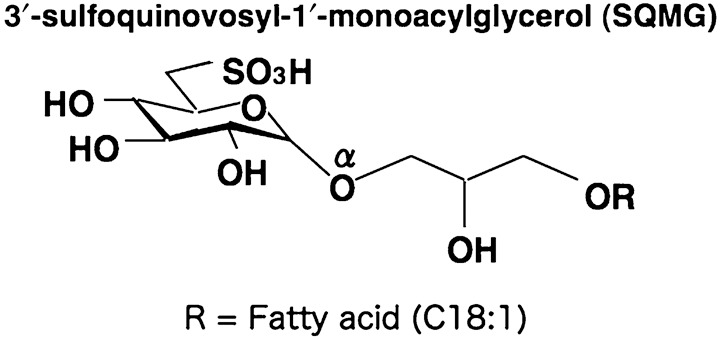
Structure of 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG). SQMG contains a single fatty acid, R = C18:1.
Cell lines. Human breast adenocarcinoma MDA‐MB‐231, lung adenocarcinoma A549, colon adenocarcinoma WiDr, prostate adenocarcinoma PC‐3, tongue squamous cell carcinoma SAS, esophagus squamous cell carcinoma TE‐8, lung small cell carcinoma LU65 lines, and mouse normal fibroblast NIH3T3 were used in the present study. MDA‐MB‐231, A‐549, WiDr, PC‐3, and NIH3T3 were provided by the Japanese Cancer Research Resources Bank. SAS, Lu65, and TE‐8 cells were obtained from Health Science Research Resources Bank (Sendai, Japan). A549, WiDr, PC‐3, SAS, TE‐8, and Lu65 cells were cultured with RPMI1640 supplemented with 10% fetal calf serum, 200 U/mL penicillin, 200 µg/mL streptomycin, and 2 mM l‐glutamine. MDA‐MB‐231 was cultured with Leibovitz's L15 supplemented with 10% fetal calf serum, 200 U/mL penicillin, 200 µg/mL streptomycin, and 2 mM l‐glutamine. Human umbilical vein endothelial cells (HUVEC) were purchased from Cambrex (Walkerville, MD, USA) and maintained according to the provider's instructions. Cells in passage numbers three to five were used for this study.
In vivo assessment of antitumor assay. Inbred mice, female BALB/c nu/nµ mice (20–22 g, 7 weeks of age) were obtained from Japan SLC (Shizuoka, Japan). All procedures were carried out in compliance with the guidelines of the Animal Research Center of Sapporo Medical University. Human tumor cells from lines MDA‐MB‐231, A549, WiDr, PC‐3, SAS, TE‐8, and LU65 (106 cells/mouse) suspended in phosphate‐buffered saline (PBS) were injected subcutaneously into a dorsal side of the mice. After implantation, the tumor sizes in all of these mice were measured at 2‐day intervals. When the solid tumors grew to 30–40 mm3 in tumor volume (tumor volume = length × [width]2× 0.5), SQMG was administrated every day for 14 days, and the tumor growth was observed. Each type of tumor was divided randomly into two or three groups (n = 4/group). A control group was injected intraperitoneally with 0.2 mL saline solution, and test groups were injected intraperitoneally with SQMG at a dose of 5 or 20 mg/kg every day for 14 days. On the next day after the last administration of SQMG, the tumor size was measured, and tumors were excised and prepared for further study. The mean ± SE tumor volume from each group (n = 4/group) is shown (Fig. 2). The growth of each tumor was analyzed using Student's t‐test.
Figure 2.
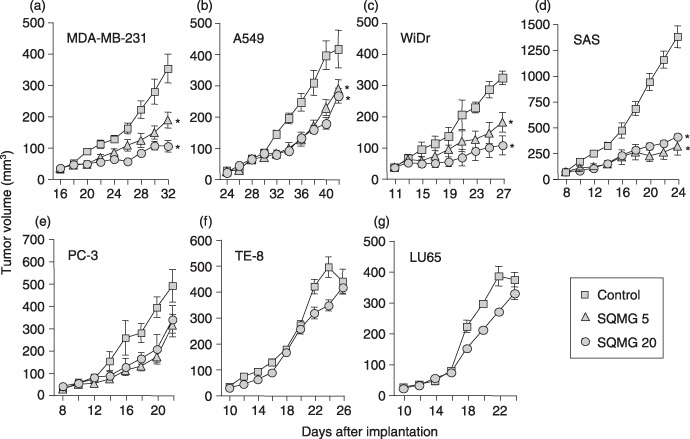
In vivo study of the antitumor effects of 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG). Human tumor cells (106) of the cell lines (a) MDA‐MB‐231, (b) A549, (c) WiDr, (d) SAS, (e) PC‐3, (f) TE‐8, and (g) LU65 were injected subcutaneously into mice, and when tumors grew to 30–40 mm3, mice were injected with saline (control), 5 mg/kg (SQMG 5), or 20 mg/kg (SQMG 20) every day for 14 days. The means ± SE of tumor volumes from each group (n = 4/group) are shown. *P < 0.01.
Immunohistochemical study. All tumors excised from mice (n = 4/group) were embedded in Tissue‐Tek OCT Compound (Sakura Finetek USA, Torrance, CA, USA) and frozen. Acetone‐fixed cryosections were stained with an antimouse CD31 monoclonal antibody, and then antirat IgG conjugated with AlexaFlour 488 (BD Bioscience Pharmingen, CA, USA) as a secondary antibody. Nuclei were counterstained with propidium iodide (PI) (Vector Laboratories, Burlingame, CA, USA). The CD31‐positive ring‐form blood vessels in 500‐mm2‐section areas of these samples were counted at ×100 magnification under a fluorescence microscope (Olympus AX80; Olympus, Tokyo, Japan) and is represented as the mean ± SE of four section areas from each group. The results were analyzed using Student's t‐test.
