Abstract
AIB1 (amplified in breast cancer 1) is frequently overexpressed in esophageal squamous cell carcinoma (ESCC), but the significance of AIB1 expression in chemoradiotherapy (CRT) sensitivity and its effect on prognosis are still unclear. In this study, the expression of AIB1 was examined by immunohistochemistry in 98 biopsy specimens of primary ESCC patients treated with definitive CRT. AIB1 overexpression was found in 63/98 (64.3%) of the ESCCs. There was a significant association between AIB1 overexpression and distant lymph node metastases (P = 0.011), but not regional lymph node metastases. In the M0 subgroup, overexpression of AIB1 was observed more frequently in stage T4 than in stage T2–3 (66.7%vs 38.5%, P = 0.031). In addition, AIB1 expression was the only factor that showed a significant correlation with CRT response, in which overexpression of AIB1 was observed more frequently in the CRT resistant group than in the CRT effective group (86.5%vs 50.8%, P < 0.001). Univariate analysis revealed that AIB1 overexpression was associated with poor progression‐free survival (PFS) (P < 0.001) and poor disease‐specific survival (DSS) (P <0.001). Furthermore, AIB1 expression could stratify patient survival in stages T2–3, T4, N1, and M0 (P < 0.05), as well as in the CRT effective group (P < 0.05), and AIB1 overexpression and CRT resistance were evaluated as significant independent prognostic factors for both PFS and DSS in multivariate analysis. These findings suggest that overexpression of AIB1 is a useful predictor of CRT resistance and an independent molecular marker of poor prognosis for ESCC patients. (Cancer Sci 2009; 100: 1591–1596)
Esophageal squamous cell carcinoma (ESCC) is a common human malignancy worldwide with an unfavorable prognosis.( 1 , 2 ) Most patients present with locally advanced disease, for which definitive chemoradiotherapy (CRT) could be considered as the standard treatment.( 3 , 4 ) However, the clinical outcomes of ESCC are heterogeneous. Even if the ESCC patients with the same clinical stage treated with the same regimen of CRT, the therapeutic effects are often different. Moreover, even after a good response to CRT, a significant proportion of ESCC patients will suffer from regional recurrence and/or distant metastasis.( 5 , 6 ) The reason is most likely due to inherent heterogeneity in the biology of the tumors.( 7 , 8 ) Currently, only the TNM staging system and CRT response are widely accepted as prognostic factors, which are not as precise as desired, leading to inefficient application of treatment. Thus, identification of specific molecular markers that could serve as predictors for CRT response and prognostic factors in ESCC would be highly desirable and has long been sought. To date, the expression of some genes has been reported to be associated with CRT sensitivity and/or prognosis of ESCC, such as epidermal growth factor receptor (EGFR), human epidermal receptor‐2 (HER‐2), cyclin D1, Ki‐67, P53, Bax, and Bcl‐2.( 9 , 10 , 11 , 12 , 13 , 14 ) However, some of the results obtained by different studies remain conflicting,( 10 , 12 , 15 ) as such reliable markers are still substantially limited.
Recently, the AIB1 (amplified in breast cancer 1) gene, also known as SRC‐3, p/CIP, RAC3, ACTR, and TRAM‐1, has been found to be involved in a number of biological processes, such as cell proliferation, cell apoptosis, cell migration, and others.( 16 ) The oncogenic role of AIB1 has been reported in breast, prostate, ovarian, pancreatic, gastric, colorectal, hepatocellular, nasopharyngeal, and bladder cancers,( 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ) and the abnormalities of this gene were observed to correlate closely with an ascending clinical stage and/or poor patient prognosis. In our previous study of 221 ESCC patients treated with surgery, we found a significant positive association of AIB1 overexpression with increased cell proliferation and advanced T stage.( 26 ) To date, however, little is known about the significance of AIB1 expression in CRT sensitivity and its effect on prognosis of ESCC.
