Abstract
Ezrin, the linker between membrane protein and cytoskeleton, plays an important role in the cellular morphology, cytoskeleton reorganization, adhesion, invasion and metastasis. In this study, ezrin was found to express in high levels either in nasopharyngeal carcinoma tissues or in nasopharyngeal carcinoma 5‐8F cells and the knockdown of ezrin expression in the 5‐8F cells by RNA interferance could reduce invasive ability, suggesting that ezrin is involved in the progression and invasion of nasopharyngeal carcinoma. Nasopharyngeal carcinoma‐associated gene 6 is a novel candidate suppressor gene of tumor metastasis, which was originally cloned in nasopharyngeal carcinoma high‐frequency heterozygosity loss region 9p21‐22 and is down‐regulated in nasopharyngeal carcinoma. In the present study, we hypothesize that nasopharyngeal carcinoma‐associated gene 6 plays an inhibitory role in the migration and invasion of nasopharyngeal carcinoma cells via modulating the function of ezrin. Firstly, different mutants of NGX6 were constructed and transfected into nasopharyngeal carcinoma 5‐8F cells. The invasion and migration of 5‐8F cells overexpressing nasopharyngeal carcinoma‐associated gene 6 or mutants were measured. The results showed that enhanced expression of nasopharyngeal carcinoma‐associated gene 6 could reduce invasive and migratory abilities of 5‐8F cells, and the cytoplasmic domain was essential for nasopharyngeal carcinoma‐associated gene 6 to modulate cell migration and invasion. Further experiment results showed that nasopharyngeal carcinoma‐associated gene 6 protein was associated with ezrin by its cytoplasm region, and it could down‐regulate the expression level of ezrin. These results demonstrated that nasopharyngeal carcinoma‐associated gene 6 was probably involved in the modulation of invasive and adhesive ability of nasopharyngeal carcinoma cells by down‐regulating the expression level of ezrin. (Cancer Sci 2007; 98: 341–349)
Abbreviations:
- NGX6
nasopharyngeal carcinoma‐associated gene 6
- LOH
loss of heterozygosity
- EGF
epidermal growth factor
- CYTO
cytoplasm
- TM1
transmembrane 1
- TM2
transmembrane 2
- G418
neomycin
- NPC
nasopharyngeal carcinoma
- DAPI
4′,6‐Diamidino‐2‐phenylindole, FAK, focal adhesion kinase
- EGFR
epidermal growth factor receptor
- RNAi
RNA interfere
- shRNA
small hair RNA, siRNA, small interfere RNA
- TMA
tissue microarray
- GFP
enhanced green fluorescent protein
Nasopharyngeal carcinoma (NPC) is rare in most countries but occurs with relatively high frequency in southern China, Kenya, the Philippines, Singapore, Tunisia, Sudan and Uganda. In contrast to this, the prevalence of NPC in neighboring oriental countries, including northern China and Japan is quite low. Emigrants from endemic countries to nonendemic areas such as the US, continue to carry this high risk, while first and second generation descendants carry progressively lower risks. The most frequently suspected etiologic factors are viral, genetic and environmental.( 1 )
Many scholars reported that the genetic factor is critical in the development and progression of NPC next to Epstein‐Barr virus (EBV) infection and environmental factors. It is very important to clone and screen the NPC‐susceptible gene to elucidate its mechanism. NPC is characteristic as poorly differentiated and highly metastatic in clinical pathology.( 2 )
Loss of heterozygosity of chromosomes 3, 5, 6, 7, 8, 9, 11, 13, 17, 18, 20, 21 in NPC has been reported. High frequency loss of heterozygosity (LOH) region 9p21‐22 is closely associated with NPC.( 3 , 4 , 5 , 6 ) Nasopharyngeal carcinoma‐associated gene 6(NGX6) was isolated from this region by positional candidate clone strategy in 1999.( 7 , 8 ) Bioinformatics investigation reveals that the full length of NGX6 cDNA is 2134 bp, encoding a protein of 338 amino acids with a predicated molecular weight of 37 kDa. NGX6 protein includes two transmemberane regions, an extracellular domain and a short cytoplasmic region. There is an EGF‐like domain and three potential N‐glycosylation sites in the extracellular domain. The short cytoplasmic region contains a tyrosine residue that is a potential phosphorylation site of tyrosine kinase.
In normal nasopharyngeal epithelial tissues, NGX6 was expressed in high levels, while it was expressed in very low or undetectable levels in NPC biopsies and cells. NGX6 was also found down‐regulated in colorectal carcinomas, and the down‐regulation ratio of NGX6 in colorectal carcinoma tissues with lymph‐node or distant metastasis (15/16) was significantly higher than those without metastasis (25/34) (P < 0.05). Transfection of NGX6 into nasopharyngeal and colorectal carcinoma cells could induce the reversion of some malignant phenotypes and down‐regulate the molecules of EGFR pathway. Subcellular localization of NGX6 protein is mostly in membrane by using a GFP‐NGX6 fusion protein in COS7 and COS1 cells. It was also found that NGX6 could interact with ezrin by immunoprecipitation assay in COS7 cells.( 9 , 10 )
Ezrin, a membrane cytoskeleton linker, is involved in cellular functions, including epithelial cell morphogenesis and adhesion. The ezrin, radixin and moesin (ERM) family consists of three closely related proteins, ezrin, radixin and moesin.( 11 ) Ezrin was first identified as a constituent of microvilli( 12 ) radixin as a barbed, end‐capping actin‐modulating protein from isolated junctional fractions( 13 ) and moesin as a heparin binding protein.( 14 ) ERM molecules contain three domains, an N‐terminal globular domain; an extended alpha‐helical domain; and a charged C‐terminal domain.( 11 ) The N‐terminal domain is highly conserved, and is also found in merlin, band 4.1 proteins and members of the band 4.1 superfamily. ERM proteins crosslink actin filaments with plasma membranes.
In order to elucidate the role of the interaction between NGX6 and ezrin in the process of invasion and migration of NPC, four domain‐deleted mutants of NGX6 were successfully constructed and transfected into 5‐8F cells, respectively.( 15 , 16 ) The role of NGX6 or mutants on migration and invasion of 5‐8F cells was measured by matrigel migration assay and scraping wound healing assay. The relationship of NGX6 and ezrin was demonstrated by immunoprecipitation assay. The effect of NGX6 on the expression level of ezrin was detected by immunoblot. Tissue microarray (TMA) and immunohistochemistry technology were performed to demonstrate whether ezrin was involved in the development and progression of NPC.