3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenylthtrazolium bromide assay and annexin V labeling assay. To investigate the cytotoxicity of SQMG, the 3‐(4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenylthtrazolium bromide (MTT) assay was carried out using HUVEC and NIH3T3 cells according to methods described previously.( 19 , 20 ) Briefly, cells (5 × 103 cells/well) were cultured in 96‐well plates for 24 h and then various amounts of SQMG suspended in PBS were added to the wells. Following cultivation for 48 h, 50 µg MTT was added to cells and incubation was continued for 3 h. Then 4% HCl in 2‐propanol was added to each well and mixed by pipette to disrupt the cells. The absorbance of each well was measured using a multiwell scanning photometer (Micro ELISA MR600; Dynatech Laboratories, Alexandria, VA, USA) at a wavelength of 570 nm. Results were represented as the mean ± SE of triplicate wells in one of three independent experiments. The annexin V labeling assay was carried out for detection of apoptotic HUVEC. HUVEC (106 cells) cultured in six‐well plates for 48 h in the presence or absence of various amounts of SQMG were suspended in PBS. Following cultivation, cells were double‐stained with annexin‐V–fluorescein and PI using the Annexin‐V‐FLUOS staining kit according to the manufacturer's instructions (Roche Applied Science, Penzberg, Germany). The percentage of apoptotic (annexin V and PI double positive) cells was determined by flow cytometric analysis (FACS Calibur and Cell Quest software; BD Bioscience). The results are represented as the mean ± SE of three independent experiments.
Angiogenesis assay. The angiogenesis assay was done using an Angiogenesis Kit (Kurabo, Osaka, Japan) according to the manufacturer's instructions. Briefly, HUVEC grown on human diploid fibroblast sheets on Matrigel (Kurabo, Osaka, Japan) were cultured with or without SQMG at the indicated concentrations in growth medium containing 10 ng/mL VEGF‐A for 14 days. Fresh growth medium with or without SQMG was replaced 4, 7, and 9 days after incubation. After incubation, cells were fixed in 70% ethanol and immunostained with an anti‐CD31 antibody for 1 h and detected using the alkaline phosphate method. The tube‐formation areas of HUVEC were quantitated using Image ++ software downloaded from the internet (http://www.pluto.dti.ne.jp/~horie‐ms/index‐j.html). The results are represented as the mean ± SE of five independent areas. In some experiment, HUVEC grown on human diploid fibroblast sheets on Matrigel were cultured for 14 days, and then in the presence or absence SQMG for 2 days. Subsequently, the cell‐derived total RNA was harvested and quantitated for the amount of mRNA copy of human VEGF receptor‐1 (Flt‐1), VEGF receptor‐2 (KDR), and Tie2 by quantitative real‐time reverse transcription (RT)–polymerase chain reaction analysis (PCR).
Quantitative real‐time RT‐PCR analysis. Tumor‐derived total RNA was prepared using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and then reverse transcribed to cDNA with a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). Measurement of gene expression by quantitative analysis was carried out using a LightCycler system (Roche Applied Science). Primers and hybridization probes were synthesized by Nihon Gene Research Laboratory (Sendai, Japan). Quantitative real‐time RT‐PCR analysis of human VEGF165 , mouse angiopoitin‐1 (Ang1), mouse angiopoitin‐2 (Ang2), human Flt‐1, human KDR, human Tie2, human CD31, and human glucose‐6‐phosphate dehydrogenase (G6PDH) gene expression was carried out using a LightCycler FastStart DNA MasterPLUS SYBR Green I system (Roche Applied Science) with primer sets described in Table 1. Detection of gene expression of mouse Flt‐1, VEGF receptor‐2 (Flk‐1), Tie1, Tie2, CD31, SM22 α, and G6PDH was carried out using a LightCycler FastStart DNA Master HybProbe system (Roche Applied Science) with primer sets and probes described in Table 1. PCR amplification of the housekeeping gene G6PDH was carried out for each sample as a control for sample loading and to allow normalization among samples. To determine the absolute copy number of the target transcripts, the amplified fragments of G6PDH or target genes amplified by PCR using the above‐described primer sets were constructed using the pCR4‐TOPO cloning vector (Invitrogen, Carlsbad, CA, USA), and the concentrations of these purified plasmids were measured. The absorbance at 260 nm and copy numbers were calculated from the concentrations of samples. A standard curve was created by plotting the threshold cycle versus the known copy number for each plasmid template in the dilutions. The copy numbers for all unknown samples were determined according to the standard curve using LightCycler software 3.5.3 (Roche Applied Science). To correct differences in both RNA quality and quantity between samples, each target gene was first normalized by dividing the copy number of the target by the copy number of G6PDH (copy number of target/copy number of G6PDH = normalized target gene). The initial value corrected for the amount of G6PDH was indicated as 100% to evaluate the sequential alteration of the mRNA expression level. For mouse genes, each sample was corrected with the copy number of the murine CD31 or SM22α gene as markers for endothelial cells and pericytes, respectively.( 22 , 23 ) Thus, this represents the amount of target gene expression in an endothelial cell or pericyte, not in a human tumor cell. Results were represented as the mean ± SE of four RNA samples from each group (n = 4/group), and analyzed using Student's t‐test.
Table 1.