In the present study, the method of immunohistochemistry (IHC) was used to examine the expression of AIB1 in 98 biopsy specimens of primary ESCC patients treated with definitive CRT. The correlation between AIB1 expression and patient clinical/prognostic factors was evaluated to determine whether expression of AIB1 could predict ESCC response to CRT and patient survival.
Materials and Methods
Patients and tissue specimens. In this study, a total of 98 ESCC patients treated with definite CRT were selected from the Department of Radiotherapy, Cancer Center, Sun Yat‐Sen University, between January 2002 and December 2008. All the patients were selected consecutively and fulfilled the following criteria: (a) histologically proven primary ESCC with available biopsy specimens; (b) no previous malignant disease or a second primary tumor; (c) no previous treatment; (d) karnofsky performance status (KPS) ≥70; (e) no severe complications; (f) no distant metastases except for supraclavicular or celiac lymph nodes; (g) received the same regimen of definitive CRT; and (h) adequate clinical information and follow‐up data. Tumor staging was evaluated with physical examination, esophagography, computed tomography (CT), esophagogastroscopy, chest X‐ray, abdominal ultrasonography, and bone scan when necessary before therapy. The tumor biopsy specimens were recruited from paraffin blocks of the 98 primary ESCCs from the Department of Pathology at our institute. In addition, 30 samples of normal esophageal mucosa were used for controls. The study was approved by the medical ethics committee of our institute.
Chemotherapy. After completion of diagnostic workup, chemotherapy with PF (cisplatin/5‐fluorouracil) regimen and radiotherapy were started on the same day. Cisplatin was administered as an i.v. drip at a dose of 80 mg/m2 on day 1; 5‐fluorouracil 3 g/m2 was administered as a continuous i.v. infusion for 48 h on days 1 to 2. Two cycles of chemotherapy were done during radiotherapy at 4‐week intervals.
Radiotherapy. All the patients received external beam radiotherapy by 8‐MV linear accelerator. The initial treatment volume included the primary tumor with a radial margin of 1.5 cm and a proximal and distal margin of 3–4 cm and enlarged lymph nodes. Two‐dimensional or three‐dimensional treatment plans using CT scans were done. A total radiation dose of 60–70 Gy (1.8–2 Gy/fraction, 5 days a week) was delivered with the three‐field technique. The dose to the spinal cord was limited within 45 GY, and the treatment field was reduced after 40–46 Gy.
Evaluation of CRT response. Esophagography was done every 2 weeks during the treatment. The effect of CRT was evaluated clinically for primary lesions based on esophagography and CT 4 weeks after CRT according to the following criteria. Complete response (CR) was defined as the complete resolution of all assessable lesions. Partial response (PR) was defined as a reduction by 50% or more of the sum of the lesions and no progression of assessable lesions. No change (NC) was indicated by a reduction <50% or increase <25% in tumor size. All these conditions had to last for at least 4 weeks without the appearance of new lesions. Progressive disease (PD) was defined as an increase ≥25% in tumor size or the appearance of new lesions.
The patients were followed up in outpatient clinics, where diagnostic examinations consisting of esophagography, CT, chest X‐ray, abdominal ultrasonography, and bone scan when necessary were performed every 3 months to detect recurrence and/or metastasis.