Materials and Methods
Tissue specimens and TMA construction. Nasopharyngeal biopsy specimens including 148 NPC and 164 non‐cancerous nasopharyngeal epithelia (NCNPE) were collected in the ENT department at Xiangya Hospital (Changsha, China). All biopsied tissues immediately were fixed in 4% buffered paraformaldehyde, routinely processed, and embedded with paraffin. The TMA was assembled with a tissue array instrument (Beecher Instruments, Silver Springs, MD, US). Two composite high‐density TMA receptive blocks including 390 spots were designed. Three 0.6‐mm diameter tissue cores were taken from each NPC and two 0.6‐mm diameter tissue cores were taken from each NCNPE. The sections were covered with thin paraffin and stored at 4°C before immunohistochemistry assay.( 17 , 18 )
Immunohistochemistry and immunostaining evaluation. Protein expression of ezrin on TMA was analyzed immunohistochemically by the labeled streptavidin/peroxidase (SP) method. TMA sections were deparaffinized and immersed in 0.01 M citrate buffer (PH 6.0), heated in a domestic microwave oven at full power for 15 min, then immersed in methanol containg 0.3% H2O2 for 30 min to block endogenous peroxidase activity. TMA slides were immunohistochemically stained using the SP staining method as previously described.( 19 ) The polyclonal rabbit antihuman ezrin antibodies (Upstate, NY, USA) in 1:500 were used. Color was developed using 3.3′‐diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin. Slides were also stained in the absence of primary antibodies to evaluate non‐specific secondary antibody reactions. Immunohistochemical staining of TMA sections was scored microscopically at ×400 magnification in all available tumor or epithelial cells meeting the typical morphological criteria by two pathologists using the qualitative scale described in the literatures.( 20 ) The number of cells staining was scored as 0 (no staining), 1 + (< 1/3 positive cells), 2 + (> 1/3 and < 2/3 positive cells) and 3 + (> 2/3 positive cells). The intensity of staining was scored from 1 + (weak) to 3 + (strong). The immunoreactive score was categorized into three groups by comprehensive evaluation of the percentage of positive cells and staining intensity as reported previously. No staining was considered negative (0 score), weakly, moderately and strongly staining were considered positive (1 score, 2 scores and 3 scores, respectively).
Immunoprecipitation and immunoblot analysis. Extraction of proteins from cultured cells was performed as previously described with a lysis buffer consisting of 50 mM Tris‐HCl (pH 7.5), 0.1% sodium dodecyl sulfate (SDS), 1% Triton X‐100, 150 mM NaCl, 1 mM dithiothreitol, 0.5 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, leupeptin (12 mg/mL), aprotinin (20 mg/mL), 100 mM sodium vanadate, 100 mM sodium pyrophosphate, and 1 mM sodium fluoride.( 16 ) Cell extracts were clarified by centrifugation at 12 000 rpm for 10 min, and the supernatants (1.5 mg of protein/mL) were subjected to immunoprecipitation with myc antibodies (BD, Franklin Lakes, NJ, USA). After incubation overnight at 4°C, protein A‐ or G‐agarose (Santa) beads were added and left for an additional 3 h, then agarose beads were gently washed with lysis buffer.( 21 ) The agarose beads were resuspended with 20 µL loading buffer. All cell lysates and the suspension were subjected to 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS‐PAGE) under reducing conditions (with 2.5% 2‐mercaptoethanol) and transferred to PVP (polyvinylpyrrolidone) membranes. The membranes were blocked and probed with the appropriate ezrin‐antibody (Upstate, NY, US) and rabbit IgG, followed by chemiluminescence with the ECL reagent (Pierce, FL, US).
RNA extraction and RT‐PCR. Total RNA was isolated from COS7.HNE1,6‐10B and 5‐8F cells using Trizol Reagent (Life Technologies, Inc., Taipei City, Taiwan) according to the manufacturer's protocol. A 3‐mg aliquot of total RNA from each specimen was reverse‐transcribed into single‐strand cDNA using oligo (dT)15 primer and Superscript II (Life Technologies, Inc.). Each single‐strand cDNA was diluted for subsequent polymerase chain reaction (PCR) amplification of NGX6 and GAPDH with the latter used as an internal quantitative control. The PCR was carried out in a reaction volume of 25 µL for 5 min at 95°C for initial denaturing, followed by 25 (for GAPDH) or 30 (for NGX6) cycles of 94°C for 40 30 s, 55°C for 40 s, and 72°C for 40 s on the Gene Amp PCR system 9600 (Perkin‐Elmer Corp., Foster City, CA, USA). The primer sequences used for amplification were 5′‐Gtcatccatgacaactttggtatc‐3′ and 5′‐ctgtagccaaattcgttgtcatac‐3′ for GAPDH and 5′‐caatgcgagctccgtgcgcc‐3′ and 5′‐ggaaccaggtccccgtct‐3′ for NGX6. PCR products were resolved in 2% agarose gels and visualized by staining with ethidium bromide. To quantify PCR products, the bands representing amplified products were analyzed by Pharmacia Biotect Image MASTER VDS.
RNAi. The vector used in this study is pRNAT‐U6 (Genscript) which contains separate GFP and shRNA expression elements.( 22 , 23 , 24 , 25 , 26 ) It carries a neomycin resistance gene as the selectable marker, which can be used for establishing stable cell lines. The GFP marker (coral GFP, cGFP) under CMV promoter control can be used to track the transfection efficiency. Double‐stranded DNA oligonucleotides coding for two different shRNAs designed to target ezrin were each ligated downstream of a U6 promoter (Fig. 1). To test the efficacy of each of the shRNAs in knocking down ezrin expression, we transduced 5‐8F cells with vector carrying shRNA or control vectors and then transiently transfected into the cells. Twenty‐four hours after tranfection, the effects of RNAi on the expression of ezrin protein were analyzed by Western blotting. The siRNA vector which effectively inhibits the expression of ezrin was used to proceed the matrigel invasion assay.
Figure 1.
