Abstract
Bcl11b encodes a zinc‐finger transcription factor and functions as a haploinsufficient tumor suppressor gene. Bcl11b KO/KO mice exhibit differentiation arrest of thymocytes during β‐selection as has been observed with other mouse models involving knockouts of genes in the Wnt/β‐catenin signaling pathway. Recurrent chromosomal rearrangement at the BCL11B locus occurs in human T‐cell leukemias, but it is not clear how such rearrangement would contribute to lymphomagenesis. To address this issue, we studied clonal cell growth, cell number, and differentiation of thymocytes in Bcl11b KO/+ mice at different time points following γ‐irradiation. Analysis of D‐J rearrangement at the T cell receptor β‐chain (TCRβ) locus and cell surface markers by flow cytometry revealed two distinct populations of clonally growing thymocytes. In one population, thymocytes share a common D‐J rearrangement but retain the capacity to differentiate. In contrast, thymocytes in the second population have lost their ability to differentiate. Since the capacity to self renew and differentiate into multiple cell lineages are fundamental properties of adult stem cells, the differentiation competent population of thymocytes that we have isolated could potentially function as cancer stem cells. We also demonstrate increased expression of β‐catenin, a well‐known oncogenic protein, in Bcl11b KO/+ thymocytes. Collectively, the Bcl11b KO/+ genotype contributes to clonal expansion and differentiation arrest in part through an increase in the level of β‐catenin. (Cancer Sci 2010)
Cancer development is a complex, multistep process involving the acquisition of capabilities of cell autonomous proliferation and resistance to apoptosis.( 1 ) This could be a consequence of a sequence of 4–6 mutations that are associated with different stages of the tumor progression.( 2 ) Leukemia and lymphoma are malignancies of hematopoietic cells, and chronic myelogenous leukemia (CML) is among the malignancies characterized with frequently having the bcr/abl chimeric gene.( 3 ) A two‐step process is seen from CML to a subset of acute lymphoblastic leukemia (ALL) bearing bcr/abl, an aggressive blast crisis phase.( 3 , 4 , 5 ) This transition requires an arrest of differentiation. Interestingly, CML already possesses intrinsic self‐renewal capability like adult tissue stem cells and differentiate to mature, nontumorigenic blood cells.( 6 )
Bcl11b is a haploinsufficient tumor suppressor gene that was isolated from analyses of γ‐ray induced mouse thymic lymphomas.( 7 , 8 , 9 ) Bcl11b KO/+ mice are susceptible to the development of thymic lymphomas,( 9 ) suggesting that loss or decrease of Bcl11b function contributes to lymphomagenesis. Recurrent chromosomal rearrangement at the human BCL11B locus has been reported in T‐cell leukemias,( 10 , 11 , 12 ) but the effects of the rearrangement are not clear. Bcl11b encodes a zinc‐finger transcription factor that is expressed in thymocytes, neurons and other tissues.( 13 , 14 , 15 , 16 , 17 ) Bcl11b KO/KO and Bcl11b lox/lox mice show differentiation arrest of thymocytes during β‐selection( 13 , 14 ) and positive selection,( 18 ) respectively; the arrest in the former seen at CD4 and CD8 double‐negative (DN) and immature CD8 single‐positive (ISP) cell stages before the CD4 and CD8 double‐positive (DP) cell stage.( 13 , 14 ) Bcl11b KO/+ mice exhibit a substantial impairment of thymocyte differentiation in mouse embryos, although not as profound as that in Bcl11b KO/KO animals.( 19 ) The arrest during β‐selection is seen in many gene‐knockout mice,( 20 ) including genes affecting Wnt/β‐catenin signaling.( 21 , 22 , 23 ) As with oncogenesis, differentiation arrest may be a mechanism through which Bcl11b deficiency contributes to tumor development. However, Bcl11b KO/KO mice also show thymocyte apoptosis, and this anti‐apoptotic property of Bcl11b seems to contradict a predicted proapoptotic function of tumor suppressors. The differentiation arrest and apoptosis are at least in part due to the decrease of pre‐T cell receptor (TCR) signaling.( 13 , 14 )
Identical rearrangements of the TCRβ locus are seen in thymic lymphomas and this establishes clonality of the lymphomas.( 24 ) Our previous studies demonstrated that such identical rearrangements were also found in γ‐ray induced mouse atrophic thymuses, indicating the existence of clonally expanded thymocytes.( 24 , 25 ) A significant percentage of those thymuses exhibited allelic loss of Bcl11b. These findings raise the question of how and at which stage does the Bcl11b heterozygous genotype contributes to lymphoma development. Here we studied the effect of Bcl11b KO/+ genotype on β‐catenin expression and on clonal cell proliferation of thymocytes in γ‐irradiated mice. Our results provide an implication that the genotype contributes to clonal cell expansion and differentiation arrest, and the contribution may, in part, occur through an increase in β‐catenin expression.
Materials and Methods
Mice and induction of atrophic thymus. Bcl11b KO/+ mice with a BALB/c background were generated as described.( 13 ) MSM mice were kindly supplied from Dr Shiroishi, National Institute of Genetics (NIG) (Mishima, Japan). Bcl11b KO/+ mice were mated with MSM mice and their progeny were subjected to γ‐irradiation of 3 Gy at 8 or 10 weeks of age. Left and right thymic lobes were separately isolated at 30, 60, or 80 days after the irradiation and subjected to analyses. Mice used in this study were maintained under specific pathogen‐free conditions in the animal colony of Niigata University. All animal experiments complied with the guidelines for animal experimentation from the University animal ethics committee.
