Abstract
The retinoids (vitamin A and its biologically active derivatives) are essential for the health and survival of the individual. Several studies have reported a strong rationale for the use of retinoids in cancer treatment and chemoprevention. It has been discovered that expression of retinoic acid receptor (RAR) β is frequently silenced in epithelial carcinogenesis, which has led to the hypothesis that RARβ could act as a tumor suppressor. However, the status of RARβ in human endometrial carcinoma has not been examined. In the present study, we initially studied the effects of retinoic acid on cell proliferation and the expression of RARα, RARβ, and RARγ using AM580 (a RAR‐specific agonist) in the Ishikawa endometrial cancer cell line. We also examined the expression of RAR in human eutopic endometrium (30 cases), endometrial hyperplasia (28 cases), and endometrial carcinoma (103 cases) using immunohistochemistry. Finally, we correlated these findings with the clinicopathological parameters. In vitro, cell growth was inhibited and RARβ and RARγ mRNA was significantly induced by AM580, compared with vehicle controls, whereas RARα mRNA was significantly attenuated by AM580, compared with vehicle. RARβ was detected predominantly in endometrial hyperplasia, compared with endometrial carcinoma. No statistically significant correlation was obtained between the expression of any other RAR subtypes and clinicopathological parameters in human endometrial carcinoma. The results of our study demonstrate that AM580 inhibits cell growth and induces RARβ mRNA expression in the Ishikawa cell line, and the expression level of RARβ in endometrial carcinoma is significantly lower than that in endometrial hyperplasia. AM580 might therefore be considered as a potential treatment for endometrial carcinoma. (Cancer Sci 2008; 99: 267–271)
The retinoic acids are natural and synthetic derivatives of vitamin A that regulate a variety of important cellular functions. A strong rationale exists for the use of retinoids in cancer therapy and chemoprevention based on preclinical, epidemiological, and clinical findings.( 1 , 2 , 3 )
All‐trans‐retinoic acid (ATRA) activates the classical nuclear retinoic acid receptors (RAR), whereas 9‐cis‐retinoic acid activates the RAR and non‐classical nuclear retinoid X receptors (RXR).( 4 , 5 ) There are six genes encoding retinoid receptors: RARα, RARβ, and RARγ, as well as RXRα, RXRβ, and RXRγ. Multiple receptor isoforms exist through the alternate usage of splice sites and promoters. The ligand‐binding domains of RAR and RXR are distinct, and can be pharmacologically targeted individually. RAR can heterodimerize with RXR, and RXR can heterodimerize with other nuclear receptors, including the thyroid hormone receptors, vitamin D receptor, and peroxisomal proliferator activated receptor.( 5 )
Endometrial carcinoma is one of the most common female pelvic malignancies in the world, and its incidence has increased recently.( 6 ) Although little is known about the molecular events involved in the pathogenesis, a close relationship has been observed between estrogenic stimulation of the endometrium and the appearance of endometrial hyperplasia.( 7 ) Studies in experimental animals have shown that retinoids, particularly ATRA, may play an important role in regulating the effects of estrogens on the endometrium. Studies with vitamin A‐deficient rats demonstrated that physiological levels of retinoic acids suppress endometrial hyperplasia and metaplasia associated with chronic estrogen administration.( 8 ) In immature ovariectomized rats, pharmacological doses of retinoic acids suppressed estrogen‐induced endometrial stromal‐cell proliferation.( 9 )
In the normal human endometrium, intracellular retinoic acid concentrations in both epithelial and stromal cells are elevated during the secretory phase.( 10 ) Kumarendran et al. reported the expression of RARα, RARβ, RARγ, and RXRα mRNA in human eutopic endometrium using northern blotting.( 11 ) Siddiqui et al. also reported the presence of RAR and RXR mRNA in endometrioid endometrial carcinoma using northern blotting.( 12 ) However, the details of the status of these retinoid receptors and the correlation between retinoid receptors and clinical outcomes have not been studied in normal and diseased human endometrium.
