Abstract
Nectin‐like molecule‐5 (Necl‐5) is an immunoglobulin (Ig)‐like molecule that is up‐regulated in many types of cancer cells. It was shown experimentally that Necl‐5 enhances cell migration, proliferation, and metastasis, but its clinical significance has not been documented. The aim of this study was to observe the expression of Necl‐5 in surgically resected primary lung adenocarcinomas and to investigate its clinical significance. A total of 63 surgically resected primary pulmonary adenocarcinoma tissues were investigated by immunohistochemistry for the expression of Necl‐5. The relationship between expression of Necl‐5 and clinicopathological features was analyzed, and the influence of Necl‐5 expression on outcomes in these patients was assessed. A strong expression of Necl‐5 by cancer cells was observed in 43 of the 63 tumors. The overexpression of Necl‐5 by cancer cells was significantly associated with lymph node metastasis (P = 0.0398), TNM staging (P = 0.0367), and the bronchioloalveolar carcinoma ratio of tumors (P = 0.0423). Furthermore, the disease‐free survival rate in patients with positive Necl‐5 overexpression was significantly lower than that in patients with negative Necl‐5 overexpression (P = 0.0004). Multivariate survival analysis revealed Necl‐5 expression to be an independent risk factor for an unfavorable outcome (P = 0.0294). Additionally, an analysis including only the stage I cases revealed that the disease‐free survival rate of the Necl‐5‐positive group was significantly lower than that of the Necl‐5‐negative group (P = 0.0192). These results indicate that Necl‐5 plays a role in mediating tumor cell invasion and that the overexpression of Necl‐5 in cancer cells has clinical significance for prognostic evaluation of patients with primary pulmonary adenocarcinoma.
(Cancer Sci 2010; 101: 1326–1330)
Necl‐5/poliovirus receptor (PVR)/CD155/Tage4 is an immunoglobulin(Ig)‐like molecule with a domain structure consisting of one extracellular region with three Ig‐like loops, one transmembrane region, and one cytoplasmic region.( 1 ) Human PVR/CD155 was originally identified as the PVR in a screen of genomic DNA from cells susceptible to poliovirus infection.( 2 , 3 ) Rodent Tage4 was originally identified in the rat by monoclonal antibody screens to isolate tumor‐specific markers of hepatocellular carcinoma( 4 ) and colon carcinoma.( 5 , 6 , 7 , 8 ) Tage4 was also found to be expressed in fetal rat liver.( 4 ) PVR/CD155 was subsequently shown to be overexpressed in many human cancer cells.( 9 , 10 , 11 ) The PVR/CD155 gene thus far has been found only in primates and the Tage4 gene has been found only in rodents, but these genes are likely to be derived from a common ancestral gene;( 12 , 13 ) therefore, PVR/CD155 and Tage4 were renamed nectin‐like molecule‐5, or Necl‐5.( 1 , 14 ) Nectins are Ca2+‐independent Ig‐like cell–cell adhesion molecules.( 1 ) Nectin‐like molecules (Necls) have been designated as a group of Ig‐like molecules with domain structures similar to, but slightly different from, those of nectin.( 1 )
The physiological roles of Necl‐5 except that of PVR have remained unknown for a long time, but recently some of these roles have been elucidated. Necl‐5 colocalizes with integrin avβ3 at leading edges of migrating cells and enhances serum‐ and platelet‐derived growth factor (PDGF)‐induced cell migration in an integrin‐dependent manner.( 15 ) Cdc42 and Rac small G proteins are activated by the action of Necl‐5,( 15 ) causing the formation of filopodia and lamellipodia, respectively,( 16 , 17 ) which are necessary for cell motility.( 18 ) Necl‐5 enhances the serum‐induced activation of Ras‐Raf‐MEK‐ERK signaling, up‐regulating cyclins D2 and E and down‐regulating p27kip1, shortening the period of the G0/G1 phase of the cell cycle. Similarly, Necl‐5 enhances PDGF‐induced cell proliferation.( 19 ) Necl‐5 has also heterophilic cell–cell adhesion activity selectively with nectin‐3.( 14 , 20 ) The interaction of Necl‐5 with nectin‐3 promotes recruitment of E‐cadherin to nectin‐3 in the initial stages of cell–cell adhesion( 21 ) and causes the endocytosis‐mediated down‐regulation of Necl‐5 from the cell surface at the cell–cell contact, resulting in reduction of cell movement and proliferation.( 22 ) Such down‐regulation of Necl‐5 might be, at least in part, one mechanism that underlies contact inhibition of cell movement and proliferation. It was also demonstrated that Necl‐5 expression increased and then promptly decreased in regenerating liver after partial hepatectomy or acute injury.( 23 )