Flow cytometry. Flow cytometric analysis was performed as previously described.( 13 ) In brief, single cell suspensions of thymocytes were prepared from thymus and 1–2 × 106 cells were incubated with antibodies in phosphate‐buffered saline containing 2% fetal calf serum and 0.2% NaN3 for 20 min at 4°C. The monoclonal antibodies (mAbs) used were: anti‐CD4‐PerCP‐Cy5.5 or ‐APC (RM4‐5), anti‐CD8‐PE (53‐6.7), anti‐TCRβ‐FITC (H57‐597; BioLegend, San Diego, CA, USA), anti‐β‐catenin‐FITC (14; BD Biosciences, San Jose, CA, USA), and IL‐7Rα‐PE (SB/199, BioLegend, San Diego, CA, USA). They were purchased from eBioscience. To prevent nonspecific binding of mAbs, we added CD16/32 (93; eBioscience) before staining with labeled mAbs. Dead cells and debris were excluded from the analysis by appropriate gating of forward scatter (FSC) and side scatter (SSC). Cells were analyzed by a FACScan (Becton‐Dickinson, Franklin Lakes, NJ, USA) flow cytometer, and data were analyzed using the Flow‐Jo software (Tree‐Star, Ashland, OR, USA).
For BrdU incorporation experiments, we injected mice intraperitoneally with 100 μL of BrdU solution (10 mg/mL) and thymus was isolated 1 h after. Thymocytes were prepared from the thymus and analyzed with the use of the BrdU Flow Kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s instructions. In brief, cells were suspended at a concentration of 1–2 × 106 cells/mL, fixed, permeabilized, treated with DNase to expose incorporated BrdU, and incubated with a murine anti‐BrdU antibody for 20 min at room temperature. After washing, cells were resuspended in 1 mL of PBS containing 20 μL of the 7AAD solution. Cells were resuspended in staining buffer and analyzed with the FACScan flow cytometer.
DNA isolation and PCR analysis. DNA was isolated from brain, thymocytes, and thymic lymphomas using the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). To determine D‐J rearrangement patterns in the TCRβ locus, polymerase chain reaction (PCR) was performed as described.( 24 , 25 ) Of allelic loss analysis at the Bcl11b locus, D12Mit53 and D12Mit279 markers were used for PCR as described previously.( 7 ) The PCR reaction was processed through 32 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min in most cases. The products were analyzed by 8% polyacrylamide gel electrophoresis. PCR bands were stained with ethidium bromide and band intensities were quantitated with a Molecular Imager FX (Bio‐Rad Laboratories, Hercules, CA, USA) to determine the allele ratio of BALB/c and MSM alleles or of MSM and BALB/c alleles.
Results
Decrease in the thymocyte number in γ‐irradiated Bcl11b KO/+ mice. We subjected 8‐week‐old Bcl11b KO/+ and Bcl11b +/+ mice to 3 Gy of γ‐radiation and examined both left and right lobes of the thymus separately at 14, 30, 60, and 80 days after irradiation (the respective thymic lobes are designated as 14‐, 30‐, 60‐, and 80‐day thymuses). The earliest time at which fully malignant thymic lymphomas were observed was approximately 100 days after irradiation.( 7 , 8 , 9 ) Figure 1 shows the cell number in the thymuses. In Bcl11b +/+ mice, the number at 14 days post radiation was not restored to the level in unirradiated mice but restored to the level or more at 30 days after. The cell number was maintained until 80 days after. On the other hand, Bcl11b KO/+ mice showed impairment in the recovery of cellularity. The cell number at 30 days after was not restored to the normal level in most thymuses and the average was 1.8 × 107 in Bcl11b KO/+ thymuses which was lower than 5.0 × 107 in Bcl11b +/+ thymuses (P < 0.0001). Also, the cell number was not well maintained at 60 or 80 days after. These results suggest an impairment in the maintenance of thymocyte number in Bcl11b KO/+ mice after γ‐irradiation.
Figure 1.
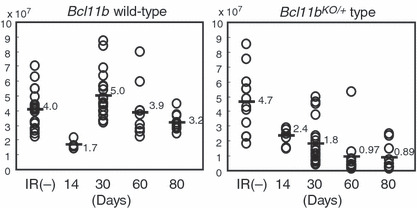
Cell number in thymuses at various days after γ‐irradiation. Bcl11b +/+ mice, left; Bcl11b KO/+ mice, right. Average cell number in Bcl11b +/+ mice was 4.0, 1.7, 5.0, 3.9, and 3.2 × 107 cells for unirradiated, 14, 30, 60, and 80 days after irradiation, respectively. Average cell number in Bcl11b KO/+ mice was 4.7, 2.4, 1.8, 0.97, and 0.89 × 107 cells for unirradiated, 14, 30, 60, and 80 days after irradiation, respectively.