Primer and probe for quantitative real‐time reverse transcription–polymerase chain reaction
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | 5′ LCRed640 probe (5′–3′) | 3′ Fluorescein probe (5′–3′) |
|---|---|---|---|---|
| Mouse Flt‐1 | GGCTTTGCAGATCCACAT | GCAGAGTGCTAGTGTCCAG | TCCAGGCTCATGAATTTGAAAGCGTTTACAT | TCGGTGAAAGCTCCTCAAAGGTTTTGATTCT |
| Mouse Flk‐1 | AAGCGGGACGAGGAGAGA | CCCATGTGGACCGATGTT | AAGCTTGTACCACGTGAGGTTCTCAAACG | ATTTCTGTCTGCAGTGCACAACAGGGACA |
| Mouse Tie1 | GAAGGAGGAGAAGGAGGCT | CAGGAAACCATCACCGGA | CAGCAAAGGCTCAGGACAGTCAGTAGTCAT | ACGTGCCAGCTCTCTAGCCAGGGCT |
| Mouse Tie2 | CGGACTGACTACGAGCTGT | GTTGTTGCAGGGATCGC | CTCTTGGAGGAGGGAGTCCGATAGACG | TGTTGTAAATCGTCTCACAGGCCCAGGA |
| Mouse CD31 | CACCTTATGAAAGCAAAGAG | AATCACAACTTCATCCACTG | TGTTGCTGGGTCATTGGAGGTCACCT | TTCCATGTCTCTGGTGGGCTTATCTGTGA |
| Mouse SM22α | GGAGATCCCAACTGGTTTATG | AGAGCTGTCAGG GCTAAGAAC | GAGGGGAAGCACGTCATTGGCCTT | AGAGGGACTTCACAGACAGCCAACTGCA |
| Mouse G6PDH | GCACAAGATTGATCGAGA | GAGGCAGAGTATAGATGGTGTA | CTTGTAGGTACCCTCGTACTGGAAGCCCA | TCTCTTCATCAGCTCATCTGCCTCTGTGG |
| Mouse Ang1 | GCATCTGGAGCATGTGATG | TAGCAGTTGTATTTCAAGTCG | ||
| Mouse Ang2 | GAAGAGCGTGGACAGCACAGG | GAGTCGTCGTAGTCGAGGGG | ||
| Human VEGF165 | AGAGCAAGACAAGAAAATCC | TACAAACAAATGCTTTCTCC | ||
| Human Flt‐1 | GACTGACAGCAAACCCAAG | AGCGTGGTCGTAGGTGAAC | ||
| Human KDR | TGGTCTCTCTGGTTGTGTATG | AAGGGTATGGGTTTGTCACTG | ||
| Human Tie2 | CAAACCCGTTAATCACTATG | TCCGATAGAAGCTGTTGTG | ||
| Human CD31 | CAACTTTTAAAAACAAGTAA | AATCTGGACCTCATCCACCG | ||
| Human G6PDH | CTGCGTTATCCTCACCTTC | CGGACGTCATCTGAGTTG |
Results
In vivo assessment of antitumor effects of SQMG. Seven human tumor cell lines, MDA‐MB‐231, A549, WiDr, PC‐3, SAS, TE‐8, and LU65, were injected subcutaneously into mice, and then these mice bearing solid tumors that grew to 30–40 mm3 in tumor volume were injected intraperitoneally with saline or SQMG every day for 14 days. As shown in Figure 2, SQMG treatment of mice bearing MDA‐MB‐231, A549, WiDr, and SAS solid tumors, injected with 5 and 20 mg/kg SQMG showed significant inhibition of tumor growth as compared with the control group on the next day after the last injection date. None of the mice showed any significant loss of bodyweight throughout the experiment period (data not shown). In contrast, mice bearing PC‐3, TE‐8, and LU65 solid tumors injected with SQMG did not show tumor growth inhibition as compared with the control on the day after the last injection. These data demonstrate that four tumor lines, MDA‐MB‐231, A549, WiDr, and SAS, were sensitive to SQMG (SQMG‐sensitive), but three lines, PC‐3, TE‐8, and LU65 were resistant (SQMG‐resistant).
Antiangiogenesis activity of SQMG in vivo. We previously indicated that SQMG treatment results in hemorrhagic necrosis in tumors. To investigate the mechanism of the antitumor effects of SQMG, we first carried out immunohistochemical analysis to determine the angiogenesis profiles in tumors. Tumors were excised from mice on the next day after the last injection and cryosections of these acetone‐fixed tumors were stained with antimouse CD31 monoclonal antibody as an endothelial cell marker. Representative photos showing immunohistochemical staining of MDA‐MB‐231 tumors treated with or without SQMG are presented in Figure 3, in which CD31‐positive ring‐form blood vessels are clearly observed for both the control (Fig. 3a,b) and SQMG treatment (Fig. 3c,d). Therefore, the CD31‐positive ring‐form blood vessels of all samples in 500‐mm2‐section areas were counted under a fluorescence microscope. Consequently, as shown in Table 2, in all four SQMG‐sensitive tumors treated with 20 mg/kg SQMG, the numbers of blood vessels were significantly decreased (P < 0.01) with SQMG treatments, as compared with controls. In contrast, in all three of the SQMG‐resistant tumors treated with 20 mg/kg SQMG, there were no significant differences in the number of blood vessels between controls and SQMG treatments. These data suggested that the antitumor effect of SQMG could be attributed to the inhibition of tumor angiogenesis.
Figure 3.
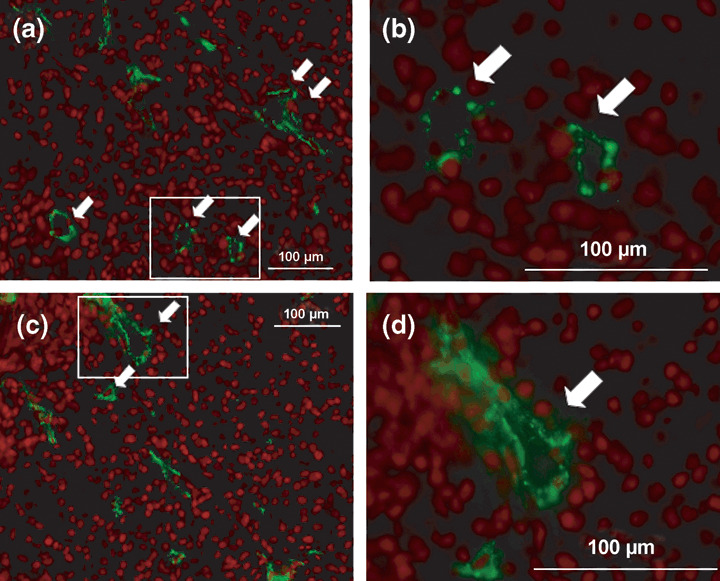
Antiangiogenesis assessment by immunohistochemical analysis. Cryosections of MDA‐MB‐231 treated (a,b) without and (c,d) with 3′‐sulfoquinovosyl‐1′‐monoacylglycerol were stained with antimouse CD31 monoclonal antibody and antirat IgG conjugated with AlexaFlour 488, and nuclei were counterstained with propidium iodide. Arrows indicate CD31‐positive blood vessels. Insets in (a) and (c) are magnified and shown in (b) and (d), respectively. Scale bar = 100 µm.