Immunohistochemistry (IHC). IHC staining was performed on 5‐µm tissue sections rehydrated through graded alcohols. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 15 min. For antigen retrieval, tissue slides were boiled in 10 mM citrate buffer (pH 6.0) in a pressure cooker for 10 min. Nonspecific binding was blocked with 10% normal rabbit serum for 20 min. The tissue slides were incubated with anti‐AIB1 (a monoclonal antibody directed at amino acids 376–389 of AIB1; 1:50 dilution; Transduction Laboratories, San Jose, CA, USA) for 60 min at 37°C in a moist chamber. Subsequently, the slides were sequentially incubated with biotinylated rabbit antimouse immunoglobulin at a concentration of 1:100 for 30 min at 37°C and then reacted with a streptavidin–peroxidase conjugate for 30 min at 37°C and 3′‐3′ diaminobenzidine as a chromogen substrate. The nucleus was counterstained using Meyer's hematoxylin. A negative control was obtained by replacing the primary antibody with a normal murine IgG. Positive expression of AIB1 in ESCC and normal esophageal mucosa cells was primarily a nuclear pattern (Fig. 1). Known immunostaining positive slides were used as positive controls. The malignant and non‐malignant tissues were scored for AIB1 by assessing the site of positive staining in the nucleus. Since the expression of AIB1 in the normal esophageal mucosa of each case was observed as negative staining or no more than 10% of epithelium with positive staining of AIB1, positive staining of AIB1 in ESCC cells that ranged from 0%–10% was depicted as normal expression of AIB1, and overexpression of AIB1 was designated when more than 10% of tumor cells were positively stained in the nuclei.( 19 , 21 , 22 , 26 ) Two independent observers blinded to the clinicopathologic information performed the scoring.
Figure 1.
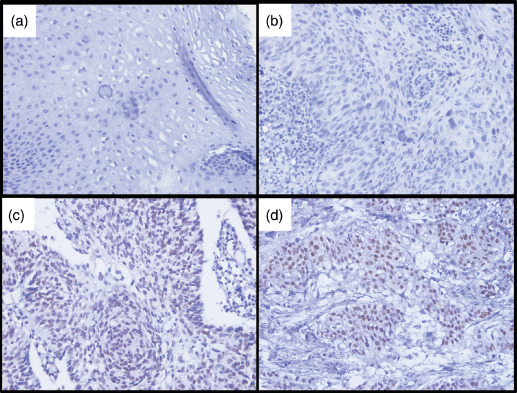
Immunohistochemical staining of AIB1 (amplified in breast cancer 1) in human esophageal tissues. (a) Normal esophageal mucosa showed normal expression of AIB1 protein with a negative staining of AIB1 in the nuclei of all esophageal squamous cells. (b) An esophageal squamous cell carcinoma (ESCC) case (case 19) demonstrated normal expression of AIB1, in which all tumor cells showed negative staining of AIB1. (c) Overexpression of AIB1 was detected in an ESCC (case 53), in which more than 60% ESCC cells showed positive staining of AIB1 protein in the nuclei. (d) Another ESCC (case 71) showed overexpression of AIB1, in which all ESCC cells showed positive staining of AIB1 protein in the nuclei.
Statistical analysis. Statistical analysis was performed with SPSS software (SPSS Standard version 13.0, SPSS, Chicago, IL, USA). The association of AIB1 expression with ESCC patient clinicopathological features was assessed by the χ2‐test. A binary logistic regression model was used for the analysis of variables correlating with AIB1 expression. Disease‐free survival (DFS) and disease‐specific survival (DSS) were assessed with the Kaplan–Meier method and compared by the log‐rank test. Multivariate survival analysis was performed on all parameters that were found to be significant on univariate analysis using the Cox regression model. P‐values of <0.05 were considered significant.
Results
Patient characteristics. The clinicopathological characteristics of the 98 patients studied are summarized in Table 1. According to the 6th edition of the TNM classification of the International Union Against Cancer (UICC, 2002), eight patients were classified into stage II, 51 cases were stage III, and 39 cases were stage IV. All patients received the same regimen of concurrent CRT described above. Sixty‐eight patients received a total dose of 60GY; the other 30 cases received 62–70 GY. At the evaluation time, CR, PR, NC, and PD were achieved in 19 patients, 42 patients, 36 patients, and one patient, respectively. Of the 79 patients who did not achieve CR, 22 cases received adjuvant chemotherapy, and two cases received radical esophagectomy. The other patients did not receive any anti‐tumor treatments until tumor progression.
Table 1.