Clonal cell expansion. Clonality was determined by assaying specific V(D)J rearrangements with three primer sets designed for the TCRβ locus.( 24 , 25 ) Figure 2(A) shows PCR patterns of 30‐ and 80‐day thymuses. Unirradiated thymus (lane Th) gave six different bands corresponding to possible recombination sites between D and J regions by Dβ1‐Jβ1, Dβ2‐Jβ2, and Dβ1‐Jβ2 probe sets and one band for germ‐line DNA by the former two probe sets. On the other hand, thymic lymphoma DNA (Ly) gave one band only by the Dβ2‐Jβ2 probe set used, indicating an identical rearrangement, and brain DNA (Br) gave the germ‐line DNA band by Dβ1‐Jβ1 and Dβ2‐Jβ2 probe sets. Two of the 20 30‐day thymuses in Bcl11b KO/+ mice exhibited only a few bands or limited numbers of bands different from the normal thymus pattern, indicating the existence of clonally expanded thymocytes (C‐type thymus). Most others showed rearrangement patterns identical or similar to the control thymus (classified as T‐type thymus). There was one thymus that was classified as C/T‐type thymus due to the difficulty of distinction between C‐ and T‐type thymus. An additional experiment showed a consistent result, one C/T‐type thymus detected in 12 30‐day thymuses examined (data not shown). All 20 Bcl11b +/+ mouse thymuses were T‐type thymus (data not shown). On the other hand, the 60‐day Bcl11b KO/+ thymuses showed two C‐type and four C/T‐type thymuses in 10 thymuses examined, whereas the 80‐day Bcl11b KO/+ thymuses showed six C‐type and two C/T‐type thymuses in 10 thymuses examined (Fig. 2B). These indicate increase in the incidence of C‐type thymus with the time after irradiation. Those results suggest that Bcl11b KO/+ genotype promotes the development of clonally expanding thymocytes in γ‐irradiated mice.
Figure 2.
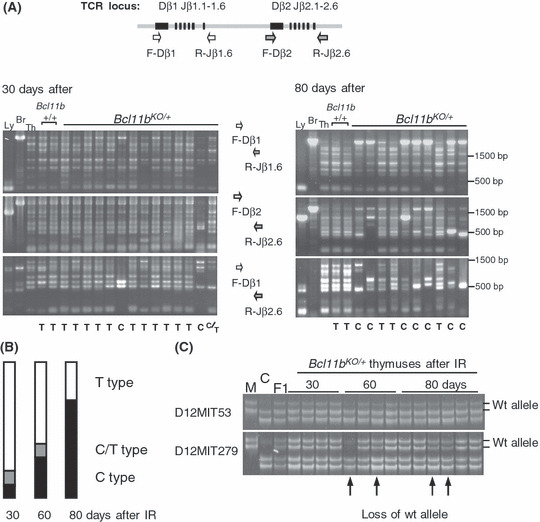
Clonal growth of thymocytes in thymuses after γ‐irradiation of 8‐week‐old Bcl11b KO/+ mice. (A) D‐J rearrangement patterns at the T cell receptor β‐chain (TCRβ) locus in thymuses at 30 and 80 days after irradiation. The upper diagram shows part of the TCRβ locus and the relative location of PCR primers used. The lower panel shows gel electrophoresis of PCR products with three different sets of primers, F‐Dβ1 and R‐Jβ1.6 (top), F‐Dβ2 and to‐Jβ2.6 (middle), and F‐Dβ1 and R‐Jβ2.6 (bottom). T below the panel indicates T‐type thymus that shows identical or similar rearrangement patterns to the control thymus, and C indicates C‐type thymus that shows a few bands more prominent than the other bands or limited numbers of bands. C/T indicates C/T‐type thymus between the T‐type and C‐type patterns. Size markers are shown at right. (B) Incidences of C‐ (black box), C/T‐ (gray box) and T‐type (white box) thymuses in 30, 60, and 80 days after γ‐irradiation in Bcl11b KO/+ mice. (C) Allelic losses at the Bcl11b locus in irradiated thymuses. Two panels show polyacrylamide gel electrophoresis for PCR products of D12Mit53 and D12Mit279 primer pairs. Chromosomal location of D12Mit53, Bcl11b, and D12Mit279 is 108.69, 109.15–24, 109.69 Mb from the centromere, respectively. We determined the allele ratio of BALB/c and MSM bands and judged the thymus as allelic loss‐positive when the allele ratio was more than 2 or less than 0.5.
We examined loss of the wild‐type Bcl11b allele in C‐ and T‐type thymuses using Massachusetts Institute of Technology (MIT) microsatellite markers flanking the Bcl11b locus (Fig. 2C). Of the 40 Bcl11b KO/+ thymuses examined, four exhibited loss of the wild‐type allele. All of these were C‐type thymus. Their average cell number was as low as 0.20 × 107, and this decrease may be due to a loss of Bcl11b function because Bcl11b KO/KO thymocytes exhibit profound apoptosis.( 13 )
Cell cycle and cell size. We examined the cell cycle distribution of irradiated thymocytes that were isolated from mice at 1 h after intraperitoneal injection of BrdU. We determined the percentage of S‐phase cells, size of G1‐phase cells, and percentage of a fraction containing large thymocytes in the G1 phase. Figure 3(A) shows examples of flow cytometric analysis. The large cells in the G1 phase (indicated by a horizontal bar) were designated as middle‐sized cells because their size was between the size of normal G1 cells (small size) and the size of S phase cells (large size). Figure 3(B) summarizes the percentage of S‐phase cells at the vertical axis and the percentage of the middle‐sized G1 cells at the horizontal axis in the two groups of 30‐day thymuses and 60‐ plus 80‐day thymuses. As for the 30‐day thymuses, the percentage of S‐phase cells or middle‐sized G1 cells did not much differ between Bcl11b KO/+ and Bcl11b +/+ thymuses except for one thymus. On the other hand, there were eight Bcl11b KO/+ thymuses possessing more than 20% middle‐sized G1 cells among the 60/80‐day Bcl11b KO/+ thymuses, which were all C type. They showed a considerable variation in the percentage of the S phase. The middle‐sized thymocytes may be related with premalignancy because cell‐size enlargement is a characteristic of thymic lymphomas.( 24 ) Those thymocytes are probably cells pausing at the G1 stage, and growing and progressing toward the S phase.