High levels of Necl‐5 expression have been observed in a wide range of transformed and cancer cell lines.( 3 , 4 , 7 , 8 , 11 , 14 ) In various human cancer tissues, up‐regulated Necl‐5 expression also has been observed.( 9 , 10 , 11 ) Experiments using transformed or cancer cell lines that strongly express Necl‐5 have shown that the down‐regulation of Necl‐5 in these cells decreases migration,( 11 , 15 ) proliferation,( 19 ) and metastasis.( 15 ) It was also shown that colitis‐associated neoplasia induced by dimethylhydrazine and/or dextran sodium sulphate treatment was significantly less frequent in Necl‐5‐deficient mice than in wild‐type mice, suggesting that Necl‐5 plays a role in cancer development.( 24 ) Furthermore, an experiment in which colon adenocarcinoma cells were injected into the tail vein of mice showed that up‐regulated expression of cell surface Necl‐5 increased the number of metastatic foci in the lungs.( 25 )
According to previous reports and the elucidated biological properties of Necl‐5, Necl‐5 up‐regulation in cancer cells may play a role in their invasion and metastasis, and possibly influence prognosis in patients. But an analysis of Necl‐5 expression using a large number of surgically resected human cancer tissues has not been reported. Neither has the clinical significance of Necl‐5 been documented. We analyzed the expression of Necl‐5 by immunohistochemistry and observed the mode of expression of Necl‐5 in surgically resected human primary pulmonary adenocarcinoma tissue from 63 patients. We also investigated whether Necl‐5 expression in tumor tissues was associated with outcomes in these patients.
Materials and Methods
Patients. We analyzed data on 63 patients (39 males, 24 females) who underwent operations for primary lung cancer at our institution and whose tumor specimens after surgery were histologically classified as adenocarcinoma according to the criteria of the World Health Organization. This group included 18 consecutive patients who underwent an operation between January and August 2002 and 45 consecutive patients who underwent an operation between January and December 2004. They ranged in age from 42 to 86 years, and the mean age at the time of surgery was 67.5 years. All cancers were TNM classified and staged using the criteria of the seventh edition of the International Union Against Cancer (UICC) Staging System for Lung Cancer. Staging was based on data obtained from imaging, bronchoscopy, invasive diagnostic techniques, operative findings, and pathologic examinations. Of the 63 patients, 23, 14, four, three, 15, two, and two had stage IA, IB, IIA, IIB, IIIA, IIIB, and IV tumors, respectively (Table 1). Lobectomy, segmentectomy, and complete pneumonectomy with dissection of hilar and mediastinal lymph nodes were performed on 52, six, and one patient, respectively, and four patients had partial resection with sampling of hilar and mediastinal lymph nodes. Of the total patient group, two, one, and five patients received induction chemotherapy, radiation, and chemoradiation, respectively. Two patients were administered postoperative adjuvant chemotherapy. The postoperative clinical course was assessed by analyzing outpatient medical records and by making telephone inquiries. The day of surgery was considered Day 0 in calculating postoperative survival. Follow‐up ranged from 1 to 80 months (mean, 44 months).
Table 1.
Association between Necl‐5 overexpression and clinico‐pathologic characteristics
| Variable | Total | Necl‐5 | P‐values | |
|---|---|---|---|---|
| Positive | Negative | |||
| No. patients | 63 | 43 | 20 | |
| Age (years), mean and range | 67.5 (42–86) | 67.3 (42–81) | 68.0 (48–86) | 0.7774 |
| Gender | ||||
| Male/Female | 39/24 | 26/17 | 13/7 | 0.7301 |
| T factor | ||||
| T1/T2/T3/T4 | 29/27/6/1 | 17/19/6/1 | 12/8/0/0 | 0.2076 |
| N factor | ||||
| N0/N1/N2/N3 | 41/5/16/1 | 23/5/14/1 | 18/0/2/0 | 0.0398 |
| M factor | ||||
| M0/M1 | 61/2 | 41/2 | 20/0 | 0.3270 |
| Stage | ||||
| I/II/III/V | 37/7/17/2 | 20/6/15/2 | 17/1/2/0 | 0.0369 |
| P factor | ||||
| 0/1/2/3 | 42/11/6/4 | 28/7/5/3 | 14/4/1/1 | 0.8325 |
| PA invasion | ||||
| Positive/Negative | 12/51 | 11/32 | 1/19 | 0.0528 |
| PV invasion | ||||
| Positive/Negative | 27/36 | 22/21 | 5/15 | 0.0508 |
| LY invasion | ||||
| Positive/Negative | 28/35 | 22/21 | 6/14 | 0.1156 |
| BAC ratio, mean | 26.0% | 19.3% | 40% | 0.0423 |
BAC, bronchioloalveolar carcinoma; LY, lymph duct; Necl‐5, nectin‐like molecule‐5; PA, pulmonary artery; PV, pulmonary vein.