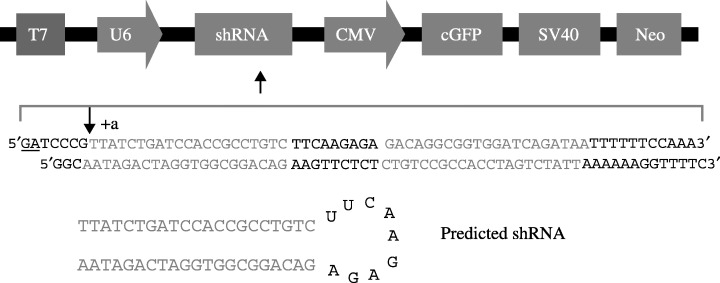
Ezrin small hair RNA construct design. DNA oligonucleotides containing ezrin small hair RNA a (shown) and b (not shown) were inserted downstream of the U6 promoter in pRnat‐U6. The blue sequence is equivalent to the ezrin mRNA target sequence. The presumed U6 transcription start site designated as +a (shown) and the predicted small hair RNA structure is shown. U6, Pol III promoter; shRNA, small hairpin RNA; GFP, enhanced green fluorescent protein.
Plasmid construction cells and culture conditions. NGX6 mutants including ΔEGF (EGF‐like domain deleted), ΔTM1 (transmembrane‐1 deleted) and ΔTM2 (transmembrane‐2 deleted) and ΔCYTO (cytoplasm region deleted) were generated by one‐step PCR with plasmid pCMV‐NGX6 (constructed by Dr Ma Jian) as a template.( 27 ) The primers were designed from the two termini of deletion regions as follows: EGF left primer: gacagtgcagatgcgctcac; EGF right primer: ggacaggaaggtgcgcatc; Cyto left primer: atgttcttctcccggtatgcg; Cyto right primer: actccgaatggccaggacc; TM1 left primer: gatatgtgctggaagctgcagcagtctac; TM1 right primer: gcagctggaatccataggtgagcgc; TM2 left primer: gtctgtatttccaccacgtt ctccg; TM2 right rimer: ggtgaaggtgtagactgcagcttcc. PCR products were purified with gel extraction kit (Sangon, Shanghai, China), and phosphated by T4PNK and cyclized by T4DNA ligase (TakaRa, Biotechnology, Dalian, China). The reconstituted plasmids were transformed into Ecoli JM109. The plasmids were confirmed by restriction enzyme digestion and DNA sequencing. So we successfully constructed the vectors of pCMV‐NGX6, pCMV‐NGX6ΔEGF, pCMV‐NGX6ΔTM1, pCMV‐NGX6ΔTM2, and pCMV‐NGX6ΔCYTO. To construct the vectors of the pcDNA3.1 system, the ORF of NGX6 or mutants were confirmed plasmids and were used as templates for producing NGX6 and its deletion mutant ORF. NGX6 and mutants ORF were amplified by PCR with common primers pcDNA3.1 left primer: ttggatccaaccatggccctggtcc ctggagc and pcDNA3.1 right primer: cccaagctgcgcgatttactccaatgtgtc. PCR products were inserted into vector pcDNA3.1(‐)B‐myc‐his with T4DNA ligase after digestion by restriction enzymes, then transformed into Ecoli JM109, and positive clones were confirmed by restriction enzyme digestion and DNA sequencing. They were named as pcDNA3.1(‐)B‐NGX6, pcDNA3.1(‐)B‐ΔEGF,pcDNA3.1(‐)B‐ΔTM1,pcDNA3.1(‐)B‐ΔTM2, pcDNA3.1(‐)B‐ΔCYTO, (Fig. 2). These vectors were transfected into 5‐8F cells, respectively, by lipofectamine 2000, (Invitrogen, US), which were established from the metastatic nodule of human nasopharyngeal carcinoma, and vector pcDNA3.1(‐)B‐myc‐his (Invitrogen, US) was used as control plasmid. The cells transfected with the above plasmids, respectively, were grown and isolated in G418‐containing medium (400 µg/mL) and G418‐resistant clones were established for 2 weeks. The pcDNA3.1(‐)B‐myc‐his vector alone was also transfected into 5‐8F cells to generate control clones, designated as 5‐8F‐pcDNA3.1.
Figure 2.
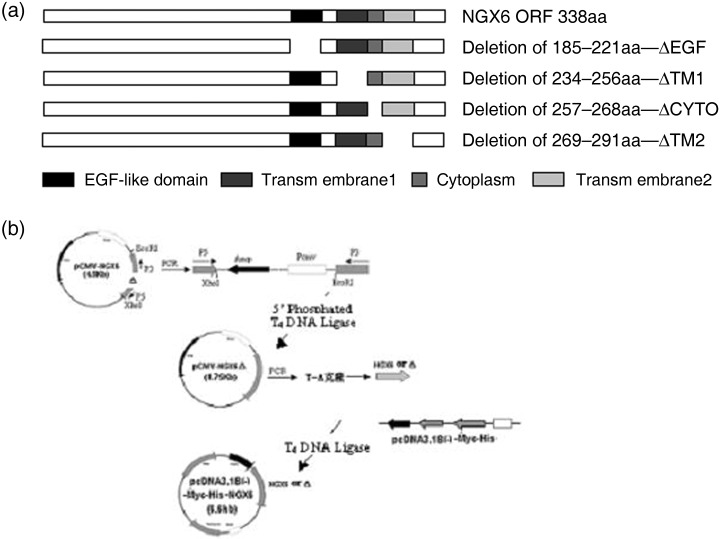
Construction of deleted mutants of nasopharyngeal carcinoma‐associated gene 6 and expression in 5‐8F cells. (a) Schematic representation of NGX6 mutants deleted different domains, respectively; (b) Construct design of expression vector of the mutants.
Matrigel invasion assay. Cell invasion was measured by a Matrigel invasion chamber assay( 28 ) which was performed using 8‐µm pore size Transwell chambers (Corning Incorporated, Corning, NY, USA). Matrigel (BD, Franklin Lakes, NJ, USA) was diluted in cold distilled water (200 mg/mL), 0.1 mL added to the upper well of the Transwell chamber, then dried in a sterile hood. The Matrigel was reconstituted with medium for 1 h at 37°C before the addition of cells. 5‐8F cells transfected with pcDNA3.1(‐)B‐NGX6, pcDNA3.1(‐)B‐ΔEGF, pcDNA3.1(‐)B‐ΔTM1, pcDNA3.1(‐)B‐ΔTM2, pcDNA3.1(‐)B‐ΔCYTO or pcDNA3.1(‐)B‐myc‐his, respectively, were starved overnight in serum‐free medium and resuspended at a concentration of 2.5 × 105 cells/mL in serum‐free medium containing 0.1% bovine serum albumin; 0.2 mL of cell suspension was added to the top of each well, then 5 mg/mL fibronectin solution was added to the bottom well of the chamber as a chemo‐attractant. After 24 h, the cells that had not invaded were removed from the upper surface of the filters using a cotton swab. The cells that had invaded to the lower surface of the filter were fixed with methanol, stained with H&E and five high‐power fields (200×) were counted.