Table 2.
Number of tumor‐induced blood vessels in tumor tissues
| Criteria | Tumor | No. blood vessels (500 mm2) | |
|---|---|---|---|
| Control | SQMG | ||
| MDA‐MB‐231 † | 10.2 ± 1.0 | 2.3 ± 1.8* | |
| A549 | 6.3 ± 1.1 | 2.9 ± 0.3* | |
| Sensitive | WiDr | 13.4 ± 4.2 | 6.6 ± 2.6* |
| SAS | 15.2 ± 1.3 | 7.2 ± 1.6* | |
| PC‐3 | 10.7 ± 1.7 | 11.9 ± 2.7 | |
| Resistant | TE‐8 | 11.4 ± 2.4 | 11.7 ± 1.9 |
| LU65 | 6.2 ± 2.2 | 3.8 ± 1.1 | |
Data are presented as mean ± SE.
P < 0.01. SQMG, 3′‐sulfoquinovosyl‐1′‐monoacylglycerol.
Inhibitory effect of SQMG on endothelial cell‐derived capillary formation in vitro. To investigate whether SQMG directly affected the growth profile of endothelial cells, we assessed proliferation and cytotoxicity by using the human endothelial cell line HUVEC. SQMG was added to HUVEC in concentrations from 0 to 100 µM, and cell proliferation and apoptosis were analyzed using the MTT assay and annexin V labeling assay, respectively. As shown in Figure 4a, there was no obvious the inhibitory effect on the proliferation of HUVEC up to the concentration of 25 µM. When 50 or 100 µM SQMG was added to cells, the cell proliferation was inhibited to 71.5 ± 5.6 or 55.3 ± 4.5%, respectively. Meanwhile, when SQMG at concentrations from 0 to 50 µM was added to cells, 8.5–13.3% of cells were observed to be apoptotic. However, when 100 µM SQMG was added to cells, apoptotic effects were increased to 33.8 ± 11.6%, suggesting that there was minimal weak influence on the apoptosis of HUVEC up to the concentration of 50 µM SQMG (Fig. 4b).
Figure 4.
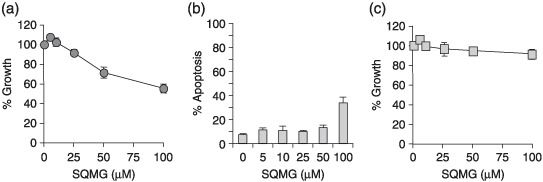
Influence of 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG) on cell proliferation and apoptosis. (a) Human umbilical vein endothelial cells (HUVEC) and (c) NIH3T3 were cultured in the presence or absence of SQMG at the indicated concentrations. Cell proliferation was examined by MTT assay. Results represent means ± SE of triplicate wells on one of three independent experiments. (b) HUVEC were cultured in the presence or absence of SQMG at the concentrations indicated for 48 h, harvested, and double‐stained with annexin‐V–fluorescein and propidium iodide (PI). The percentage of apoptotic (annexin V and PI double positive) cells was determined by flow cytometric analysis. Results represent means ± SE of three independent experiments.
We further considered that it would be important to evaluate the antiangiogenic activity of SQMG using an angiogenesis model in vitro that is considered to closely represent in vivo situations. It is well known that HUVEC cocultured with fibroblast cells on Matrigel form capillary networks with tube‐like structures and adopt characteristics of newly formed blood vessels.( 21 ) Before investigating the influence of SQMG on the formation of these structures, we studied fibroblast cell proliferation and cytotoxicity using mouse NIH3T3 instead of human normal fibroblasts by MTT assay. Consequently, there was no obvious cytotoxic potential up to the concentration of 100 µM SQMG (Fig. 4c). Meanwhile, when 50 µM SQMG was added to these cells, capillary network formation was markedly inhibited compared with the control (Fig. 5a–c). Quantitation of these capillary areas by Image ++ software showed that the capillary formation treated with 50 µM SQMG was reduced approximately 70% compared with the control (Fig. 5d). Thus, these data suggest that SQMG could influence capillary formation.
Figure 5.
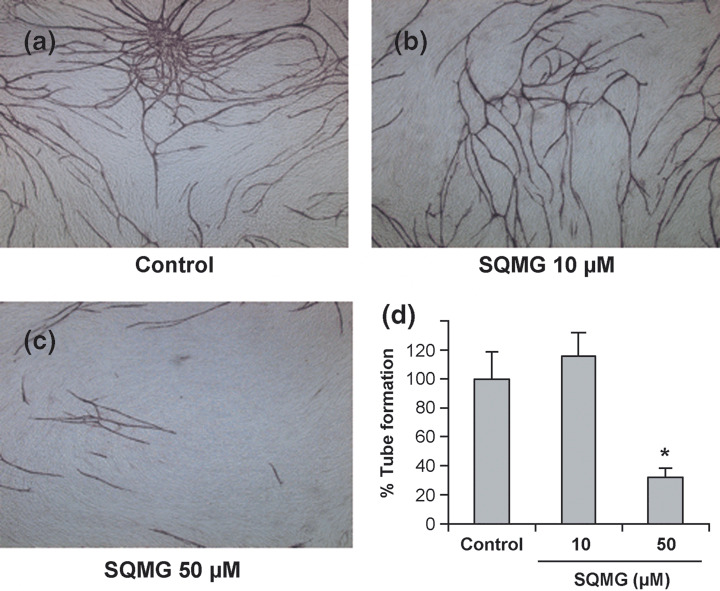
Effect of 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG) on angiogenesis in vitro. (a–c) Human umbilical vein endothelial cells (HUVEC) grown on a fibroblast sheet on Matrigel were cultured (a) without and (b,c) with SQMG at the indicated concentrations. After cultivation, the cells were fixed and stained with an antihuman CD31 antibody for 1 h. CD31 molecules were detected using the alkaline phosphate method. (d) The tube formation of HUVEC was quantitated using Image ++ software. Results represent means ± SE of five independent areas on one of three independent experiments. *P < 0.01.