In the present study, we initially examined the effects of retinoic acid on cell proliferation and the expression of RARα, RARβ, and RARγ using AM580 (a synthetic RAR‐specific ligand) in the Ishikawa endometrial cancer cell line. We then examined the expression of RAR in human eutopic endometrium, endometrial hyperplasia, and endometrial carcinoma using immunohistochemistry. Finally, we correlated these findings with clinicopathological parameters in endometrial carcinoma.
Materials and Methods
Cell culture. The human endometrial cancer cell line Ishikawa was obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM)/F‐12 (Gibco/BRL, Grand Island, NY, USA) containing 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin B (250 ng/mL) (growth medium). Fresh suspensions of stromal cells were plated in culture dishes and incubated at 5% CO2 and 37°C. Media was changed at 72‐h intervals until the cells became 70–80% confluent. Confluent cells were serum‐deprived for 16 h in serum‐free, phenol red‐free DMEM/F‐12 before being subjected to the following treatments for 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay and real‐time reverse transcription (RT)‐polymerase chain reaction (PCR): MTT assay, serum‐free and phenol red‐free medium with ethanol as the baseline control, or serum‐containing, phenol red‐free medium with AM580 (10−8–10−6 M) for 24, 48, 72, and 96 h; real‐time RT‐PCR, serum‐free and phenol red‐free medium with ethanol as the baseline control, or serum‐free and phenol red‐free medium with AM580 (0.1 µM) for 1, 3, and 6 h.
MTT cell‐proliferation assay. Cell proliferation was assessed by a colorimetric assay using MTT. The MTT assay that detects the formation of dark‐blue formazan produced from MTT in active mitochondria was carried out as reported previously.( 13 ) At 4 h before the end of each experiment, 10 µL MTT solution was added into each well of 96‐well plates. The optical absorbance at 570 nm was read within 30 min with a microplate reader (Thermomax, Molecular Devices, Menlo Park, CA, USA). Data are expressed in optical density units. The last column of each 96‐well plate did not contain cells and was used as a blank.
Isolation of total RNA and real‐time RT‐PCR. Total RNA was isolated from the Ishikawa cells using TRI Reagent (Sigma‐Aldrich, St Louis, MO, USA). The concentration and quality of total RNA were determined spectrophotometrically and by electrophoresis on denaturing formaldehyde–agarose gels.
Reverse transcription reactions were carried out using the SUPERSCRIPT III First‐Strand Synthesis System for RT‐PCR (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The expression levels of mRNA for RARα, RARβ, and RARγ were measured by real‐time RT‐PCR using a standard TaqMan PCR kit protocol on an Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Primers and probes were obtained from the ABI TaqMan Gene Expression Assay catalog (Applied Biosystems). The probes contained a 6‐carboxy‐fluorescein phosphoramidite (FAM dye) label at the 5′ end and a minor groove binder and non‐fluorescent quencher at the 3′ end, and were designed to hybridize across exon junctions. For each sample, triplicates were run for each gene in a 384‐well format plate. Template cDNA and TaqMan Gene Expression Assays, which contain PCR primers and probes, were added to TaqMan Universal PCR Mastermix (Applied Biosystems) to a final volume of 20 µL. The reactions were incubated at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The threshold cycle (CT) was defined as the fractional cycle number at which the fluorescence passed the fixed threshold. TaqMan CT values were converted into absolute copy numbers. All RNA samples were normalized based on the TaqMan Gene Expression Assays for the human glyceraldehyde‐3‐phosphate dehydrogenase endogenous control (Applied Biosystems).