In vitro scratch wound healing assay. 5‐8F‐NGX6, 5‐8F‐ΔEGF, 5‐8F‐ΔTM1, 5‐8F‐ΔTM2, 5‐8F‐ΔCYTO and 5‐8F‐pcDNA3.1 cells (2.5 × 105) were cultured in cover slips to confluence without vacant space. Cell migration was measured by the in vitro scratch wound healing assay.( 29 ) Monolayer cells were scratched with a sterilized pipette tip in 6‐well plastic dishes. After 18 h of culture in RPMI 1640 supplemented with 2% serum, the cell migration was evaluated by measuring the width difference of the wounds between 0 h and 18 h.
Results
Clinicopathological significance of ezrin in NPC with or without lymph node metastasis. Since the present study showed that the role of NGX6 expression was associated with ezrin in NPC 5‐8F cells, we investigated the association between ezrin protein expression and the clinicopathological factors (lymph node metastasis) of NPC patients using immunohistochemistry with an NPC TMA containing 312 nasopharyngeal tissue samples. The positive expression rate of ezrin was 69% (69/100) in the group of 100 NPC patients with lymph node metastasis, while it was 45.83% (22/48) in the group of 48 NPC patients without lymph node metastasis, and 46.34% (76/164) in the group of 164 non‐NPC patients (Fig. 3, 1, 2). Statistical analysis revealed that the positive ratio of ezrin expression was lower in the non‐metastasis group than that in the metastasis group. Similarly, the positive ratio of ezrin expression was lower in the non‐NPC group than that in NPC groups. Meanwhile, it was found that the expression of ezrin was located cytoplasm and membrane in NPC cells. Furthermore, the immunoblot analysis was performed to detect the expression of ezrin in COS7, HNE1, 6‐10B and 5‐8F cells. The results indicated that ezrin was expressed at the highest level in 5‐8F cells which have high potential to metastasize, relatively lower level in HNE1 and 6‐10B nasopharyngeal carcinoma cells, and basical level in COS7 cells (Fig. 4). Inversely, NGX6 was expressed in COS7 cells, followed by 6‐10B, HNE1 and 5‐8F cells.
Figure 3.
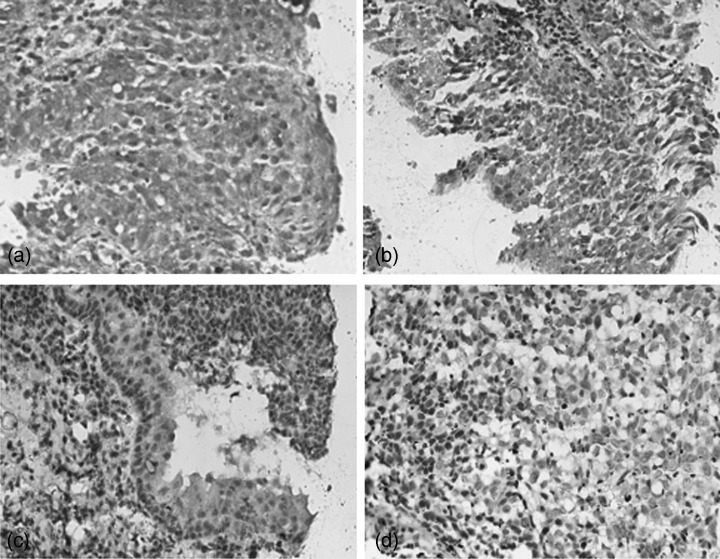
Expression of the ezrin protein on the tissue microarray including human nasopharyngeal carcinoma and non‐cancerous nasopharyngeal epithelium was detected by immunohistochemistry. (a) Positive expression of ezrin in nasopharyngeal carcinoma (3,3’‐Diaminobenzidine [DAB] staining, 40×); (b) Positive expression of ezrin in dysplastic nasopharyngeal epithelia (DAB staining, 20×); (c) weak positive expression of ezrin in inflammatory nasopharyngeal epithelia (DAB staining, 20×); (d) Ezrin protein expression was negative in nasopharyngeal carcinoma (DAB staining, 20×).
Table 1.
Ezrin expression in NPC and inflammatory nasopharyngeal epithelium
| Positive (%) | Negative (%) | |
|---|---|---|
| NPC | 91 (61.49) | 57 (38.51) |
| NCNPE | 76 (46.34)* | 88 (53.66) |
Note: χ2 test revealed that the higher positive rate of ezrin expression in NPC compared with chronically inflammatory nasopharyngeal epithelium (P < 0.05), (*P < 0.05). NPC, nasopharyngeal carcinoma: NCNPE, non‐cancerous nasopharyngeal epithelium
Table 2.
Relationship between expression of ezrin protein and nasopharyngeal carcinoma clinicopathological characteristics
| Positive (%) | Negative (%) | |
|---|---|---|
| Clinical phase I | 19 (42.22)*** | 26 (57.78) |
| II | 31 (62.00) | 19 (48.00) |
| III and IV | 41 (77.36) | 12 (32.64) |
| NPC with node metastasis | 69 (69.00)** | 31 (31.00) |
| NPC without node metastasis | 22 (45.83) | 26 (54.17) |
Note: χ2 test revealed that the metastasis group had higher ezrin expression positive ratio than the non−metastasis group (P < 0.01). (**P < 0.01, *P < 0.05); the expression of ezrin in clinical phase I of nasopharyngeal carcinoma patients was lower positive rate than that of in clinical phase ΙΙορΙΙΙ or IV of NPC patients (P < 0.01). (***P < 0.01). NPC, nasopharyngeal carcinoma
Figure 4.
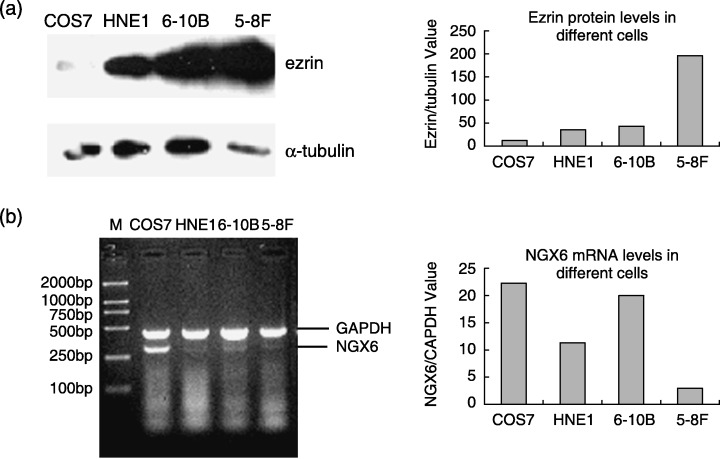
Expression of ezrin and NGX6 in COS7, HNE1, 6‐10B, 5‐8F cells. (a) Ezrin protein was expressed at the higher level in 5‐8F cells which had high potential of metastasis than in 6‐10B cells which has the same parentage as 5‐8F cells but no metastasis potential in immunoblot assay; (b) Nasopharyngeal carcinoma‐associated gene 6 mRNA was expressed at the higher level in 6‐10B cells than 5‐8F cells by RT‐PCR experiment.