Influence of SQMG on VEGF gene expression in tumor tissues. It is known that angiogenesis is basically dependent on VEGF.( 1 , 2 ) We next quantified the mRNA copy number of tumor‐derived VEGF165 in mice bearing tumors treated with or without 5 and 20 mg/kg SQMG by quantitative real‐time RT‐PCR. G6PDH expression was used as a housekeeping gene control. Consequently, as indicated in Table 3, in all four SQMG‐sensitive and the three resistant models, the mRNA copy number of human VEGF165 did not show any overt difference between controls and SQMG treatment groups, suggesting that SQMG did not influence VEGF gene expression. In our preliminary experiments using enzyme‐linked immunosorbent assay, VEGF secretion in SQMG‐sensitive MDA‐MB‐231 and SQMG‐resistant TE‐8 tumor tissues did not differ between controls and SQMG treatment groups (data not shown).
Table 3.
Human vascular endothelial growth factor ( VEGF ) gene expression in tumor tissues treated without or with 3′‐sulfoquinovosyl‐1′‐monoacylglycerol (SQMG)
| Criteria | Tumor | mRNA copy number (human VEGF165/G6PDH) | ||
|---|---|---|---|---|
| Control | SQMG‐5‡ | SQMG‐20‡ | ||
| MDA‐MB‐231 † | 2.71 ± 0.63 | 2.76 ± 0.61 | 2.91 ± 0.81 | |
| Sensitive | A549 | 0.15 ± 0.01 | 0.21 ± 0.06 | 0.18 ± 0.03 |
| WiDr | 4.43 ± 0.61 | 2.40 ± 0.05 | 2.37 ± 0.42 | |
| SAS | 1.89 ± 0.14 | 1.99 ± 0.04 | 1.67 ± 0.1 | |
| PC‐3 | 0.58 ± 0.11 | 0.63 ± 0.04 | 0.66 ± 0.01 | |
| Resistance | TE‐8 | 1.54 ± 0.56 | ND | 1.76 ± 0.58 |
| LU65 | 3.23 ± 0.43 | ND | 3.25 ± 0.82 | |
Data are presented as mean ± SE. G6PDH, glyceraldehyde‐6‐phosphate dehydrogenase; ND, not done; SQMG 5, treatment with 5 mg/kg SQMG; SQMG 20, treatment of 20 mg/kg SQMG.
Influence of SQMG on angiopoietin expression in tumor tissues. We next quantified the mRNA copy numbers of Ang1 and Ang2, which are known as important factors for vascular remodeling. Because Ang1 and Ang2 are expressed mainly in pericytes and endothelial cells, respectively,( 10 ) the amounts of their expression in mice were adjusted with the amounts of G6PDH‐normalized SM22α and CD31, respectively ([mRNA copy number of target gene/mRNA copy number of G6PDH]/[mRNA copy number of SM22α or CD31/mRNA copy number of G6PDH]).( 23 ) In the SQMG‐sensitive tumors MDA‐MB‐231, A549, and SAS, the mRNA copy numbers of Ang1 in SQMG‐treated tumors appeared to have a tendency to increase two‐ to three‐fold (Table 4), whereas this was not true for Ang2.
Table 4.
Mouse angiopoietin (Ang) gene expression in tumor tissues
| Tumor | Treatment | mRNA copy number ratio | |
|---|---|---|---|
| Ang‐1/SM22α | Ang‐2/CD31 | ||
| Control | 0.045 ± 0.015 | 0.158 ± 0.053 | |
| MDA‐MB‐231 | SQMG 5 | 0.187 ± 0.095* | 0.312 ± 0.078 |
| SQMG 20 | 0.120 ± 0.047* | 0.300 ± 0.103 | |
| Control | 0.028 ± 0.003 | 1.838 ± 1.087 | |
| A549 | SQMG 5 | 0.041 ± 0.008* | 1.422 ± 0.746 |
| SQMG 20 | 0.054 ± 0.028* | 4.346 ± 5.406 | |
| Control | 0.021 ± 0.001 | 0.400 ± 0.021 | |
| WiDr | SQMG 5 | 0.017 ± 0.002 | 0.409 ± 0.053 |
| SQMG 20 | 0.022 ± 0.005 | 0.458 ± 0.059 | |
| Control | 0.035 ± 0.025 | 0.355 ± 0.167 | |
| SAS | SQMG 5 | 0.093 ± 0.047* | 0.390 ± 0.185 |
| SQMG 20 | 0.091 ± 0.051* | 0.448 ± 0.109 | |
| Control | 0.008 ± 0.001 | 0.228 ± 0.067 | |
| TE‐8 | SQMG 5 | ND | ND |
| SQMG 20 | 0.009 ± 0.001 | 0.182 ± 0.100 | |
| Control | 0.008 ± 0.001 | 0.237 ± 0.184 | |
| LU65 | SQMG 5 | ND | ND |
| SQMG 20 | 0.011 ± 0.001 | 0.225 ± 0.046 | |
| Control | 0.572 ± 0.212 | 0.295 ± 0.054 | |
| PC‐3 | SQMG 5 | 0.172 ± 0.024 | 0.340 ± 0.176 |
| SQMG 20 | 0.301 ± 0.077 | 0.392 ± 0.040 | |
P‐values are in the range of 0.22–0.54.
Data are presented as mean ± SE. ND, not done; SQMG 5, treatment of 5 mg/kg SQMG; SQMG 20, treatment of 20 mg/kg SQMG.