Figure 3.
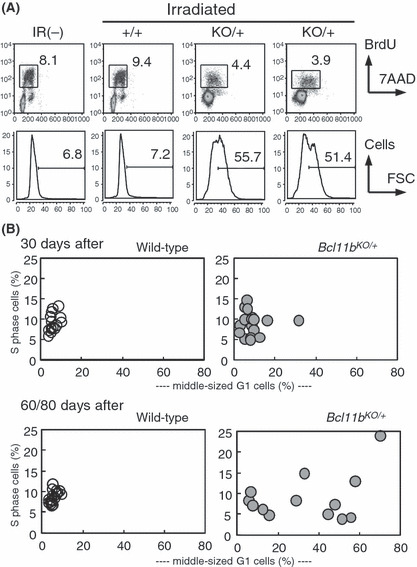
Cell proliferation and cell size. (A) Flow cytometry of cell cycle in unirradiated and irradiated thymuses. (upper) The vertical axis shows BrdU incorporation levels and the horizontal axis displays 7‐AAD staining for DNA contents. A square marks a fraction of thymocytes in the S‐phase, and the number gives the percentage of S‐phase cells. (lower) The vertical axis shows the cell number and the horizontal axis displays forward scatter (FSC) values reflecting the cell size in G1‐phase thymocytes. The bar shows a fraction of thymocytes in large size (middle‐sized G1 cells) and the number above the bar indicates the percent of those thymocytes. The percentage was determined in each thymus by the criterion where the percentage in normal thymus was set to approximately 5% of the FSC value. (B) The vertical axis shows the percentage of S‐phase cells and the horizontal axis displays the percentage of middle‐sized G1 cells. Thirty‐day thymuses, upper; groups of 60‐ and 80‐day thymuses, lower; Bcl11b +/+ thymuses, left; Bcl11b KO/+ thymuses, right.
Differentiation arrest. Thymocytes from Bcl11b KO/KO mice show differentiation arrest at DN and ISP stages to lack DP cells,( 13 , 14 ) and hence C‐type thymocytes or possibly T‐type thymocytes may exhibit differentiation arrest. We examined 12 30‐day and 10 80‐day thymuses with flow cytometry using CD4, CD8, and TCRβ cell surface markers (Fig. 4A,B). We defined the gated region on the FSC versus SSC dot plot to exclude debris and dead cells. Although the cell percentage in the gated region did not much differ in the 30‐day thymuses, it markedly differed among 80‐day thymuses. The fraction of debris and dead cells increased in C‐ but not in T‐type thymuses (data not shown). Analysis of CD4 and CD8 markers revealed that almost all T‐type thymocytes of 30‐day Bcl11b KO/+ (also Bcl11b +/+) mice except for one (d) showed a pattern similar to unirradiated normal thymus, mainly consisting of DP cells. However, analysis of TCRβ showed lower percentages of TCRβhigh mature CD8+ cells in Bcl11b KO/+ thymocytes than Bcl11b +/+ thymocytes (Fig. 4C). These together indicated a small impairment of differentiation in Bcl11b KO/+ thymocytes. On the other hand, all eight C‐ and C/T‐type thymuses of 80‐day Bcl11b KO/+ mice showed marked differentiation impairment. For instance, (f) in Figure 4(B) shows thymocytes at the DN fraction by CD4 and CD8 expression, and (g) and (h) show thymocytes mainly at the DN/ISP and CD4 fractions, respectively. CD4+ SP cells in (h) mostly showed low expression of the TCRβ protein, different from normal CD4+ SP cells. These results suggest that the Bcl11b KO/+ genotype confers differentiation impairment of thymocytes in γ‐irradiated mice.
Figure 4.
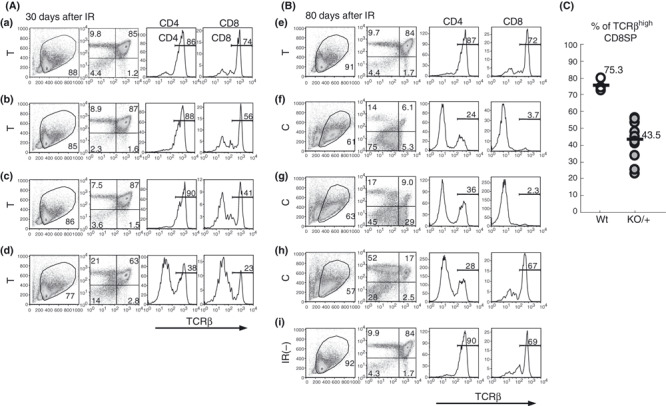
Flow cytometry of CD4, CD8, and T cell receptor β‐chain (TCRβ) expression on thymocytes. Thymocytes at 30 days (A) and 80 days (B) after irradiation. (from left to right) The vertical axis shows side scatter (SSC) values and the horizontal axis displays forward scatter (FSC) values (the gated region marked by a circle); the vertical axis shows CD4 expression and the horizontal axis displays CD8 expression; the vertical axis shows cell number and the horizontal axis displays TCRβ expression of thymocytes in the CD4 quadrant; the vertical axis shows cell number and the horizontal axis displays TCRβ expression of thymocytes in the CD8 quadrant. (a) in (A) is a thymus in irradiated Bcl11b +/+ mice and (b–d) are thymuses in irradiated Bcl11b KO/+ mice. All four thymuses are T type. (e–h) in (B) are irradiated Bcl11b KO/+ mice and (i) is an unirradiated Bcl11b KO/+ mice. (e) is T‐type and (f–h) are C‐type thymuses. (C) The percentage of TCRβhigh CD8SP thymocytes in Bcl11b +/+ (75.3%) and Bcl11b KO/+ (43.5%) mice.