Knockdown of ezrin reduced the invasion of 5‐8F cells by RNAi. Cells transfected with pRNAT‐U6 or pRNAT‐U6‐E1,2 exhibited almost complete ezrin expression, while those transfected with pRNAT‐U6‐E3−4 exhibited almost complete suppression of ezrin expression based on Western blot analysis (Fig. 5b). Taking advantage of the pRNAT‐U6 system, four targeted sequences against ezrin mRNA were cloned into the siRNA expression vector. Among them, two sequences were verified as effective in inhibiting the expression of ezrin by Western blot analysis (Fig. 5b). The effective siRNA expression vector was transfected into 5‐8F cells. The transient transfection rate was about 50–70%. The positive cells expressing GFP protein showed green fluorescence under fIuorescence microscope. The invasive ability of 5‐8F cells with or without knockdown of ezrin was measured by matrigel invasion assay. Results showed that after knockdown of ezrin, the number of cells which moved through the base membrane decreased (Fig. 5).
Figure 5.
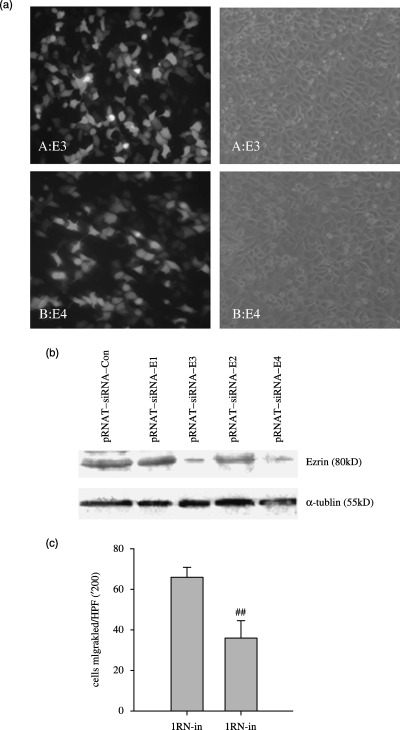
Knockdown of ezrin expression blocks the in vitro invasive activities of different 5‐8F cells. (a) the transient transfection of pRNAT‐siRNA‐E3 into 5‐8F cells; (b) siRNA‐mediated knockdown of ezrin protein expression. Lysates prepared from cells with pRNAT‐siRNA‐E were analyzed by immunoblotting using antibody as indicated. Cells transfected with pRNAT‐U6 or pRNAT‐U6‐E1,2 exhibited almost complete ezrin expression, while those transfected with pRNAT‐U6‐E3 or pRNAT‐U6‐E4 exhibited almost complete suppression of ezrin expression based on western blot analysis. (c) Data collection and presentation are described in Materials and Methods. Results shown are mean ± SEM of three experiments. The knockdown of ezrin by RNA interference reduced the invasive ability of 5‐8F cells (P < 0.05) (## P < 0.05).
Expression of NGX6 or mutants in 5‐8F cells. The eukaryotic expression plasmids of NGX6 or mutants were transfected into NPC 5‐8F cells, respectively. The lyzed protein of each group cells after selection with G418 for 2 weeks was analyzed with immunoblotting (Fig. 6).
Figure 6.
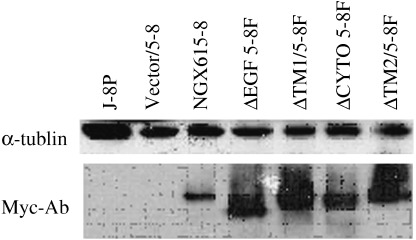
Protein expression of NGX6 and the mutants in 5‐8F cells. The fusion protein of NGX6 or mutants with Myc‐tag were detected with antibody against Myc‐tag. No detective positive signals were found in the 5‐8F cells or 5‐8F cells transfected with pcDNA3.1‐myc‐his. The molecular weight of NGX6 or mutants was 37–40 kDa.
Effects of NGX6 and its mutants on invasion and migration of 5‐8F cells. Matrigel invasion experiments was performed based on the principle of the Boyden chamber assay. The matrigel served as a reconstituted basement membrane in vitro. The number of cells migrated through the matrigel was counted and the result is shown in Fig. 7 The 5‐8F‐NGX6 cells showed significant reduction of invasion as compared with 5‐8F‐pcDNA cells (P < 0.05). These data indicated that enhanced expression of NGX6 in 5‐8F cells was associated with reduced invasive ability. The deletion mutant ΔCYTO has no inhibitory role in the invasion and mobility of NPC 5‐8F cells. This suggested that the CYTO is an essential domain for NGX6 to modulate the migration and invasion of 5‐8F cells. The results showed that ΔEGF, ΔTM1 and ΔTM2 also had the effects of inhibiting the migration and invasion of 5‐8F cells, which are similar to NGX6.
Figure 7.
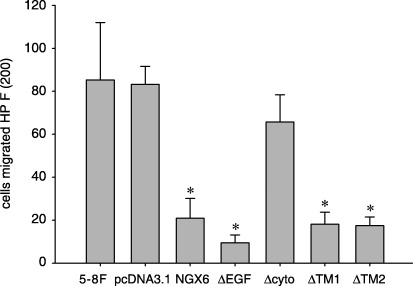
Effects of NGX6 and its deletion mutants on in vitro invasion of nasopharyngeal carcinoma 5‐8F cells. Cells were plated in invasion Transwell with filters coated with matrigel. After incubation for 24 h, cells that migrated through the filters were stained with H&E and counted in five randomly selected fields. The invasive cell number of NGX6/5‐8F: ΔEGF/5‐8F, ΔTM1/5‐8F, ΔTM2/5‐8F decreased significantly compared with control group pcDNA3.1/5‐8F (P < 0.05). It showed that the cytoplasm region was essential to modulate the migration ability for nasopharyngeal carcinoma‐associated gene 6.