Downregulation of Tie2 gene expression in SQMG‐sensitive tumors. We demonstrated that although SQMG had less effect on cytotoxic activity against endothelial cells in vitro and on VEGF secretion in vivo, it strongly inhibited angiogenesis in vitro and in vivo. To investigate the reasons why the number of mouse‐derived blood vessels was decreased by SQMG treatment, we next quantified the mRNA copy numbers of receptor genes related to angiogenesis that are expressed on the endothelial cell surface, namely, Flt‐1, Flk‐1, Tie1, and Tie2, by quantitative real‐time RT‐PCR. The expression profiles of these genes were calculated with the following formula using the mRNA copy number of each gene, as shown previously:( 23 )
| (target gene/G6PDH)/(CD31/G6PDH). |
As shown in Table 5, the mRNA copy number of the mouse Flt‐1 gene per copy of the CD31 gene, which is expressed on mouse endothelial cells in tumor tissues, was similar in controls and after SQMG treatment. This was also true for the mRNA copy number of mouse Flk‐1 in most tumor tissues other than SAS tumors. Only in A549 did Tie1 gene expression seem to be influenced by SQMG treatment. In contrast, the mRNA copy number of mouse Tie2 in tumor tissues was significantly downregulated in all SQMG‐sensitive tumors but not in SQMG‐resistant tumors, suggesting that SQMG might affect mouse Tie2 gene expression in the endothelial cells.
Table 5.
Angiogenic receptor gene expression on mouse endothelial cells in tumors
| Tumor | Treatment | mRNA copy number ratio (target gene/CD31 gene) | |||
|---|---|---|---|---|---|
| Flt‐1 | Flk‐1 | Tie1 | Tie2 | ||
| Control | 0.22 ± 0.02 | 0.80 ± 0.07 | 0.56 ± 0.10 | 0.10 ± 0.01 | |
| MDA‐MB‐231 | SQMG 5 | 0.22 ± 0.02 | 0.82 ± 0.06 | 0.59 ± 0.06 | 0.07 ± 0.01 |
| SQMG 20 | 0.26 ± 0.04 | 0.69 ± 0.06 | 0.64 ± 0.01 | 0.06 ± 0.02** | |
| Control | 0.21 ± 0.01 | 0.82 ± 0.02 | 0.64 ± 0.09 | 0.20 ± 0.01 | |
| A549 | SQMG 5 | 0.20 ± 0.01 | 0.67 ± 0.02 | 0.58 ± 0.10 | 0.08 ± 0.01** |
| SQMG 20 | 0.24 ± 0.01 | 0.79 ± 0.01 | 1.21 ± 0.38* | 0.14 ± 0.012* | |
| Control | 0.33 ± 0.02 | 0.69 ± 0.02 | 0.58 ± 0.03 | 0.08 ± 0.01 | |
| WiDr | SQMG 5 | 0.31 ± 0.03 | 0.64 ± 0.01 | 0.59 ± 0.02 | 0.07 ± 0.01 |
| SQMG 20 | 0.33 ± 0.01 | 0.64 ± 0.02 | 0.63 ± 0.02 | 0.06 ± 0.01* | |
| Control | 0.36 ± 0.01 | 1.04 ± 0.02 | 1.96 ± 0.58 | 0.20 ± 0.01 | |
| SAS | SQMG 5 | 0.35 ± 0.02 | 0.93 ± 0.05 | 2.16 ± 0.73 | 0.18 ± 0.01 |
| SQMG 20 | 0.25 ± 0.01 | 0.52 ± 0.03** | 1.94 ± 0.43 | 0.11 ± 0.01** | |
| Control | 0.16 ± 0.01 | 0.93 ± 0.02 | 1.42 ± 0.03 | 0.08 ± 0.01 | |
| PC‐3 | SQMG 5 | 0.11 ± 0.13 | 0.73 ± 0.06 | 1.26 ± 0.17 | 0.07 ± 0.05 |
| SQMG 20 | 0.15 ± 0.02 | 0.78 ± 0.07 | 1.40 ± 0.04 | 0.09 ± 0.01 | |
| Control | 0.34 ± 0.03 | 0.74 ± 0.05 | 0.43 ± 0.11 | 0.08 ± 0.01 | |
| TE‐8 | SQMG 5 | ND | ND | ND | ND |
| SQMG 20 | 0.30 ± 0.02 | 0.70 ± 0.04 | 0.44 ± 0.11 | 0.10 ± 0.01 | |
| Control | 0.23 ± 0.02 | 0.55 ± 0.06 | 0.43 ± 0.01 | 0.04 ± 0.01 | |
| LU65 | SQMG 5 | ND | ND | ND | ND |
| SQMG 20 | 0.21 ± 0.21 | 0.69 ± 0.01 | 0.42 ± 0.02 | 0.04 ± 0.01 | |
Data are presented as mean ± SE. ND, not done; SQMG 5, treatment of 5 mg/kg SQMG; SQMG 20, treatment of 20 mg/kg SQMG.
P < 0.05.
P < 0.01.
We further investigated whether Flt‐1, KDR, and Tie2 gene expression in capillary‐formed HUVEC were influenced by SQMG in vitro. As shown in Figure 6, although Flt‐1 and KDR expression were not influenced, Tie2 gene expression in capillary‐formed HUVEC was also downregulated to approximately 50% lower than the control level when 50 µM SQMG was added to cells. Taken together, the data suggest that SQMG plays a role in downregulating Tie2 gene expression in vivo and in vitro.
Figure 6.
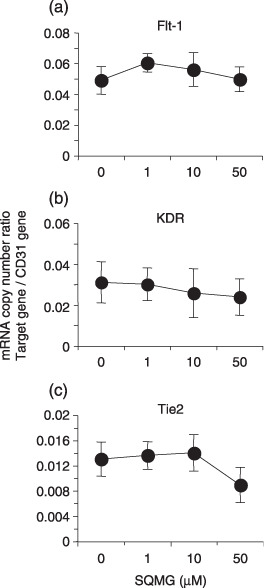
Angiogenic receptor gene expression on capillary‐formed human umbilical vein endothelial cells (HUVEC) in vitro. Total RNA of capillary‐formed HUVEC was assessed as to the amount of mRNA copy number of human (a) Flt‐1, (b) KDR, and (c) Tie2 by quantitative real‐time reverse transcription–polymerase chain reaction analysis. Results represent means ± SE of triplicate wells on one of three independent experiments.