Immunohistochemistry. Expression of Necl‐5 was analyzed in 63 surgically resected primary lung adenocarcinoma specimens. Using the immunoperoxidase technique, formalin‐fixed paraffin‐embedded sections 3‐mm thick were stained. The primary antibody used in this study was a rabbit polyclonal antibody to polio virus receptor (ab60115; Abcam, Cambridge, UK) (diluted 1:100). Sections were deparaffinized in xylene, heat‐treated for 20 min in 10 mm citrate‐phosphate buffer (pH 6.0) for antigen retrieval, incubated with the primary antibody, and stained using EnVision/HRP Universal Kit Rabbit/Mouse (DAB) (K1390; Dako, Glostrup, Denmark). Optical microscopic observation of each specimen was performed. We regarded the specimens as Necl‐5 positive if 5% or more cancer cells within a tumor were strongly stained.
The sections were also immunostained with mouse monoclonal antibody to Vimentin (M0725) (diluted 1:100) to detect stroma cells.
The frozen specimens of surgically resected primary lung adenocarcinoma were embedded and cut on cryostat. The frozen sections 5‐mm thick were dried after preparation, fixed in dry acetone for 10 min, and immunostained with mouse monoclonal antibody to human integrin avβ3 (CD51/CD61) (MAB3050; R&D Systems, Minneapolis, MN USA) (diluted 50 mg/mL) and a rabbit polyclonal antibody to polio virus receptor (ab60115; Abcam) (diluted 1:50).
Statistical analysis. Associations between Necl‐5 overexpression on cancer cells and clinicopathological features were determined using the χ2‐test. Survival was examined using the Kaplan–Meier method, and the significance of difference was evaluated by a log‐rank test. Variable effects on survival time were investigated using Cox’s regression model. Statistical significance was determined as P < 0.05. All statistical analyses were performed using Stat View (version 5.0; SAS Institute, Cary, NC, USA).
Results
Optical microscopic observation of Necl‐5 expression in tissues. Necl‐5 expression in surrounding normal lung tissue in the tumor specimens was not observed (Fig. 1a). In two bronchioloalveolar carcinomas classified as Noguchi type A, in which there is no focus of collapse of the alveolar structure,( 26 ) cancer cells were not stained or were stained very weakly (Fig. 1b,c). The Necl‐5 overexpression in the stroma of the alveolar wall was observed in the peripheral area of the bronchioloalveolar carcinomas (Fig. 1c). Many of the cancer cells strongly expressed Necl‐5 at the invasive sites of tumors; and the stroma around the cancer cells at these sites also was stained strongly (Fig. 1d,e,g). In such areas, stroma cells and cancer cells were mixed in a disorderly pattern. On the other hand, in several specimens, solitary cancer cells or small numbers of cancer cells were stained in a scattered fashion (Fig. 1f), although in many specimens many cancer cells were stained diffusely.
Figure 1.