In vitro scratch wound healing assay and the effects of NGX6 on cell migration in vitro are shown in Fig. 8. The migration distance of the cells transfected with NGX6 was 1.97 ± 0.33 (which was significantly smaller than those of the pcDNA group (3.85 ± 0.22) (1/200 cm) (P < 0.01). Therefore, enhanced expression of NGX6 could inhibit the migration of 5‐8F cells. The migration distances of 5‐8F cells transfected with ΔEGF, ΔTM1 and ΔTM2 cells were similar to that of the NGX6 transfected cells, while 5‐8F cells transfected with ΔCYTO had no difference compared to control cells. The results demonstrated that the CYTO region was essential for NGX6 to modulate the migration of 5‐8F cells.
Figure 8.
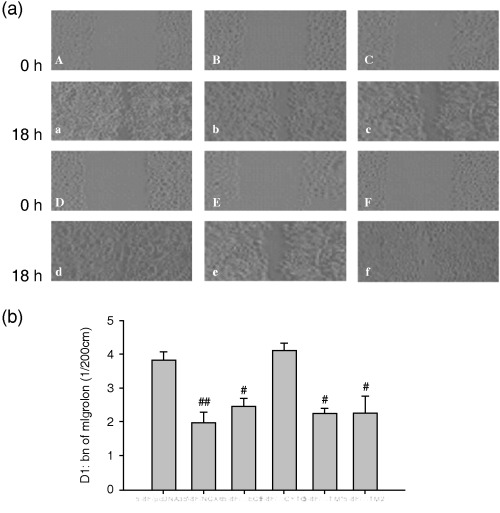
Role of nasopharyngeal carcinoma‐associated gene 6 and its different mutants on the mobility of nasopharyngeal carcinoma cells 5‐8F. Relative cell motility was calculated as the wound width at t = 0 h minus the wound width at t = 18 h, as indicated. There is no difference between ΔCYTO/5‐8F and pcDNA3.1/5‐8F cells. The mobility ability of NGX6/5‐8F, ΔEGF/5‐8F, ΔTM1/5‐8F, ΔTM2/5‐8F reduced when compared with pcDNA3.1/5‐8F cells. (a) Effect of nasopharyngeal carcinoma‐associated gene 6 and mutants on invasion and migration of NPC 5‐8F Cells a–f: pcDNA3.1/5‐8F, NGX6/5‐8F, ΔEGF/5‐8F, ΔCyto/5‐8F, ΔTM1/5‐8F, ΔTM2/5‐8F at 0 h, A–F: pcDNA3.1/5‐8F, NGX6/5‐8F, ΔEGF/5‐8F, ΔCyto/5‐8F, ΔTM1/5‐8F, ΔTM2/5‐8F at 18 h. (b) Data collection and presentation are described in Materials and Methods. Results shown are mean ± SEM of three experiments (# P < 0.05, ## P < 0.01)
NGX6 inhibits the invasion of 5‐8F cells by association with ezrin. Previous study has shown that NGX6 interacted with ezrin in COS7 cells by immunoprecipitation and immunofIuorescence testing. Our present experiment demonstrated that NGX6 interacted with ezrin through its cytoplasm region examined by immunoprecipitation assay.
In this experiment, we determined whether NGX6, ΔEGF, ΔTM1, ΔTM2 or ΔCYTO could associate with ezrin in 5‐8F cells, respectively. 5‐8F cells were transiently transfected with vectors of Myc‐NGX6 (or ΔEGF or ΔTM1 or ΔTM2 or ΔCYTO) or pCMV‐myc (as the negative control). Cell lysates were immunoprecipitated with antibody against myc‐tag, and then Western blot analysis was performed using anti‐ezrin as the primary antibody. We determined that NGX6, ΔEGF, ΔTM1 and ΔTM2 could associate with ezrin, but ΔCYTO cannot (Fig. 9). The results suggested that NGX6 could associate with ezrin by its cytoplasm region. The lysates of cells overexpressing NGX6 or mutants were also directly used to measure the expression of ezrin by Western blot analysis with ezrin as the primary antibody. The expression of ezrin was down‐regulated in 5‐8F cells transfected with NGX6, ΔEGF, ΔCYTO, ΔTM1 and ΔTM2; however, the expression of ezrin in 5‐8F cells transfected with ΔCYTO was higher than the other cells (Fig. 10).
Figure 9.
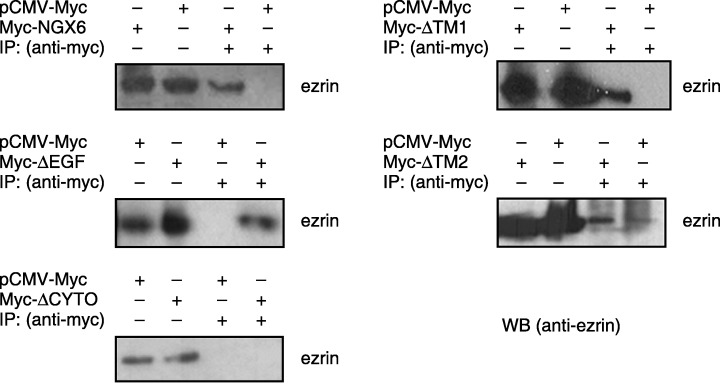
Co‐immunoprecipitation of ezrin with nasopharyngeal carcinoma‐associated gene 6 or its mutants in 5‐8F cells. Transiently expressed Myc‐NGX6 or its mutant protein were immunoprecipitated with antibody against Myc‐tag. Immunoprecipitates were then separated on 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis, transferred and probed with an antiezrin antibody. Nasopharyngeal carcinoma‐associated gene 6, ΔEGF, ΔTM1 and ΔTM2 could interact with ezrin, but ΔCYTO could not. This suggests that nasopharyngeal carcinoma‐associated gene 6 could associate with ezrin by its cytoplasm region.
Figure 10.