Discussion
In the present study, we demonstrated that SQMG induced antiangiogenic effects in tumor xenografts, resulting in inhibition of solid tumor growth. As significant decreases in tumor angiogenesis were observed in SQMG‐sensitive tumors but not in SQMG‐resistant tumors, it was speculated that the antitumor effects of SQMG could be attributed to the inhibition of tumor angiogenesis. To analyze this mechanism, we first investigated the influence of endothelial cell proliferation and apoptosis in vitro by SQMG. Although the proliferation of HUVEC cells treated with 50 µM SQMG was inhibited, the apoptosis of these cells were not clearly observed. Consequently, our study implied that the inhibitory effects of SQMG shown in the MTT assay are attributed to a slower proliferation of HUVEC cells but not apoptosis.
Thus, SQMG appears to have only a weak inhibitory effect on cell proliferation and apoptosis activity as compared with other chemotherapeutic compounds for cancer.( 24 , 25 ) However, the capillary formation, consisting of HUVEC treated with 50 µM SQMG, was significantly reduced approximately 70% as compared with the control. As Tie2 but not Flt‐1 and KDR gene expression in capillary‐formed HUVEC was decreased selectively, it is possible that some synergistic effects between slow proliferation and downregulation of Tie2 gene expression exist. This was comparable with our observation that xenografted tumors treated with SQMG showed downregulation of Tie2 gene expression in the endothelial cells. In addition, we recently found that the SQMG‐derivative α‐SQMG (c18:0) binds to the extracelluar domains of Tie2 using phage display screening and surface plasmon resonance analysis (Sakimoto I, Ohta K, Yamazaki T, et al. 2006 unpublished data in Discussion of( 21 )). Such binding may influence antiangiogenesis through downregulation of Tie2.
The receptor tyrosine kinase Tie2 is highly expressed in endothelial cells, and plays a critical role in normal vascular development via the regulation of vascular remodeling and endothelial cell interactions with supporting pericytes and smooth muscle cells.( 26 , 27 , 28 , 29 , 30 , 31 , 32 ) In particular, Tie2 is essential for the development of embryonic vasculature but not hematopoietic cell development( 33 ) because Tie2 −/– mice die between embryonic days 9.5 and 12.5 due to lack of remodeling of the primary capillary plexus.( 28 , 29 ) However, Tie2 is constitutively expressed and phosphorylated at a low level in adult mice, suggesting that Tie2 activation is required in adult tissue to maintain the mature quiescent phenotype of vasculature.( 34 ) Interruption of Tie2 signaling with a soluble receptor can significantly inhibit tumor growth in mice, suggesting that Tie2 is important for tumor angiogenesis as well.( 11 ) However, the molecular mechanism by which SQMG induces the downregulation of Tie2 gene expression in vivo and in vitro was not demonstrated. The regulatory mechanism of Tie2 gene expression by SQMG is currently under investigations.
We also observed increased Ang1 gene expression in three of the four SQMG‐sensitive tumors. Ang1 and Ang2 are known to function as ligands for Tie2. Ang1, mainly secreted from pericytes, acts as an agonist of Tie2, whereas Ang2, mainly secreted from endothelial cells, is known to act as an antagonist as well as an agonist, depending on the experimental system.( 10 ) Ang1 specifically induces tyrosine phosphorylation of Tie2, which results in multiple activities related to angiogenesis such as endothelial cell migration,( 35 ) tube formation,( 15 ) sprouting,( 36 , 37 ) and survival( 38 , 39 ) but not proliferation of endothelial cells in vitro.( 40 ) Thus, Ang1 basically act as a factor of angiogenesis. However, it was reported that Ang1‐overexpressing human tumor xenografts could not grow due to inhibition of angiogenesis,( 41 , 42 ) proposing an inhibitory mechanism whereby the antiangiogenic effects of Ang1 overexpression are mediated in part by increased support by vascular pericytes that results in overall vessel stabilization and therefore inhibition of the initiation of tumor angiogenesis. In the current study, although an upregulation of Ang1 in SQMG‐treated tumor xenograft was observed, it remains undefined whether Tie2 phosphorylation levels were influenced by SQMG. The further regulatory mechanism of antiangiogenesis between the Ang1 and Tie2 molecules and how SQMG regulates this needs further study.
In conclusion, as little is known about chemical compounds inducing downregulation of Tie2, SQMG could be a promising candidate for the treatment of tumor‐induced angiogenesis targeting Tie2.
Acknowledgments
This research was supported by The Special Coordination Funds on Science and Technology of the Ministry of Education, Culture, Sports, Science of Japan and a research grant from Toyo Suisan.