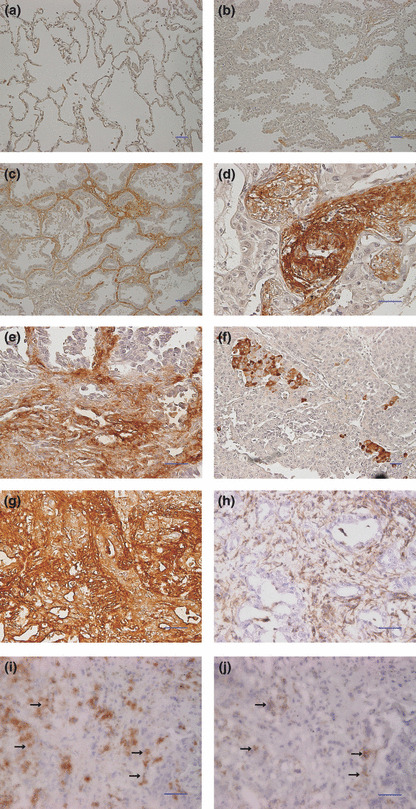
Nectin‐like molecule‐5 (Necl‐5) expression in primary lung adenocarcinoma tissues. Normal lung tissue and primary pulmonary adenocarcinoma tissues were stained with the anti‐Necl‐5 antibody using the immunoperoxidase technique. (a) Necl‐5 was not expressed in normal lung tissue. (b) Cancer cells were not stained in the bronchioloalveolar carcinomas classified as Noguchi type A. (c) Necl‐5 was expressed only in the stroma in the peripheral area of the bronchioloalveolar carcinoma. (d–f) Cancer cells strongly expressed Necl‐5. (g,h) The serial sections were stained with the anti‐Necl‐5 antibody (g) and the anti‐Vimentin antibody (h) to verify stroma cells expressing Necl‐5. Vimentin was expressed in the stroma cells but not in the cancer cells (h). Comparison of these two slides revealed that Necl‐5 was expressed in both cancer and stroma cells. (i,j); The serial frozen sections were stained with the anti‐Necl‐5 antibody (i) and the anti‐integrin avβ3 antibody (j). Comparison showed that both Necl‐5 and integrin avβ3 were expressed at the same sites (arrow in i and j). It suggested the colocalization of Necl‐5 and integrin avβ3 in the invasive front of lung cancer samples. Scale bar, 50 mm in (a–j).
The samples were immunostained with antibody to vimentin, a marker of stromal cells, to verify stroma cells expressing Necl‐5 at the invasive site. The comparative studies of the vimentin‐stained (Fig. 1h) and Necl‐5‐stained (Fig. 1g) serial sections demonstrated that Necl‐5 was expressed in both stroma cells, which were stained by anti‐Vimentin antibody, and cancer cells.
The immunostaining of the serial sections of frozen samples with antibodies to integrin avβ3 and Necl‐5 showed that the Necl‐5‐expressing cancer cells also expressed integrin avβ3 (Fig. 1i,j). This result supported a previous report that Necl‐5 colocalizes with integrin avβ3 at leading edges of migrating cells( 15 ).
Relationship between Necl‐5 expression and clinicopathological characteristics of patients. We regarded specimens as Necl‐5 positive if 5% or more cancer cells within a tumor were strongly stained; all other specimens were regarded as negative. Five percent was used as the cut‐off value because of the statistical advantage in this study. As a result, 43 specimens were classified as Necl‐5 positive and 20 specimens as Necl‐5 negative. The relationships between Necl‐5‐positive cases and various clinicopathological characteristics at the time of surgery are shown in Table 1. Expression of Necl‐5 was significantly associated with lymph node metastasis (P = 0.0398), TNM stage (P = 0.0367), and the bronchioloalveolar carcinoma ratio of tumor (P = 0.0423). Necl‐5 overexpression was not significantly related to age (P = 0.7774), gender (P = 0.7301), T factor in the criteria of the seventh edition of the UICC Staging System for Lung Cancer (P = 0.2056), distant metastasis (P = 0.3270), cancer invasion to the pulmonary artery (P = 0.0528), cancer invasion to the pulmonary vein (P = 0.0508), or cancer invasion to lymphatic ducts (P = 0.1156).
Relationship between Necl‐5 expression and disease outcome. A survival analysis based on immunochemical examinations revealed that the 5‐year disease‐free survival rate of the Necl‐5‐positive group was 33.7%, while that of the Necl‐5‐negative group was 94.7%. A log‐rank test showed that the difference was statistically significant (P = 0.0004), with the lifespan curve for the Necl‐5‐negative patients above that for the Necl‐5‐positive patients (Fig. 2). A univariate analysis indicated that among clinicopathological factors, tumor classification, lymph node metastasis, and Necl‐5 overexpression correlated with outcomes (Table 2). Further assessment with Cox’s multivariate analysis demonstrated that lymph node metastasis and Necl‐5 overexpression were independent risk factors that affected outcome (Table 2).
Figure 2.