Tissue preparation. Thirty cases of eutopic endometrium, 28 cases of endometrial hyperplasia, and 103 cases of endometrial endometrioid adenocarcinoma (well‐differentiated, 49 cases; moderately differentiated, 32 cases; poorly differentiated, 22 cases) were retrieved from the surgical pathology files of Tohoku University Hospital, Sendai, Japan. This study was approved by the Ethical Committee of the Tohoku University School of Medicine. We obtained non‐pathological endometria from hysterectomy specimens carried out due to carcinoma in situ of the uterine cervix. Endometrial hyperplasia and carcinoma specimens were obtained from total dilatation and curretage, and hysterectomy, respectively. None of the patients had undergone irradiation or chemotherapy before surgery. The lesions were classified according to the Histological Typing of Female Genital Tract Tumors by the World Health Organization and staged according to the International Federation of Gynecology and Obstetrics system.( 14 ) All specimens were processed routinely (i.e. 10% formalin fixed for 24–48 h), paraffin embedded, and thin sectioned (3 µm).
Immunohistochemistry. Polyclonal antibodies for RARα (sc‐551), RARβ (sc‐552), and RARγ (sc‐550) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Immunohistochemical analysis was carried out using the streptavidin–biotin amplification method with a Histofine kit (Nichirei, Tokyo, Japan), which has been described previously in detail.( 15 ) The dilutions of the primary antibodies used in our study were as follows: RARα, 1/500; RARβ, 1/5; and RARγ. 1/500. The antigen–antibody complex was visualized with 3.3′‐diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris‐HCl buffer [pH 7.6], and 0.006% H2O2), and counterstained with hematoxylin. Tissue sections of skin were used as positive controls for RARα and RARγ and those of human breast cancer were used for RARβ.
Scoring of immunohistochemistry. Evaluation of RAR was carried out in high‐power fields (×400) using a standard light microscope. Two of the authors (K. T. and M. T.) simultaneously searched the entire tissue sections and determined the most representative areas using a double‐headed microscope. For evaluation of immunoreactivity of the RAR, we determined the labeling index (LI; the percentage of positive cells) according to the report by Sasano and colleagues.( 15 ) After completely reviewing the immunostained sections of each lesion, a total of more than 500 tumor cells from three different representative fields were counted independently. Cases with discordant results (interobserver differences of more than 5%) were reevaluated simultaneously by the same authors using a double‐headed light microscope. Interobserver differences were less than 5% in the present study.
Statistical analysis. The statistical significance of the relationship between immunoreactivity for the RAR and clinical stage, histological grade, myometrial invasion, vascular involvement, recurrence rate, and overall survival were evaluated using the t‐test. P‐values less than 0.05 were considered significant.
Results
Effect of AM580 on Ishikawa cell proliferation. The proliferative effects of AM580 (10−8–10−6 M) on Ishikawa cells were assessed using the MTT colorimetric assay. Ishikawa cells were treated with AM580 for 24–96 h. Following treatment for 24 h, there were no significant differences between the groups. However, at 48 h and thereafter, both of the AM580 groups (10−7 M and 10−6 M) had significantly decreased proliferation compared with the control group (P < 0.05; Fig. 1).
Figure 1.
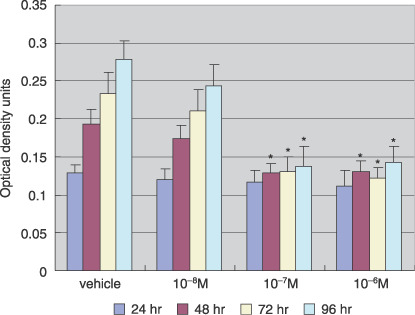
Effect of AM580 on Ishikawa cell proliferation. Cells were treated with AM580 (10−8–10−6 M) or with dimethylsulfoxide (DMSO; vehicle), as a control, for 24–96 h. Cell proliferation was analyzed in 96‐well microplates by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide colorimetric assay. Values are expressed as mean ± SEM of eight wells for each group. Following 24 h of treatment, there were no significant differences between the groups. However, at 48 h and thereafter, both of the AM580 groups (10−7 M and 10−6 M) had significantly decreased proliferation compared with the control group. *P < 0.05 versus vehicle.