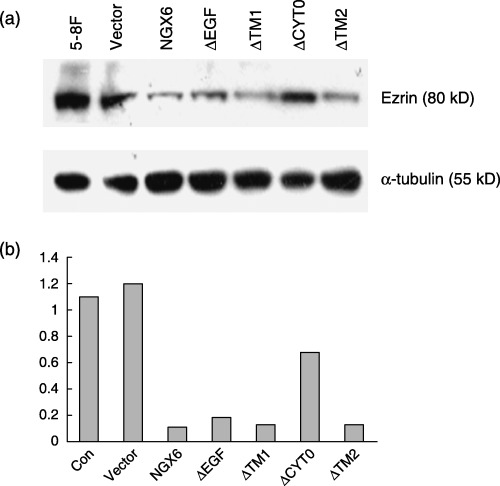
(a) Western blot analysis of ezrin in 5‐8F cells in the presence of nasopharyngeal carcinoma‐associated gene 6 or constructed mutants. α‐tubulin was used as the internal control. (b) Quantitative analysis of the protein levels were measured by scanning densitometry. The protein expression levels of ezrin was decreased in 5‐8F cells transfected with nasopharyngeal carcinoma‐associated gene 6, ΔEGF, ΔTM1 and ΔTM2, but not altered in 5‐8F cells transfected with the vector. The protein expression level of ezrin in 5‐8F cells transfected with ΔCYTO was higher than that in 5‐8F cells transfected with nasopharyngeal carcinoma‐associated gene 6, but lower than that in control cells.
Discussion
Our results demonstrated that NGX6 could interact with ezrin by its cytoplasmic region. Ezrin, a linker between membrane protein and cytoskeleton, played an important role in cell morphology, cytoskeleton reorganization, adhesion, invasion and metastasis.( 20 , 30 , 31 , 32 , 33 ) The basic expression of ezrin is essential to support the morphology and function of cells; however, the high expression of ezrin is an important factor for invasion and metastasis of many malignant tumors such as endometrial cancer, uterine endometrioid adenocarcinoma, breast carcinoma and osteosarcoma.( 34 , 35 , 36 , 37 ) Our results showed that the positive expression ratio of ezrin in NPC patients was greater than that in non‐NPC patients, and the positive ratio of ezrin in NPC patients with lymph nodes metastasis was much greater than that in NPC patients without metastasis. So the positive ratio of ezrin expression may be associated with the clinicopathological phase. Furthermore, the expression of ezrin was higher in 5‐8F cells which had high potential of metastasis compared to 6‐10B cells, which is low, but are derived from the same parentage as 5‐8F cells. It is noteworthy that Yang et al. reported similar results in these two different cell lines.( 38 ) Moreover, knockdown of ezrin expression by RNAi could reduce the invasive ability of 5‐8F cells. In conclusion, the results demonstrated that the high expression of ezrin was a promoting factor to accelerate the development and progression of NPC. In the present study, we examined the role of NGX6 and its mutants in the migration and invasion of 5‐8F cells which were derived from biopsies carrying Epstein‐Barr virus from NPC patients with node metastasis. 5‐8F cells are epidermoid carcinoma cells with poor differentiation in pathology and have high metatstasis potential to lungs and lymph nodes. NGX6 is a tumor suppressor gene located at the high frequency heterozygosity loss region of NPC 9p13.1. It could inhibit the invasion and migration of 5‐8F cells. It was suggested that the down‐regulation of NGX6 in NPC, especially in NPC with metastasis of lymph nodes was probably an important molecular event in the development and metastasis of nasopharyngeal carcinoma. NGX6 protein contains an EGF‐like domain, two transmembrane and a cytoplasmic region. We had presumed that the EGF‐like domain of NGX6 would be essential for NGX6 to modulate the invasion and metastasis of 5‐8F cells, but in fact, the cytoplasmic region seems more critical for the role of NGX6. There is a tyrosine residue in the cytoplasmic region of NGX6 which can be a potential phosphorylation site of tyrosine kinase. The function of NGX6 would be associated with phosphorylation of tyrosine residue by tyrosine kinases when extra‐cellular signals were transmitted into the inside of cells.
In our study, the overexpression of NGX6 could down‐regulate the expression level of ezrin in 5‐8F cells, which was different from the results reported by Dr Majian.( 13 ) This may result from the different roles of ezrin in different stages and derivation of carcinoma cells. The mutants of NGX6, including ΔEGF, ΔTM1, ΔTM2 had similar effects to NGX6 on ezrin, while ΔCYTO showed no difference from control pcDNA3.1‐His‐Myc. In matrigel migration and scraping assay, CYTO also showed no inhibitory role in the migration and invasion of 5‐8F cells. At the same time, ΔCYTO protein could not interact with ezrin in immunoprecipitation assay. From these results, we inferred that the mechanism of NGX6 to inhibit the invasion and metastasis of 5‐8F cells is mediated by interacting with ezrin and down‐regulating the expression of ezrin.
In conclusion, our present results demonstrate that ezrin is an important promotive factor in the development and metastasis of NPC. NGX6 played an inhibitory role in the migration and invasion of NPC cells by interacting with ezrin through its cytoplasmic region and down‐regulating the expression of ezrin and ezrin‐related signaling molecules. These findings provide important clues for NGX6 to function in cell migration and metastasis modulation.
Acknowledgments
This Research was supported by grants from the National 973 Program of China (2006CB910500), the National Natural Scientific Foundation of China (No. 30300064, No. 30470965, No. 30500584), the Special Funds for Major State Basic Research of China (2005CCA03200), the Province Natural Scientific Foundation (06JJ20042, 05JJ10006) and the Science and Technology Bureau Key Project of Hunan Province (04SK2002, 05SK1001‐1).