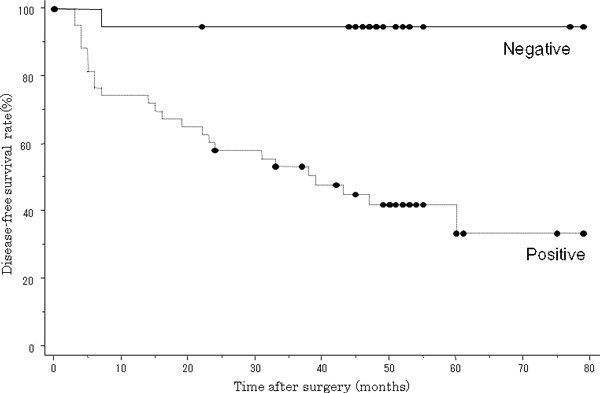
Postoperative disease‐free survival rate among patients with positive and negative nectin‐like molecule‐5 (Necl‐5) overexpression by cancer cells in primary pulmonary adenocarcinomas. The disease‐free survival rate in Necl‐5‐positive patients was significantly lower than that in Necl‐5‐negative patients (P = 0.0004).
Table 2.
Univariate and multivariate analysis of prognostic factors
| Variable | Hazard ratio | 95% Confidence interval | P‐values |
|---|---|---|---|
| Univariate | |||
| Age | 0.995 | 0.949–1.042 | 0.8286 |
| Gender (male vs female) | 0.479 | 0.201–1.144 | 0.0976 |
| T factor (T1 vs other) | 0.337 | 0.141–0.804 | 0.0142 |
| LN (positive vs negative) | 6.765 | 2.884–15.871 | <0.0001 |
| Necl‐5 (positive vs negative) | 15.055 | 2.035–111.373 | 0.0079 |
| Multivariate | |||
| Age | 1.008 | 0.953–1.065 | 0.7858 |
| Gender (male vs female) | 0.523 | 0.215–1.274 | 0.1539 |
| T factor (T1 vs other) | 0.608 | 0.243–1.520 | 0.2870 |
| LN (positive vs negative) | 3.935 | 1.576–9.825 | 0.0033 |
| Necl‐5 (positive vs negative) | 9.537 | 1.253–72.581 | 0.0294 |
LN, lymph node metastasis; Necl‐5, nectin‐like molecule‐5.
Relationship between Necl‐5 expression and disease outcome in stage I patients. Among the stage I cases, 20 patients were classified as Necl‐5 positive and 17 patients as Necl‐5 negative (Table 1). Survival analysis on only the stage I patients revealed that the 5‐year disease‐free survival rate of the Necl‐5‐positive group was 46.9%, while that of the Necl‐5‐negative group was 100%. The log‐rank test showed that the difference was statistically significant (P = 0.0192) (Fig. 3). Only the Necl‐5‐positive patients had cancer recurrence in the stage I group. The sites of recurrence were the lung, pleura, hilar lymph node, brain, and liver.
Figure 3.
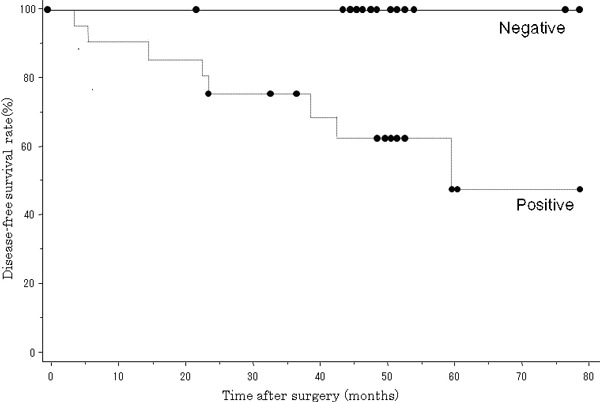
Postoperative disease‐free survival rate among patients with positive and negative nectin‐like molecule‐5 (Necl‐5) overexpression by cancer cells in stage I primary pulmonary adenocarcinomas. The disease‐free survival rate in Necl‐5‐positive patients was significantly lower than that in Necl‐5‐negative patients (P = 0.0192).
Discussion
Up‐regulated Necl‐5 expression has been reported in a wide range of transformed and cancer cell lines,( 3 , 4 , 7 , 8 , 11 , 14 ) and has also been observed in various human cancer tissues.( 9 , 10 , 11 ) Additionally, experimental studies have demonstrated that up‐regulated Necl‐5 expression in cancer cells enhances migration, proliferation, and distant metastasis.( 11 , 15 , 19 , 25 ) According to these earlier reports and the already‐reported biological properties of Necl‐5, Necl‐5 up‐regulation in cancer cells may play a role in tumor invasion. However, an analysis of Necl‐5 expression in a large number of surgically resected human cancer tissues has not been reported previously, nor has the clinical significance of Necl‐5 been ascertained. Our findings, presented herein, are the first to show that Necl‐5 overexpression in cancer tissue specimens is related to the clinicopathological features of lung carcinoma and the clinical outcome of patients.