Effects of retinoic acid on the expression of RAR. To examine the effects of the RAR‐specific ligand AM580 on the expression of RAR, Ishikawa cells were cultured for 1, 3, and 6 h with 0.1 µM AM580, and compared with vehicle‐treated cells as controls. Real‐time RT‐PCR analysis was carried out to measure the mRNA expression of the respective RAR, in the presence or absence of AM580 treatment. As shown in Figure 2, RARβ and RARγ mRNA were induced significantly by AM580, compared with vehicle control (P < 0.001), whereas RARα mRNA was attenuated significantly by AM580, compared with vehicle (P < 0.001).
Figure 2.
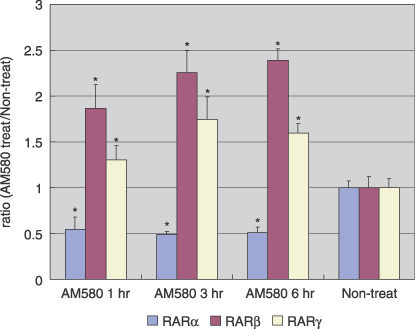
Retinoic acid receptor (RAR) β and RARγ mRNA were induced by AM580 in Ishikawa cells. Real‐time reverse transcription–polymerase chain reaction analysis was carried out to measure RAR mRNA expression in the presence or absence of AM580 treatment. Summary data for three independent experiments are shown. Results are expressed as the mean ± SE. *P < 0.001 versus non‐treated.
Cellular localization of RAR in eutopic endometrium, endometrial hyperplasia, and endometrial carcinoma. Immunohistochemistry for the RAR was carried out using serial sections of eutopic endometrium, endometrial hyperplasia, and endometrial carcinoma (Fig. 3).
Figure 3.
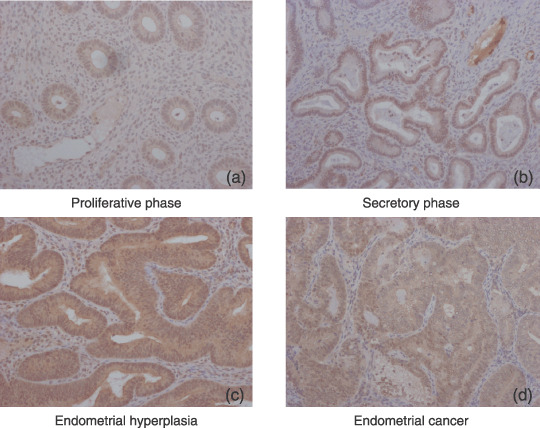
Retinoic acid receptor (RAR) β localization in eutopic endometrium, endometrial hyperplasia, and endometrial carcinoma. (a,b) In ectopic endometrium, RARβ immunoreactivities were detected in the nuclei of epithelial cells throughout all menstrual phases. (c) In endometrial hyperplasia, RARβ immunoreactivity was detected in the nuclei of 60–75% of stromal cells. In endometrial carcinoma, no RARβ immunoreactivity was detected in the stromal cells in any of the cases examined. (d) RARβ immunoreactivities were detected in the nuclei of 18–37% of carcinoma cells. Immunohistochemistry for the RAR was carried out using serial sections of eutopic endometrium, endometrial hyperplasia, and endometrial carcinoma. (Original magnification, ×200.)
In ectopic endometrium, RARβ immunoreactivity was detected in the nuclei of 25–50% of stromal cells, whereas RARα immunoreactivity was present in the nuclei of 3–5% of stromal cells throughout the phases of the menstrual cycle. RARγ immunoreactivity was not detected in any of the epithelial cells examined. RARα and RARβ immunoreactivities were detected in the nuclei of epithelial cells throughout all menstrual phases.
In endometrial hyperplasia, immunoreactivity for RAR was detected in the nuclei of both epithelial and stromal cells. RARβ immunoreactivity was detected in the nuclei of 60–75% of stromal cells, whereas RARα and RARγ immunoreactivities were present in the nuclei of 10–15% of stromal cells.
In endometrial carcinoma, no RAR immunoreactivity was detected in the stromal cells in any of the cases examined. RARα and RARβ immunoreactivities were detected in the nuclei of 18–37% of carcinoma cells.