References
- 1. Fandi A, Altun M, Armand JP, Cvitkovic E. Nasopharyngeal cancer: epidemiology, staging and treatment. Semin Oncol 1994; 21: 382–97. [PubMed] [Google Scholar]
- 2. Raab‐Traub N. Epstein‐Barr virus and nasopharyngeal carcinoma. Semin Cancer Biol 1992; 3: 297–307. [PubMed] [Google Scholar]
- 3. Wiest JS, Franklin WA, Otstot JT et al. Identification of a novel region of homozygous deletion on chromosome 9p in squamous cell carcinoma of the lung: the location of a putative tumor suppessor gene. Cancer Res 1999; 57: 1–6. [PubMed] [Google Scholar]
- 4. Deng L, Jing N, Tan G et al. A common region of allelic loss on chromosome region 3p25.3‐26.3 in nasopharyngeal carcinoma. Genes Chromosomes Cancer 1998; 23 (1): 21–5. [DOI] [PubMed] [Google Scholar]
- 5. Kim NG, Kim JJ, Ahn JY et al. Putative chromosomal deletions on 9p, 9q and 22q occur preferentially in malignant gastrointestinal stroma tumors. Int J Cancer 2000; 85 (5): 633–8. [DOI] [PubMed] [Google Scholar]
- 6. Weber TK, Conroy J, Keitz B et al. Genome‐wide allelotyping indicates increased loss of heterozygosity on 9p and 14q in early age of onset colorectal cancer. Cytogenet Cell Genet 1999; 86 (2): 142–7. [DOI] [PubMed] [Google Scholar]
- 7. Yang J, Tang X, Deng L et al. Detailed deletion mapping of chromosome 9p21∼22 in nasopharyngeal carcinoma. Chin J Oncol 1999; 21 (6): 419–21. [PubMed] [Google Scholar]
- 8. Yang J, Bing L, Li Z et al. Refined localization and cloning of a novel putative tumor suppressor gene associated with nasopharyngeal carcinoma on chromosome 9p21‐22. Chinese J Cancer 2000; 19 (1): 6–9. [Google Scholar]
- 9. Ma J, Zhou J, Fan S. et al. Role of a novel EGF‐like domain‐containing gene NGX6 in cell adhesion modulation in nasopharyngeal carcinoma ells. Carcinogenesis 2005 February; 26 (2): 281–91. [DOI] [PubMed] [Google Scholar]
- 10. Wang L, Ma J, Li J et al. NGX6 gene inhibits cell proliferation and plays a negative role in EGFR pathway in nasopharyngeal carcinoma cells. J Cell Biochem 2005 May 1; 95 (1): 64–73. [DOI] [PubMed] [Google Scholar]
- 11. Tsukita S, Yonemura SERM proteins. head‐to‐tail regulation of actin–plasma membrane interaction. Trends Biochem Sci 1997; 22: 53–8. [DOI] [PubMed] [Google Scholar]
- 12. Bretscher A. Purification of an 80 000‐dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol 1983; 97: 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsukita S, Hieda Y. A new 82‐kD barbed end‐capping protein (radixin) localized in the cell‐to‐cell adherens junction: purification and characterization. J Cell Biol 1989; 108: 2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lankes W, Griesmacher A, Grunwald J, Schwartz‐Albiez R, Keller R. A heparin‐binding protein involved in inhibition of smooth‐muscle cell proliferation. Biochem J 1988; 251: 831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song L, Wang H, Zheng M et al. Study on the tumor heterogeneity of nasopharyngeal carcinoma cell line (SUNE‐1). J Pract Oncol 2002; 17 (1): 11–3. [Google Scholar]
- 16. Song L, Yan J, Wang H et al. Molecular mechanisms tumorgenesis metastasis nasopharyngeal carcinoma cell sublines. Chin J Cancer 2002; 21 (2): 158–62. [PubMed] [Google Scholar]
- 17. Song QF, Jian M, Jie Z et al. Differential expression of Epstein‐Barr virus‐encoded RNA and several tumor‐related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Human Pathol 2006. (in press). [DOI] [PubMed]
- 18. th International Union Against Cancer (UICC) Classification. TNM Classification of Malignant Tumours, 5th edn.Germany: Springer Verlag, 997. [Google Scholar]
- 19. Wong SC, Chan JK, Lee KC, Hsiao WL. Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J Pathol 2001; 194: 35–42. [DOI] [PubMed] [Google Scholar]
- 20. Shanmugaratnam K. Histological typing of tumours of the upper respiratory tract and ear. World Health Organization International Histological Classification of Tumours, 2nd edn. Berlin: Springer‐Verlag, 1991: 32–4. [Google Scholar]
- 21. Ozawa M, Baribault H, Kemler R et al. Phosphorylation of beta‐catenin by cyclic AMP‐dependent protein Kinase. EMBO J 1989; 8: 1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown D, Jarvis R, Pallotta V, Byrom M, Ford L. RNA interference in mammalian cell culture: design, execution, and analysis of the siRNA effect. Ambion Technotes 2002; 9 (1): 3–5. [Google Scholar]
- 23. Sui G, Soohoo C, Affar EB, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector‐based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 2002; 99 (8): 5515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu J‐Y, DeRuiter SL, Turner DL. RNA interference by expression of short‐interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 2002; 99 (9): 6047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paul CP, Good PD, Winer I, Engelke DR. Effective expression of small interfering RNA in human cells. Nat Biotechnol 2002; 20: 505–8. [DOI] [PubMed] [Google Scholar]
- 26. Miyagishi M, Taira K. U6 promoter‐driven siRNAs with four uridine 3¢ overhangs effectively suppress targeted gene expression in mammalian cells. Nature Biotechnol 2002; 20: 497–500. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Shen B, Li Y et al. Applying a new PCR Method to quickly prepare human TNF‐a deletion mutants. Chin J Biochem Mol Biol 18 (5): 600–4. [Google Scholar]
- 28. Mohanam S, Sawaya R, McCutcheon I et al. Modulation of in vitro invasion of human glioblastoma cells by urokinase‐type plasminogen activator receptor antibody. Cancer Res 1993; 53: 4143–7. [PubMed] [Google Scholar]
- 29. Joyce NC, Matkin ED, Neufeld AH. Corneal endothelial wound closure in vitro. Invest Ophthalmol Vis Sci 1989; 30: 1548–59. [PubMed] [Google Scholar]
- 30. Harms BD, Bassi GM, Horwitz AR, Lauffenburger DA. Directional persistence of EGF‐induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys J 2005 February; 88 (2): 1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao X, Cheng L, Forte JG. Biochemical characterization of ezrin/actin interaction. J Biol Chem 1996; 271 (12): 7224–9. [DOI] [PubMed] [Google Scholar]
- 32. Ng T, Parsons M, Hughes WE et al. Ezrin is a downstream effector of trafficking PKC‐integrin complexes involved in the control of cell motility. EMBO J 2001; 20: 2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA. High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun 1999; 258 (2): 395–400. [DOI] [PubMed] [Google Scholar]
- 34. Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res 2005; 7 (3): R365–73. Epub 2005 March 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohtani K, Sakamoto H, Rutherford T et al. Ezrin, a membrane‐cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett 2002 May 8; 179 (1): 79–86. [DOI] [PubMed] [Google Scholar]
- 36. Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane‐cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett 1999 December 1; 147 (1–2): 31–8. [DOI] [PubMed] [Google Scholar]
- 37. Wick W, Grimmel C, Wild‐Bode C, Platten M, Arpin M. Weller. Ezrin‐dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL‐2 and transforming growth factor‐beta2. J Neurosci 2001 May 15; 21 (10): 3360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang XY, Ren CP, Wang L et al. Identification of differentially expressed genes in metastatic and non‐metastatic nasopharyngeal carcinoma cells by suppression subtractive hybridization. Cell Oncol 2005; 27 (4): 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