In order to further study the relationship between clonal expansion and differentiation arrest, we subjected 10‐week‐old Bcl11b KO/+ mice to 3‐Gy γ‐radiation and examined thymuses at 30 days after. This experimental condition was chosen based on the higher incidence (6/8, 75%) of C‐type thymus observed in mice irradiated at this age and the decrease to 10% (2/20) when mice were irradiated at 4 weeks of age (data not shown). D‐J rearrangement assay revealed C‐type thymus in six of the 12 thymuses and T‐type thymuses in the remaining six (Fig. 5A). The decrease in cell number was observed in Bcl11b KO/+ mice as predicted, the average number being 1.25 × 107. On the other hand, the average of S‐phase cells was as low as 4.0% in Bcl11b KO/+ mice, and there was one C‐type thymus possessing more than 20% middle‐sized thymocytes. Figure 5(B) shows examples of flow cytometric analysis using CD4, CD8, and TCRβ markers. Of the six C‐type thymuses, only two showed differentiation arrest (see b and c), and the remaining four C‐type thymuses showed a normal differentiation pattern but lower expression of TCRβ (d and e). This indicated that thymocytes in the four C‐type thymuses were capable of differentiating into mature cell types. This contrasts with the results of 80‐day thymuses irradiated at 8 weeks of age: all C‐ and C/T‐type thymuses showed impairment in the development of mature thymocytes. These results suggest a process from normally differentiating C‐type thymocytes to differentiation‐arrested C‐type thymocytes in irradiated Bcl11b KO/+ mice.
Figure 5.
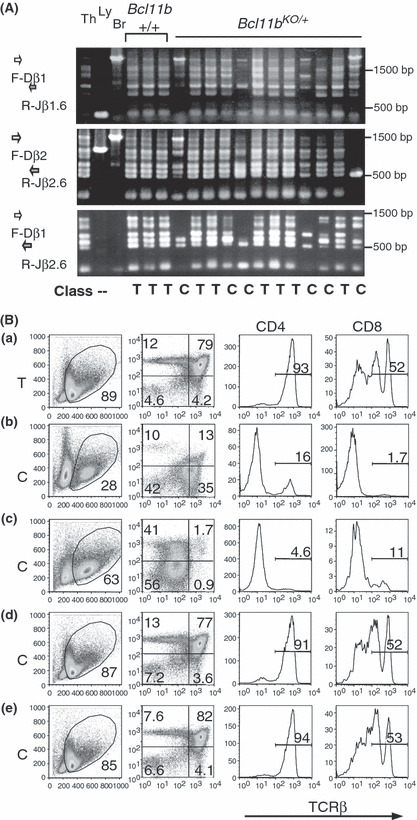
Analyses of thymuses at 30 days after irradiation of Bcl11b KO/+ mice at 10 weeks of age. (A) D‐J rearrangement patterns at the TCRβ locus, as described in the legend for Figure 3(A). (B) Flow cytometry of CD4, CD8, and T cell receptor β‐chain (TCRβ) expression in thymocytes, as described in the legend for Figure 4. T‐ or C‐type thymus is shown at left.
Elevation of β‐catenin expression in Bcl11b KO/+ thymo‐cytes. During the flow cytometric analysis using CD4, CD8, and TCRβ markers, we noted a higher percentage of TCRβ− CD8+ immature ISP cells and a lower percentage of DN cells in Bcl11b KO/+ thymocytes (data not shown). The high ISP and low DN percentages, indicative of some differentiation arrest before the DP cell stage, suggested the possibility of an abnormal increase in Wnt/β‐catenin signaling.( 21 , 22 , 23 ) Therefore, we examined the expression levels of β‐catenin and interleukin‐7 receptor (IL‐7R), a cell surface receptor downstream from β‐catenin signaling.( 26 , 27 ) Figure 6(A) shows examples of flow cytometric analysis of Bcl11b +/+ and Bcl11b KO/+ thymocytes, as well as thymocytes from Apc min/+ mice as a control. The Apc protein is a component of the degradation complex that modifies and regulates the β‐catenin protein level.( 23 ) Consistent with previous reports,( 27 , 28 )β‐catenin was expressed at higher levels in DN cells than DP cells in wild‐type mice, indicating a down‐regulation of β‐catenin in DP cells. Figure 6(A,B) shows a comparison of β‐catenin levels in DN and DP cells between thymocytes in the three different genotypes. β‐Catenin expression was higher in Bcl11b KO/+ thymocytes in both DN and DP cells and the differences were statistically significant (P = 0.0034 and P = 0.019, respectively). Elevated β‐catenin expression was also observed in the DP cells of Apc min/+ mice. DN thymocytes of Bcl11b +/+ and Bcl11b KO/+ mice at 30 days after irradiation also showed a difference in β‐catenin expression (Fig. 6C), suggesting that β‐catenin expression was not affected by irradiation. Figure 6(D) shows IL‐7R expression at the horizontal axis. Expression of IL‐7R in DN cells and its down‐regulation in DP cells were also seen, as described previously.( 26 , 27 ) The IL‐7R expression was higher in Bcl11b KO/+ DN cells than wild‐type DN cells, suggesting that IL‐7R activation is a reflection of increased β‐catenin signaling. These results suggest that elevation of β‐catenin activity in Bcl11b KO/+ thymocytes may affect the proliferation and survival of thymocytes.