Relationship between the expression of RAR subtypes and clinicopathological parameters in endometrial carcinoma. As shown in Figure 4, RARβ was detected predominantly in endometrial hyperplasia, compared with endometrial carcinoma (P = 0.014; LI in endometrial hyperplasia 68.39 ± 28.31 vs LI in endometrial carcinoma 34.67 ± 28.95). There was no significant correlation between the RARβ expression of each histological type. There was no significant correlation between the expression of each of the RAR subtypes in endometrial carcinoma (data not shown).
Figure 4.
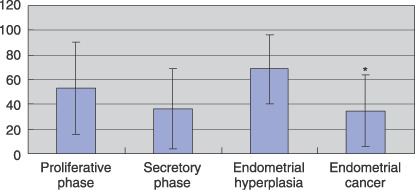
Summary of immunohistochemistry for retinoic acid receptor (RAR) β. For evaluation of RARβ expression, we determined the labeling index (LI). Results are expressed as the mean ± SE. *P < 0.02 versus endometrial hyperplasia.
The relationships between the expression of the RAR subtypes and the clinicopathological findings in endometrial carcinoma are summarized in Table 1. There was no statistically significant correlation between LI for any of the other RAR subtypes and the clinicopathological parameters, including clinical stage, histological grade, myometrial invasion, vascular involvement, recurrence rate, and overall survival.
Table 1.
Summary of the relationship between retinoic acid receptor (RAR) expression and clinicopathological findings in endometrial cancer
| Clinicopathological characteristic (number of patients) | RAR | P‐value | ||
|---|---|---|---|---|
| α | β | γ | ||
| Stage | ||||
| I (66) | 18.11 ± 10.06 | 36.62 ± 29.80 | 3.45 ± 3.17 | NS |
| II (12) | 22.33 ± 19.05 | 25.92 ± 30.19 | 3.25 ± 3.93 | |
| III (22) | 16.91 ± 11.18 | 33.50 ± 26.44 | 5.77 ± 8.69 | |
| IV (3) | 17.67 ± 5.51 | 35.33 ± 30.62 | 3.67 ± 2.08 | |
| Grade | ||||
| Well‐differentiated (49) | 17.59 ± 9.62 | 36.27 ± 28.78 | 3.88 ± 3.42 | NS |
| Moderate‐differentiated (32) | 22.19 ± 14.97 | 37.22 ± 30.36 | 3.28 ± 3.08 | |
| Poorly differentiated (22) | 14.36 ± 7.72 | 27.41 ± 27.33 | 5.00 ± 8.78 | |
| Myometrial invasion | ||||
| <1/2 (62) | 18.34 ± 11.74 | 34.08 ± 30.49 | 3.25 ± 2.98 | NS |
| 1/2 (38) | 17.76 ± 11.42 | 33.05 ± 27.30 | 3.92 ± 3.35 | |
| Vessel involvement | ||||
| + (29) | 17.62 ± 12.32 | 31.55 ± 29.74 | 3.83 ± 3.14 | NS |
| – (34) | 16.47 ± 6.72 | 35.38 ± 30.72 | 3.03 ± 2.94 | |
| Recurrence | ||||
| + (16) | 15.19 ± 8.48 | 26.31 ± 26.27 | 3.06 ± 2.82 | NS |
| – (87) | 18.91 ± 11.93 | 36.21 ± 29.30 | 4.09 ± 5.26 | |
| Prognosis | ||||
| Alive (95) | 18.52 ± 11.57 | 34.14 ± 29.31 | 9.00 ± 14.04 | NS |
| Dead (8) | 16.13 ± 11.15 | 41.00 ± 25.07 | 3.51 ± 3.11 | |
For evaluation of RARs’ expression, we determined the labeling index. Results are expressed as the mean ± SE. NS, not significant.