The analysis of the relationship between Necl‐5 overexpression in cancer cells in primary pulmonary adenocarcinoma tissues and the clinicopathological features of the patients from whom these tissues were obtained showed that Necl‐5 overexpression was correlated with lymph node metastasis and TNM stage. The degree of Necl‐5 overexpression was increased in tandem with the degree of lymph node metastasis and TNM stages, which indicated that Necl‐5 plays a role in the invasion mechanism of lung tumors. In order to define the effect of Necl‐5 overexpression on the prognosis of patients with lung cancer, a prognostic analysis was performed on follow‐up data. The results showed that the disease‐free survival rate in patients with Necl‐5 overexpression was notably lower than that in patients negative for Necl‐5 overexpression, indicating that positivity for Necl‐5 overexpression has a harmful effect on the clinical course and further indicating that Necl‐5 was correlated with tumor malignancy. Cox’s multivariate analysis proved that Necl‐5 overexpression was an independent risk factor affecting prognosis. Furthermore, a prognostic analysis including only the stage I cases revealed that the disease‐free survival rate of the Necl‐5‐positive group was significantly lower than that of the Necl‐5‐negative group. The recurrence rate of resected stage I non‐small‐cell lung cancer was reported to be about 30%.( 27 , 28 , 29 ) In our study, cancer relapse was observed only in the Necl‐5‐positive patients among the stage I cases. In these patients, dormant micrometastases may have existed at the time of surgery. The strong expression of Necl‐5 might demonstrate the invasive activity of the cancer cells. Several experimental studies have clarified that up‐regulated Necl‐5 expression in cancer cells enhances migration, proliferation, and distant metastasis.( 11 , 15 , 19 , 25 ) Our results suggest that Necl‐5 may be taken as a reference index for molecular staging to select patients at high risk of recurrence among the stage I patients who may benefit from intensive adjuvant therapy. Additionally, the earlier report, in which anti‐Necl‐5 monoclonal antibody inhibited lung metastasis of the cancer cells,( 25 ) suggested the possibility of using Necl‐5 as a molecular target. We need more studies about the meaning of overexpression of Necl‐5 in lung adenocarcinoma, integrating this parameter in prospective studies of adjuvant chemotherapy and in the setting of the palliative chemotherapy and evaluating is potential prognostic role.
Necl‐5 transcripts are detectable in many normal tissues, including the human lung, by reverse transcription–polymerase chain reaction or northern blotting after an extended exposure time,( 3 , 13 , 30 ) although the Necl‐5 expression level is very low in most adult organs, and Necl‐5 proteins are undetectable by western blotting, indirect immunofluorescence, or immunoperoxidase staining protocols in normal adult rodent tissues.( 4 , 14 ) A previous immunohistochemical analysis in human normal tissues reported no staining of Necl‐5 in many organs, although there was moderate staining in some organs, including the lung.( 11 ) Therefore, we regarded cancer tissue that was stained strongly as positive and that in which there was no clear contrast with normal tissues as negative. Up‐regulated Necl‐5 expression has been observed by immunohistochemical analysis in various human cancer tissues, including colon carcinoma,( 10 , 11 ) malignant glioma,( 9 , 11 ) prostate carcinoma, renal cell carcinoma, pancreatic carcinoma, ovarian carcinoma, non‐small‐cell lung carcinoma, and breast carcinoma.( 11 ) In summary, Necl‐5 has been reported to be overexpressed in almost all types of cancer tissue tested. In our study, we detected strongly stained cancer cells in 43 of the 63 lung adenocarcinoma specimens. One report showed an increased level of Necl‐5 in 12 of 12 colorectal adenocarcinoma samples,( 10 ) while other reports showed increased levels ranging from one of 10 samples to 19 of 25 samples in various types of cancer tissue.( 9 , 11 ) Necl‐5 overexpression may thus be useful for prognostic evaluation in other types of cancer as well. Two types of positive staining were reported in a previous study of glioblastoma cells; specifically, scattered positive cells within a predominantly negative sample or diffuse staining across many cells in the sample.( 11 ) Similarly, in our study, we observed the staining of a solitary cancer cell or a small number of cancer cells in a scattered fashion in some specimens and diffuse staining among many cancer cells in many specimens. In the specimens of diffuse staining type, the majority of cancer cells were partially positive for staining but this observation was not acquired in every part of the tumor. No cases showed 50% or more stained cancer cells, while two cases had 25% or more and 10 cases had 10% or more. There was no statistically significant difference in clinical outcomes neither in 10% or more nor in 25% or more groups. We therefore chose 5% as a criteria of positivity and considered that the presence of cancer cells overexpressing Necl‐5, even in a small proportion, was related to unfavorable prognosis.