References
- 1. Folkman J. What is the evidence that tumor are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005; 438: 932–6. [DOI] [PubMed] [Google Scholar]
- 3. O’Reilly MS, Holmgren L, Shing Y et al . Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–28. [DOI] [PubMed] [Google Scholar]
- 4. O’Reilly MS, Boehm T, Shing Y et al . Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–85. [DOI] [PubMed] [Google Scholar]
- 5. Fong TA, Shawver LK, Sun L et al . SU5416 is a potentand selective inhibitor of vascular endothelial growth factor receptor (Flk‐1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 1999; 59: 99–106. [PubMed] [Google Scholar]
- 6. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669–76. [DOI] [PubMed] [Google Scholar]
- 7. Hurwitz H, Feherenbacher L, Novotny W et al . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 8. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinicals studies. Cancer Res 2005; 65: 671–80. [PubMed] [Google Scholar]
- 9. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438: 967–74. [DOI] [PubMed] [Google Scholar]
- 10. Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context‐depedent regulators of vascular remodeling. Exp Cell Res 2006; 312: 630–41. [DOI] [PubMed] [Google Scholar]
- 11. Lin P, Polverini P, Dewhirst M, Shan S, Roao PS, Peters KG. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie‐2 in pathologic vascular growth. J Clin Invest 1997; 100: 2072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin P, Buxton JA, Acheson A et al . Anti‐angiogenic gene therapy targeting the endothelium‐specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci USA 1998; 95: 8829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siemeister G, Schirner M, Weindel K et al . Two independent mechanisms essential for tumor angiogenesis: inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie‐2 pathway. Cancer Res 1999; 59: 3185–91. [PubMed] [Google Scholar]
- 14. Stratmann A, Acker T, Burger AM, Amann K, Risau W, Plate KH. Differential inhibition of tumor angiogenesis by Tie2 and vascular endothelial growth factor receptor‐2 dominant‐negative receptor mutants. Int J Cancer 2001; 91: 273–82. [DOI] [PubMed] [Google Scholar]
- 15. Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin‐1 and its receptor Tie‐2 participate in the regulation of capillary‐like tubule formation and survival of endothelial cells. Microvasc Res 1999; 58: 224–37. [DOI] [PubMed] [Google Scholar]
- 16. White RR, Shan S, Rusconi CP et al . Inhibition of rat corneal angiogenesis by a nuclease‐resistant RNA aptamer specific for angiopoietin. Proc Natl Acad Sci USA 2003; 100: 5028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie‐2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti‐angiogenic molecule, thrombospondin‐1. Cancer Biol Ther 2004; 3: 402–5. [DOI] [PubMed] [Google Scholar]
- 18. Tournaire R, Simon M‐P, Le Noble F, Eichmann A, England P, Pouyssegur J. A short synthetic peptide inhibits signal transduction, migration and angiogenesis mediated by Tie2 receptor. EMBO Rep 2004; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sahara H, Ishikawa M, Takahashi N et al . In vivo anti‐tumour effect of 3′‐sulphonoquinovosyl 1′‐monoacylglyceride isolated from sea urchin (Strongylocentrotus intermedius) intestine. Br J Cancer 1997; 75: 324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahara H, Hanashima S, Yamazaki T et al . Anti‐tumor effect of chemically synthesized sulfolipids based on sea urchin's natural sulfonoquinovosylmonoacylglycerols. Jpn J Cancer Res 2002; 93: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakimoto I, Ohta K, Yamazaki T et al . α‐Sulfoquinovosylmonoacylglycerol is a novel potent radiosensitizer targeting tumor angiogenesis. Cancer Res 2006; 66: 2287–95. [DOI] [PubMed] [Google Scholar]
- 22. Solway J, Seltzer J, Samaha FF et al . Structure and expression of a smooth muscle cell‐specific gene, SM22α. J Biol Chem 1995; 270: 13 460–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Yang N, Park J et al . Tumor‐derived vascular endothelial growth factor up‐regulates angiopoietin‐2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 2003; 63: 3403–12. [PubMed] [Google Scholar]
- 24. Yamori T, Matsunaga A, Sato S et al . Potent antitumor activity of MS‐247, a novel DNA minor grove binder, evaluated by an in vitro and in vivo human cancer cell line panel. Cancer Res 1999; 59: 4042–9. [PubMed] [Google Scholar]
- 25. Dan S, Tsunoda T, Kitahara O et al . An integrated database of chemosensitivity to 55 anticancer drugs and gene expression profiles of 39 human cancer cell lines. Cancer Res 2002; 62: 1139–47. [PubMed] [Google Scholar]
- 26. Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. Tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in the endothelial cells and their presumptive precursors. Oncogene 1992; 7: 1471–80. [PubMed] [Google Scholar]
- 27. Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial‐specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene 1993; 8: 1293–301. [PubMed] [Google Scholar]
- 28. Dumont DJ, Gradwohl G, Fong GH et al . Dominant‐negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994; 8: 1897–909. [DOI] [PubMed] [Google Scholar]
- 29. Sato TN, Tozawa Y, Deutsch U et al . Distinct roles of the receptor tyrosine kinases Tie1 and Tie‐2 in blood vessel formation. Nature 1995; 376: 70–4. [DOI] [PubMed] [Google Scholar]
- 30. Maisonpierre PC, Suri C, Jones PF et al . Angiopoietin‐2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60. [DOI] [PubMed] [Google Scholar]
- 31. Hanahan D. Signaling vascular morphogenesis and maintenance. Science 1997; 277: 48–50. [DOI] [PubMed] [Google Scholar]
- 32. Davis S, Yancopoulos GD. The angiopoietins: ying and yang in angiogenesis. Curr Top Microbiol Immunol 1999; 237: 173–85. [DOI] [PubMed] [Google Scholar]
- 33. Hamaguchi I, Morisada T, Azuma M et al . Loss of Tie2 receptor compromises embryonic stem cell‐derived endothelial but not hematopoitic cell survival. Blood 2006; 107: 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 1997; 81: 567–74. [DOI] [PubMed] [Google Scholar]
- 35. Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin‐1 and ‐2, ligands for the endothelial‐specific receptor tyrosine kinase Tie2. J Biol Chem 1998; 273: 18 514–21. [DOI] [PubMed] [Google Scholar]
- 36. Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin‐1 induces sprouting angiogenesis in vitro . Curr Biol 1998; 8: 529–32. [DOI] [PubMed] [Google Scholar]
- 37. Kim I, Kim HG, Moon SO et al . Angiopoietin‐1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res 2000; 86: 952–9. [DOI] [PubMed] [Google Scholar]
- 38. Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin‐1 regulates endothelial cell survival through the phosphatidylinositol 3V‐kinase/Akt signal transduction pathway. Circ Res 2000; 86: 24–9. [DOI] [PubMed] [Google Scholar]
- 39. Daly C, Wong V, Burova E et al . Angiopoietin‐1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 2004; 18: 1060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis S, Aldrich TH, Jones PF et al . Isolation of angiopoietin‐1, a ligand for the TIE2 receptor, by secretion‐trap expression cloning. Cell 1996; 87: 1161–9. [DOI] [PubMed] [Google Scholar]
- 41. Hawighorst T, Skobe M, Streit M et al . Activation of the tie2 receptor by angiopoietin‐1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol 2002; 160: 1381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stoeltzing O, Ahmad SA, Liu W et al . Angiopoietin‐1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res 2003; 63: 3370–7. [PubMed] [Google Scholar]