Discussion
Retinoic acids exhibit diverse biological properties that may potentially contribute to their antitumor effect. They inhibit cell proliferation and angiogenesis, and can induce cell differentiation and apoptosis.( 16 , 17 ) RARβ repression has been reported in preneoplastic oral‐cavity lesions,( 18 ) non‐small‐cell lung cancer,( 19 , 20 , 21 ) breast cancer,( 22 ) and esophageal cancer.( 23 ) Although other retinoid receptors were expressed in these tissues, only RARβ levels were significantly lower in the premalignant and tumor tissues. RARβ expression was selectively lost in premalignant oral lesions, and was able to be restored by retinoic acid treatment.( 18 ) The restoration of RARβ expression was associated with a clinical response, suggesting a role for RARβ, both as a mediator of the retinoic acid response and as a biological marker in chemoprevention trials.( 18 ) This was confirmed in renal cancer, in which upregulation of RARβ correlated with a response to 13‐cis‐retinoic acid and interferon α‐2a.( 24 ) Thus, the correlation with RARβ repression led to the hypothesis that RARβ could act as a tumor suppressor. In addition, introduction of RAR‐β protein into retinoic acid‐insensitive breast cancer cell lines has been shown to restore retinoic acid responsiveness.( 25 ) In our study, RARβ was detected predominantly in endometrial hyperplasia, compared with endometrial carcinoma. These results suggest that suppression of RAR‐β expression may inhibit the differentiation of endometrial epithelium in endometrial carcinoma.
In recent studies, the retinoid isotretinoin was not effective for chemoprevention in stage I non‐small‐cell lung cancer or early stage head and neck squamous‐cell carcinoma.( 26 , 27 ) The retinoid‐signaling pathway was studied in normal and neoplastic tissues to determine why preclinical retinoid activity did not readily translate into clinical success. It was discovered that expression of RARβ is frequently silenced in epithelial carcinogenesis, which led to the hypothesis that RARβ acts as a tumor suppressor that is partially responsible for the limited clinical activity of classical retinoids.( 28 , 29 ) To examine the effect of the RAR‐specific ligand AM580 on RARβ expression, we carried out MTT assay and real‐time RT‐PCR analysis using the Ishikawa cell line. Although AM580 inhibited cell growth and induced RARβ mRNA expression in Ishikawa cells, no statistically significant correlation was obtained between the expression of RARβ and clinicopathological parameters in human endometrial carcinoma. RARβ has four isoforms that are generated differentially by means of the promoters P1 and P2 and alternative splicing.( 30 ) Our studies evaluated RARβ expression as a monolithic entity and did not distinguish between the various RARβ isoforms that have been identified in humans. Differential expression of different RARβ isoforms, at least in part, might underlie the contradictory associations of RARβ expression. However, it awaits further investigations for clarification.
Retinoids are useful tools for identifying critical target genes and pathways that can reduce carcinogenesis.( 31 , 32 ) Accumulating evidence suggests that retinoids play a role in regulating the function of the endometrium.( 33 , 34 ) Retinoids have also been reported to affect the expression of a number of genes in the endometrium, such as matrix metalloproteinases and interleukin‐6.( 35 ) Although the profile of retinoid receptors of epithelial cells has been elucidated,( 11 , 12 , 36 ) the effect of retinoids on the proliferation of normal epithelial cells remains unknown. In our study, AM580 inhibited cell growth and induced RARβ mRNA expression in Ishikawa cells, and the expression level of RARβ in endometrial carcinoma was significantly lower than that in endometrial hyperplasia. AM580 might possibly be used as a treatment for endometrial carcinoma. However, it awaits further investigations for clarification.
Acknowledgments
This study was supported in part by a Grant‐in‐Aid for Scientific Research on a priority area from the Ministry of Education, Science, Sports and Culture, Japan, a Grant‐in‐Aid from the Ministry of Education, Science, Sports and Culture, Japan, a Grant‐in‐Aid from the Ministry of Health, Labor and Welfare, Japan, the 21st Century COE Program Special Research Grant (Tohoku University) from the Ministry of Education Science, Sports and Culture, Japan, a Grant‐in‐Aid from the Kurokawa Cancer Research Foundation, and the Uehara Memorial Foundation.