According to our microscopic observations, Necl‐5 was strongly or moderately expressed in the stroma of all tumors, but not in that of normal lung tissues. With regard to this result, Necl‐5 expression in the stroma was suggested to have some oncological significance. The Necl‐5 overexpression in the stroma of the alveolar wall was observed in the peripheral area of the tumor, but not at the tumor center in many of the bronchioloalveolar carcinomas. The expression of Necl‐5 in the alveolar wall may play a part in tumor expansion to surrounding normal tissue along the alveolar wall. On the other hand, strongly stained cancer cells appeared to infiltrate aggressively into strongly stained stroma at the areas of invasive components of many tumors. Cancer cells possibly stimulate overexpression of Necl‐5 in stromal cells. Clinical studies reported a poor prognosis of patients with bronchioloalveolar carcinoma containing actively proliferating stromal fibroblasts.( 26 , 31 ) Tokunou et al. suggested the importance of cancer cell–stroma communication for the development of the invasive component of bronchioloalveolar carcinoma.( 31 ) Necl‐5 might have a role in the aberrant behavior of stromal fibroblasts in the invasive component of bronchioloalveolar carcinoma. Thus, because the stroma of the alveolar wall in bronchioloalveolar carcinomas as well as the stroma around cancer cells at sites of invasion was stained strongly, the significance of the expression of this protein in the stroma should be further examined.
In conclusion, our results suggest that the abnormal overexpression of Necl‐5 in cancer cells in primary lung adenocarcinomas play an important role in invasion of lung adenocarcinomas and has a negative effect on the prognosis of patients. These results support using the overexpression of Necl‐5 as a reference index of molecular staging to select patients at high risk of recurrence who may benefit from intensive adjuvant therapy.
References
- 1. Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin‐like molecules: Roles in cell adhesion, migration, and polarization. Cancer Sci 2003; 94: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: Molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 1989; 56: 855–65. [DOI] [PubMed] [Google Scholar]
- 3. Koike S, Horie H, Ise I et al. The poliovirus receptor protein is produced both as membrane‐bound and secreted forms. EMBO J 1990; 9: 3217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faris RA, McEntire KD, Thompson NL, Hixson DC. Identification and characterization of a rat hepatic oncofetal membrane glycoprotein. Cancer Res 1990; 50: 4755–63. [PubMed] [Google Scholar]
- 5. Douillard JY, Laborda J, Burg C et al. Monoclonal antibodies to a rat colon carcinoma: model for monoclonal antibody therapy of solid tumors. Cancer Res 1989; 49: 687–92. [PubMed] [Google Scholar]
- 6. Chadeneau C, Denis MG, Blottiere HM, Gregoire M, Douillard JY, Meflah K. Characterization, isolation and amino terminal sequencing of a rat colon carcinoma‐associated antigen. Int J Cancer 1991; 47: 903–8. [DOI] [PubMed] [Google Scholar]
- 7. Chadeneau C, LeMoullac B, Denis MG. A novel member of the immunoglobulin gene superfamily expressed in rat carcinoma cell lines. J Biol Chem 1994; 269: 15601–5. [PubMed] [Google Scholar]
- 8. Lim YP, Fowler LC, Hixson DC, Wehbe T, Thompson NL. TuAg.1 is the liver isoform of the rat colon tumor‐associated antigen pE4 and a member of the immunoglobulin‐like supergene family. Cancer Res 1996; 56: 3934–40. [PubMed] [Google Scholar]
- 9. Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A 2000; 97: 6803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masson D, Jarry A, Baury B et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001; 49: 236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sloan KE, Eustace BK, Stewart JK et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer 2004; 4: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baury B, Geraghty RJ, Masson D, Lustenberger P, Spear PG, Denis MG. Organization of the rat Tage4 gene and herpesvirus entry activity of the encoded protein. Gene 2001; 265: 185–94. [DOI] [PubMed] [Google Scholar]