Figure 6.
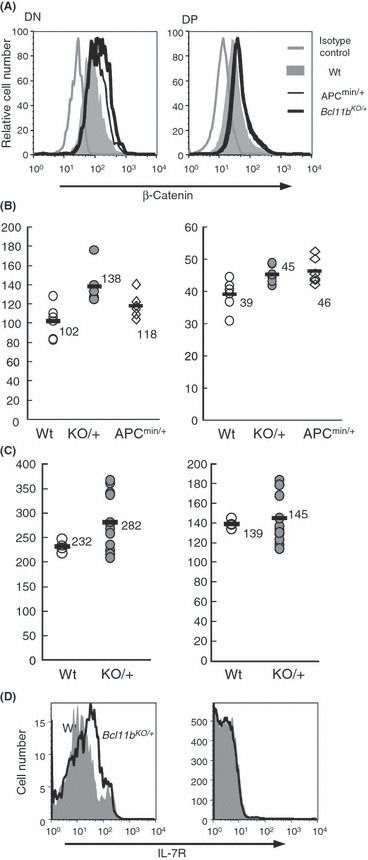
Flow cytometry of β‐catenin and interleukin‐7 receptor (IL‐7R) expression. (A) β‐catenin expression in double‐negative (DN) and double‐postive (DP) cells of Bcl11b +/+ (gray region), Bcl11b KO/+ (bold black line), and Apc min/+ (thin black line) mouse thymocytes. DN cells, left; DP cells, right. Isotype‐matched staining control for Bcl11b +/+ thymocytes is shown for comparison (gray line). The vertical axis shows relative cell number and the horizontal axis displays β‐catenin expression. (B) The mean fluorescence intensity of β‐catenin is compared between thymocytes of the three different genotypes. P‐values in DN and DP cells between Bcl11b +/+ and Bcl11b KO/+ mice were 0.0034 and 0.019, respectively. The P‐value in DP cells between wild‐type and Apc min/+ mice was 0.017. Comparison of the percent of β‐catenin‐positive cells showed similar results (not shown). (C) Mean fluorescence intensity of β‐catenin in thymocytes compared between wild‐type (gray circles) and Bcl11b KO/+ (closed black circles) mice at 30 days after irradiation. (D) IL‐7R expression in DN and DP cells of wild‐type (gray region) and Bcl11b KO/+ (black line) mouse thymocytes.
Discussion
In this paper we examined γ‐ray‐induced atrophic thymuses in Bcl11b KO/+ mice at stages prior to the time of thymic lymphoma development. Clonal expansion of thymocytes, a characteristic of lymphoma cells, was frequently detected in thymuses at 60 or 80 days after 3‐Gy γ‐irradiation, but at a lower frequency at 30 days after irradiation of 8‐week‐old mice. On the other hand, it was detected at a high frequency as early as 30 days after irradiation of 10‐week‐old mice. This age effect on clonal expansion remains to be addressed. Clonal expansion at these early time points was not observed in irradiated mice of the wild‐type genotype, but could only be detected when these mice were subjected to 4‐times fractionated whole‐body γ‐irradiation.( 25 ) These results suggest that Bcl11b heterozygosity enhances the development of clonally expanding thymocytes and contributes to lymphomagenesis by conferring an effect at an early stage before the start of or during clonal cell proliferation.
Several consequences of Bcl11b deficiency have been reported by us and other groups,( 13 , 14 , 15 , 16 , 17 , 18 ) including a loss or decrease of pre‐TCR signaling in thymocytes.( 14 , 19 ) This impairment results in differentiation arrest of thymocytes during β‐selection, which may be contributing to lymphomagenesis. The effects of pre‐TCR signaling include the stabilization or increased expression of β‐catenin via Erk activation that targets several nuclear factors such as early growth response protein (EGR), nuclear factor of activated T‐cells (NFAT), and E proteins.( 26 , 27 , 29 , 30 , 31 , 32 ) Because of the decreased pre‐TCR signaling in Bcl11b KO/+ mice, a decrease in the β‐catenin expression was predicted. However, this study demonstrated that β‐catenin expression was in fact increased in Bcl11b KO/+ mice. This increase is another consequence of the Bcl11b KO/+ genotype, probably independent of the pre‐TCR signaling. The expression level of β‐catenin is mainly regulated through the modification by a degradation complex consisting of axin, Apc, glycogen synthase kinase 3 (GSK3β), and CDK inhibitor (CKI).( 23 , 33 ) Although the mechanism is not known, Bcl11b might affect the expression of some of those proteins. We infer that the increase of β‐catenin plays a key role in lymphomagenesis, because β‐catenin is a well‐known oncogenic transcription factor and its stabilization predisposes thymocytes to malignant transformation.( 34 )β‐Catenin targets promoters of c‐myc and cyclin D1 in a complex with Tcf1 or Lef1, which positively regulate cell cycle progression.( 23 , 33 )
Differentiation arrest of thymocytes at the DN or ISP stages was observed in most thymuses that showed clonal expansion. This arrest was not seen in clonally expanded thymocytes induced in Bcl11b wild‐type mice by fractionated γ‐irradiation.( 25 ) Therefore, differentiation arrest may be due to a decrease of Bcl11b function in atrophic thymus. It may be in parallel how the arrest of thymocytes at the DN and ISP stages is a characteristic of Bcl11b KO/KO mice.( 13 ) ISP thymocytes in normal thymus are known to be highly proliferative,( 35 ) showing a high percentage of S‐phase cells (45% in our experiment; data not shown). However, the ISP cells observed in γ‐irradiated thymuses showed low percentages (approximately 5%), suggesting that the thymocytes are phenotypically similar to ISP cells but lack the property of being able to highly proliferate in the thymus. Another finding observed in irradiated Bcl11b KO/+ mouse thymuses was the decrease in cell number. This may be also ascribed to the decrease of the preTCR signaling that plays a role in survival of thymocytes.( 13 ) On the other hand, there was a group of C‐type thymuses with a low cellularity and of a high percentage of middle‐sized G1 cells. Those thymocytes of enlarged cell‐size might be the prelymphoma cells that have started to form overt thymic lymphomas.