References
- 1. Hong WK, Itri LM. Retinoids and human cancer. In: Sporn MB, Roberts AB, Goodman DS, eds. The Retinoids: Biology, Chemistry and Medicine, 2nd edn. New York: Raven Press, 1994; 597–630. [Google Scholar]
- 2. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science 1997; 278: 1073–7. [DOI] [PubMed] [Google Scholar]
- 3. Nason‐Burchenal K, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention. In: Bertino JR, ed. Molecular Biology of Cancer, 1st edn. San Diego: Academic Press, 1996; 1547–60. [Google Scholar]
- 4. Pfahl M, Piedrafita FJ. Retinoid targets for apoptosis induction. Oncogene 2003; 22: 9058–62. [DOI] [PubMed] [Google Scholar]
- 5. Pitha‐Rowe I, Petty WJ, Kitareewan S, Dmitrovsky E. Retinoid target genes in acute promyelocytic leukemia. Leukemia 2003; 17: 1723–30. [DOI] [PubMed] [Google Scholar]
- 6. Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin 1996; 46: 5–27. [DOI] [PubMed] [Google Scholar]
- 7. Kohler MF, Nishi H, Humphrey PA et al . Mutation of the p53 tumor‐suppressor gene is not a feature of endometrial hyperplasias. Am J Obstet Gynecol 1993; 169: 690–4. [DOI] [PubMed] [Google Scholar]
- 8. Bo WJ, Smith MS. The effect of retinol and retinoic acid on the morphology of the rat uterus. Anat Rec 1996; 156: 5–9. [DOI] [PubMed] [Google Scholar]
- 9. Boettger‐Tong HL, Stancel GM. Retinoic acid inhibits estrogen‐induced uterine stromal and myometrial cell proliferation. Endocrinology 1995; 136: 2975–83. [DOI] [PubMed] [Google Scholar]
- 10. Loughney AD, Kumarendran MK, Thomass EJ, Redfern CPF. Variation in the expression of cellular retinoid binding proteins in human endometrium throughout the menstrual cycle. Hum Reprod 1995; 10: 1297–304. [DOI] [PubMed] [Google Scholar]
- 11. Kumarendran MK, Loughney AD, Prentice A, Thomas EJ, Redfern CP. Nuclear retinoid receptor expression in normal human endometrium throughout the menstrual cycle. Mol Hum Reprod 1996; 2: 123–9. [DOI] [PubMed] [Google Scholar]
- 12. Siddiqui NA, Loughney A, Thomas EJ, Dunlop W, Redfern CPF. Cellular retinoid binding proteins and nuclear retinoic acid receptors in endometrial epithelial cells. Hum Reprod 1994; 9: 1410–16. [DOI] [PubMed] [Google Scholar]
- 13. Arici A, Seli E, Senturk LM et al . Interleukin‐8 in the human endometrium. J Clin Endocrinol Metab 1998; 83: 1783–7. [DOI] [PubMed] [Google Scholar]
- 14. FIGO . Stages‐1988 revision. Gynecol Oncol 1989; 35: 125–7. [Google Scholar]
- 15. Sasano H, Frost AR, Saitoh R et al . Aromatase and 17β‐hydroxysteriod dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 1996; 81: 4042–6. [DOI] [PubMed] [Google Scholar]
- 16. Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta 1980; 605: 33–91. [DOI] [PubMed] [Google Scholar]
- 17. Morriss‐kay G. Retinoic acid receptors in normal growth and development. Cancer Surv 1992; 14: 181–93. [PubMed] [Google Scholar]
- 18. Lotan R, Xu XC, Lippman SM et al . Suppression of retinoic acid receptor‐β in premalignant oral lesions and its up‐regulation by isotretinoin. N Engl J Med 1995; 332: 1405–10. [DOI] [PubMed] [Google Scholar]
- 19. Xu XC, Sozzi G, Lee JS et al . Suppression of retinoic acid receptor β in non‐small‐cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst 1997; 89: 624–9. [DOI] [PubMed] [Google Scholar]