- 13. Ravens I, Seth S, Förster R, Bernhardt G. Characterization and identification of Tage4 as the murine orthologue of human poliovirus receptor/CD155. Biochem Biophys Res Commun 2003; 312: 1364–71. [DOI] [PubMed] [Google Scholar]
- 14. Ikeda W, Kakunaga S, Itoh S et al. Tage4/nectin‐like molecule‐5 heterophilically trans‐Interacts with cell adhesion molecule nectin‐3 and enhances cell migration. J Biol Chem 2003; 278: 28167–72. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda W, Kakunaga S, Takekuni K et al. Nectin‐like molecule‐5/Tage4 enhances cell migration in an integrin‐dependent, nectin‐3‐independent manner. J Biol Chem 2004; 279: 18015–25. [DOI] [PubMed] [Google Scholar]
- 16. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–14. [DOI] [PubMed] [Google Scholar]
- 17. Takai Y, Sasaki T, Matozaki T. Small GTP‐binding proteins. Physiol Rev 2001; 81: 153–208. [DOI] [PubMed] [Google Scholar]
- 18. Mitchison TJ, Cramer LP. Actin‐based cell motility and cell locomotion. Cell 1996; 84: 371–9. [DOI] [PubMed] [Google Scholar]
- 19. Kakunaga S, Ikeda W, Shingai T et al. Enhancement of serum‐and platelet‐derived growth factor‐induced cell proliferation by Necl‐5/Tage4/poliovirus receptor/CD155 through the Ras‐Raf‐MEK‐ERK signaling. J Biol Chem 2004; 279: 36419–25. [DOI] [PubMed] [Google Scholar]
- 20. Mueller S, Wimmer E. Recruitment of nectin‐3 to cell‐cell junctions through trans‐heterophilic interaction with CD155, a vitronectin and poliovirus receptor that localizes to αvβ3 integrin‐containing membrane microdomains. J Biol Chem 2003; 278: 31251–60. [DOI] [PubMed] [Google Scholar]
- 21. Sato T, Irie K, Ooshio T, Ikeda W, Takai Y. Involvement of heterophilic trans‐interaction of Necl‐5/Tage4/PVR/CD155 with nectin‐3 in formation of nectin‐ and cadherin‐based adherens junctions. Genes Cells 2004; 9: 791–9. [DOI] [PubMed] [Google Scholar]
- 22. Fujito T, Ikeda W, Kakunaga S et al. Inhibition of cell movement and proliferation by cell‐cell contact‐induced interaction of Necl‐5 with nectin‐3. J Cell Biol 2005; 171: 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erickson BM, Thompson NL, Hixson DC. Tightly regulated induction of the adhesion molecule Necl‐5/CD155 during rat liver regeneration and acute liver injury. Hepatology 2006; 43: 325–34. [DOI] [PubMed] [Google Scholar]
- 24. Abe A, Fukui H, Fujii S et al. Role of Necl‐5 in the pathophysiology of colorectal lesions induced by dimethylhydrazine and/or dextran sodium sulphate. J Pathol 2009; 217: 42–53. [DOI] [PubMed] [Google Scholar]
- 25. Morimoto K, Satoh‐Yamaguchi K, Hamaguchi A et al. Interaction of cancer cells with platelets mediated by Necl‐5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 2008; 27: 264–73. [DOI] [PubMed] [Google Scholar]
- 26. Noguchi M, Morikawa A, Kawasaki M et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995; 75: 2844–52. [DOI] [PubMed] [Google Scholar]
- 27. Martini N, Bains MS, Burt ME et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995; 109: 120–9. [DOI] [PubMed] [Google Scholar]
- 28. Harpole DH Jr, Herndon JE 2nd, Young WG Jr, Wolfe WG, Sabiston DC Jr. Stage I nonsmall cell lung cancer : a multivariate analysis of treatment methods and patterns of recurrence. Cancer 1995; 76: 787–96. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Kattan K, Sepsas E, Fountain SW, Townsend ER. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997; 12: 380–4. [DOI] [PubMed] [Google Scholar]
- 30. Chadeneau C, LeCabellec M, LeMoullac B, Meflah K, Denis MG. Over‐expression of a novel member of the immunoglobulin superfamily in Min mouse intestinal adenomas. Int J Cancer 1996; 68: 817–21. [DOI] [PubMed] [Google Scholar]
- 31. Tokunou M, Niki T, Eguchi K et al. c‐MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol 2001; 158: 1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