We observed not only thymocytes of clonal origin showing differentiation arrest but also those showing normal differentiation in Bcl11b KO/+ mice irradiated at 10 weeks of age. The latter thymocytes are a selected clone that already possesses the capacity to self‐renew and differentiate into CD4+ and CD8+ SP cells that highly express TCRβ on the cell surface. This kind of thymocyte, possessing the self‐renewal and lineage capacity, was also observed in γ‐irradiated Bcl11b wild‐type mice.( 25 ) It may be noteworthy that CML is regarded as a cancer stem cell because of its self‐renewal and lineage capacity, fundamental properties for adult tissue stem cells.( 6 ) Though the pathogenesis is distinct between CML and the clonally expanding thymocytes, their similarity in terms of stem cell‐like properties may be of interest.( 6 ) It is probable that some of the thymocytes with lineage capacity undergo a change into thymocytes unable to differentiate during lymphoma development. Taken together, the thymocytes possessing self‐renewal and differentiation capacities demonstrated in this paper might be related with cancer stem cells or lymphoma‐initiating cells. The importance of leukemia‐initiating cells is suggested in relapsed ALL in humans because cells responsible for relapse are ancestral to the primary leukemia cells.( 36 ) Of note is that Bcl11b may play a role in the formation of lymphoma stem cells in irradiated mice. It remains open, however, what role the stem cell‐like aberrant thymocytes play in the development and completion of γ‐ray‐induced thymic lymphomas and also whether or not such stem cell‐like aberrant cells may exist in human BCL11b‐disrupted T‐cell leukemias.( 10 , 11 , 12 )
In summary, we detected two distinct populations of clonally growing thymocytes in γ‐irradiated Bcl11b KO/+ mouse thymuses. In one population, thymocytes share a common D‐J rearrangement but retain the capacity to differentiate. In contrast, thymocytes in the second population have lost their ability to differentiate. Those thymocytes are not fully malignant because of the low cell number, and therefore, the establishment of thymic lymphomas requires an additional change for proliferation to reach completion. The Bcl11b KO/+ genotype probably influences the clonal expansion and differentiation arrest of thymocytes in γ‐irradiated mice and this may be ascribed in part to an increase in the level of β‐catenin.
Acknowledgments
We thank Drs Minh To and Yuichi Wakabayashi for critical reading of this manuscript. This work was supported by Grants‐in‐Aid of Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare of Japan and for Cancer Research from the Ministry of Education, Science, Technology, Sports, and Culture of Japan.
References
- 1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2. Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer 2002; 2: 331–41. [DOI] [PubMed] [Google Scholar]
- 3. Calabretta B, Perrotti D. The biology of CML blast crisis. Blood 2004; 103: 4010–22. [DOI] [PubMed] [Google Scholar]
- 4. Mullighan CG, Goorha S, Radtke I et al. Genome‐wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007; 446: 758–64. [DOI] [PubMed] [Google Scholar]
- 5. Mullighan CG, Miller CB, Radtke I et al. BCR‐ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 2008; 453: 110–4. [DOI] [PubMed] [Google Scholar]
- 6. Clarke MF, Dick JE, Dirks PB et al. Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66: 9339–44. [DOI] [PubMed] [Google Scholar]
- 7. Wakabayashi Y, Inoue J, Takahashi Y et al. Homozygous deletions and point mutations of the Rit1/Bcl11b gene in γ‐ray induced mouse thymic lymphomas. Biochem Biophys Res Commun 2003; 301: 598–603. [DOI] [PubMed] [Google Scholar]
- 8. Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci 2006; 97: 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaminura K, Mishim O, Ohi H et al. Haploinsufficiency of Bcl11b for suppression of lymphomagenesis and thymocyte development. Biochem Biophys Res Commun 2007; 355: 538–42. [DOI] [PubMed] [Google Scholar]
- 10. Nagel S, Kaufmann M, Drexler HG, MacLeod RA. The cardiac homeobox gene NKX2‐5 is deregulated by juxtaposition with BCL11B in pediatric T‐ALL cell lines via a novel t(5;14)(q35.1;q32.2). Cancer Res 2003; 63: 5329–34. [PubMed] [Google Scholar]
- 11. MacLeod RA, Nagel S, Kaufmann M, Janssen JW, Drexler HG. Activation of HOX11L2 by juxtaposition with 3’‐BCL11B in an acute lymphoblastic leukemia cell line (HPB‐ALL) with t(5;14)(q35;q32.2). Genes Chromosomes Cancer 2003; 37: 84–91. [DOI] [PubMed] [Google Scholar]
- 12. Przybylski GK, Dik WA, Wanzeck J et al. Disruption of the BCL11B gene through inv(14)(q11.2q32.31) results in the expression of BCL11B‐TRDC fusion transcripts and is associated with the absence of wild‐type BCL11B transcripts in T‐ALL. Leukemia 2005; 19: 201–8. [DOI] [PubMed] [Google Scholar]