- 20. Picard E, Seguin C, Monhoven N et al . Expression of retinoid receptor genes and proteins in non‐small‐cell lung cancer. J Natl Cancer Inst 1999; 91: 989–91. [DOI] [PubMed] [Google Scholar]
- 21. Castillo L, Milano G, Santini J, Demard F, Pierrefite V. Analysis of retinoic acid receptor β expression in normal and malignant laryngeal mucosa by a sensitive and routine applicable reverse transcription–polymerase chain reaction enzyme‐linked immunosorbent assay method. Clin Cancer Res 1997; 3: 2137–42. [PubMed] [Google Scholar]
- 22. Widschwendter M, Berger J, Daxenbichler G et al . Loss of retinoic acid receptor β expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res 1997; 57: 4158–61. [PubMed] [Google Scholar]
- 23. Qiu H, Zhang W, El‐Naggar AK et al . Loss of retinoic acid receptor‐β expression is an early event during esophageal carcinogenesis. Am J Pathol 1999; 155: 1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berg WJ, Nanus DM, Leung A et al . Up‐regulation of retinoic acid receptor β expression in renal cancers in vivo correlates with response to 13‐cis‐retinoic acid and interferon‐α‐2a. Clin Cancer Res 1999; 5: 1671–5. [PubMed] [Google Scholar]
- 25. Liu Y, Lee MO, Wang HG et al . Retinoic acid receptor β mediates the growth‐inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol 1996; 16: 1138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lippman FR, Lee JJ, Karp DD et al . Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non‐small‐cell lung cancer. J Natl Cancer Inst 2001; 93: 605–18. [DOI] [PubMed] [Google Scholar]
- 27. Khuri FR, Lee JJ, Lippman SM et al . Randomized phase III trial of low‐dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst 2006; 98: 441–50. [DOI] [PubMed] [Google Scholar]
- 28. Petty WJ, Li N, Biddle A et al . A novel retinoic acid receptor β isoform and retinoid resistance in lung carcinogenesis. J Natl Cancer Inst 2005; 97: 1645–51. [DOI] [PubMed] [Google Scholar]
- 29. Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res 1994; 54: 3580–7. [PubMed] [Google Scholar]
- 30. Sabichi AL, Xu X, Lippman SM. RARβ1′: primed to fight retinoid resistance in lung carcinogenesis. J Natl Cancer Inst 2005; 97: 1632–3. [DOI] [PubMed] [Google Scholar]
- 31. Pitha‐Rowe I, Petty WJ, Feng Q et al . Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res 2004; 64: 8109–15. [DOI] [PubMed] [Google Scholar]
- 32. Freemantle SJ, Dragnev KH, Dmitrovsky E. The retinoic acid paradox in cancer chemoprevention. J Natl Cancer Inst 2006; 98: 426–7. [DOI] [PubMed] [Google Scholar]
- 33. Loughney AD, Redfern CP. Menstrual cycle related differences in the proliferative responses of cultured human endometrial stromal cells to retinoic acid. J Reprod Fertil 1995; 105: 153–9. [DOI] [PubMed] [Google Scholar]
- 34. Brar AK, Kessler CA, Meyer AJ, Cedars MI, Jikihara H. Retinoic acid suppresses in vitro decidualization of human endometrial stromal cells. Mol Hum Reprod 1996; 2: 185–93. [DOI] [PubMed] [Google Scholar]
- 35. Osteen KG, Keller NR, Feltus FA, Melner MH. Paracrine regulation of matrix metalloproteinase expression in the normal human endometrium. Gynecol Obstet Invest 1999; 48 (Suppl 1): 2–13. [DOI] [PubMed] [Google Scholar]
- 36. Ito K, Suzuki T, Moriya T et al . Retinoid receptors in the human endometrium and its disorders: a possible modulator of 17β‐hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 2001; 86: 2721–7. [DOI] [PubMed] [Google Scholar]