- 13. Wakabayashi Y, Watanabe H, Inoue J et al. Bcl11b is required for differentiation and survival of αβT lymphocytes. Nat Immunol 2003; 4: 533–9. [DOI] [PubMed] [Google Scholar]
- 14. Inoue J, Kanefuji T, Okazuka K, Watanabe H, Mishima Y, Kominami R. Expression of TCRβ partly rescues developmental arrest and apoptosis of αβT cells in Bcl11b‐/‐ mice. J Immunol 2006; 176: 5871–9. [DOI] [PubMed] [Google Scholar]
- 15. Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype‐specific genes that control corticospinal motor neuron development in vivo. Neuron 2005; 45: 207–21. [DOI] [PubMed] [Google Scholar]
- 16. Golonzhka O, Liang X, Messaddeq N et al. Dual role of COUP‐TF‐interacting protein 2 in epidermal homeostasis and permeability barrier formation. J Invest Dermatol 2008; 129: 1459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golonzhka O, Metzger D, Bornert JM et al. Ctip2/Bcl11b controls ameloblast formation during mammalian odontogenesis. Proc Natl Acad Sci USA 2009; 106: 4278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avurum Albu DI, Feng D, Bhattacharya D et al. BCL11B is required for positive selection and survival of double‐positive thymocytes. J Exp Med 2007; 204: 3003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okazuka K, Wakabayashi Y, Kashihara M et al. p53 prevents maturation of T cell development to the immature CD4‐CD8+ stage in Bcl11b‐/‐ mice. Biochem Biophys Res Commun 2005; 328: 545–9. [DOI] [PubMed] [Google Scholar]
- 20. Fischer A, Malissen B. Natural and engineered disorders of lymphocyte development. Science 1998; 280: 237–43. [DOI] [PubMed] [Google Scholar]
- 21. Gounari F, Aifantis I, Khazaie K et al. Somatic activation of beta‐catenin bypasses pre‐TCR signaling and TCR selection in thymocyte development. Nat Immunol 2001; 2: 863–9. [DOI] [PubMed] [Google Scholar]
- 22. Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta‐catenin impairs T cell development.Nat Immunol. Nat Immunol 2003; 4: 1177–82. [DOI] [PubMed] [Google Scholar]
- 23. Staal FJ, Clevers HC. Wnt signaling in the thymus. Curr Opin Immunol 2003; 15: 204–8. [DOI] [PubMed] [Google Scholar]
- 24. Ohi H, Mishima Y, Kamimura K, Maruyama M, Sasai K, Kominami R. Multi‐step lymphomagenesis deduced from DNA changes in thymic lymphomas and atrophic thymuses at various times after γ‐irradiation. Oncogene 2007; 26: 5280–9. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto T, Morita S, Go R et al. Clonally expanding thymocytes having lineage capability in g‐ray induced mouse strophic thymus. Int J Radiat Oncol Biol Phys (in press). [DOI] [PubMed] [Google Scholar]
- 26. Xu M, Sharma A, Wiest DL, Sen JM. Pre‐TCR‐induced β‐catenin facilitates transversal through β‐selection. J Immunol 2009; 182: 751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu M, Sharma A, Hossain MZ, Wiest DL, Sen JM. Sustained expression of pre‐TCR induced beta‐catenin in post‐beta‐selection thymocytes blocks T cell development. J Immunol 2009; 182: 759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weerkamp F, Baert MR, Naber BA et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA 2006; 103: 3322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carleton M, Haks MC, Smeele SA et al. Early growth response transcription factors are required for development of CD4‐CD8‐ thymocytes to the CD4+CD8+ stage. J Immunol 2002; 168: 1649–58. [DOI] [PubMed] [Google Scholar]
- 30. Xi H, Kersh GJ. Early growth response gene 3 regulates thymocyte proliferation during the transition from CD4‐CD8‐ to CD4+CD8+ . J Immunol 2004; 172: 964–71. [DOI] [PubMed] [Google Scholar]
- 31. Aifantis I, Gounari F, Scorrano L, Borowski C, Von Boehmer H. Constitutive pre‐TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF‐kappaB and NFAT. Nat Immunol 2001; 2: 403–9. [DOI] [PubMed] [Google Scholar]
- 32. Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J 2004; 23: 202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 2005; 19: 877–90. [DOI] [PubMed] [Google Scholar]
- 34. Guo Z, Dose M, Kovalovsky D et al. Beta‐catenin stabilization stalls the transition from double‐positive to single‐positive stage and predisposes thymocytes to malignant transformation. Blood 2007; 109: 5463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ioannidis V, Beermann F, Clevers H, Held W. The β‐catenin‐TCF‐1 pathway ensures CD4+CD8+ thymocyte survival. Nat Immunol 2001; 2: 691–7. [DOI] [PubMed] [Google Scholar]
- 36. Mullighan CG, Phillips LA, Su X et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008; 322: 1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