Association between clinicopathological characteristics, CRT response, and AIB1 expression in 98 ESCCs
| Characteristics | Cases | AIB1 protein | |
|---|---|---|---|
| Overexpression (%) | P‐values † | ||
| Age (years) ‡ | 0.762 | ||
| ≤55 | 54 | 34 (63.0) | |
| >55 | 44 | 29 (65.9) | |
| Gender | 0.463 | ||
| Male | 82 | 54 (65.9) | |
| Female | 16 | 9 (56.3) | |
| Location | 0.030 | ||
| Cervical | 24 | 11 (45.8) | |
| Thoracic | 74 | 52 (70.3) | |
| WHO grade | 0.781 | ||
| G1 | 24 | 16 (66.7) | |
| G2 | 50 | 33 (66.0) | |
| G3 | 24 | 14 (58.3) | |
| Tumor size § | 0.201 | ||
| ≤6 cm | 56 | 33 (58.9) | |
| >6 cm | 42 | 30 (71.4) | |
| T status | 0.175 | ||
| T2–3 | 47 | 27 (57.4) | |
| T4 | 51 | 36 (70.6) | |
| N status | 0.192 | ||
| N0 | 16 | 8 (50.0) | |
| N1 | 82 | 55 (67.1) | |
| M status | 0.011 | ||
| M0 | 59 | 32 (54.2) | |
| M1‐lym ¶ | 39 | 31 (79.5) | |
| M0 subgroup | 0.031 | ||
| T2–3 | 26 | 10 (38.5) | |
| T4 | 33 | 22 (66.7) | |
| M1‐lym ¶ subgroup | 0.087 | ||
| T2–3 | 21 | 17 (81.0) | |
| T4 | 18 | 14 (77.8) | |
| CRT response | <0.001 | ||
| Effective | 61 | 31 (50.8) | |
| Resistant | 37 | 32 (86.5) | |
† χ2‐test; ‡mean age; §mean tumor size; ¶distant lymph node metastases.
AIB1, amplified in breast cancer 1; CRT, chemoradiotherapy; ESCC, esophageal squamous cell carcinoma; WHO, World Health Organization.
Expression of AIB1 in ESCC. In the present study, the overexpression of AIB1 was observed by IHC in 63/98 (64.3%) of the ESCCs. Correlation analysis demonstrated that distant lymph node metastases (M‐lym) status and tumor location were the only two clinicopathologic characteristics correlated with AIB1 expression (Table 1). Further analysis of the M0 subgroup (n = 59) showed that the frequency of AIB1 overexpression in tumors in stage T4 (66.7%) was significantly larger than that in stage T2–3 (38.5%, P = 0.031, Table 1), while no significant difference of the frequency of AIB1 overexpression was observed between tumors in stages N1 and N0 (54.5%vs 46.7%, P = 0.598). Furthermore, the correlation between AIB1 expression and M‐lym status, T status and tumor location was assessed by binary logistic regression analysis. The result showed that only M‐lym status (P = 0.023, odds ratio = 3.245) was evaluated to be an independent factor.
Correlation between clinicopathologic variables, AIB1 expression, and CRT response. Of the 98 ESCC patients, 61 cases were included in the effective group (CR/PR), and the remaining 37 cases were included in the resistant group (NC/PD). The therapeutic response rate was 62.2%. AIB1 expression was the only factor that showed a significant correlation with CRT response, in which overexpression of AIB1 was observed more frequently in the CRT resistant group than in the CRT effective group (86.5%vs 50.8%, P < 0.001) (Table 1). No correlation was found between CRT response and clinicopathologic variables, such as patient age, gender and tumor grade, location, size, T status, and radiotherapy dose (P > 0.05).
Correlation between clinicopathologic variables, AIB1 expression, and ESCC patient survival. Of the 98 ESCC patients, none was lost to follow‐up. The median observation period was 17.1 months (2.3–80.7 months), with 47 local control failures, 36 new distant metastases, and 68 cancer‐related deaths. The other causes of death were censored at time of death. The median survival time was 21.9 months. The 3‐year and 5‐year DSS for the entire cohort of patients were 30% and 22.1%, respectively.
In univariate analysis, AIB1 overexpression was evaluated to correlate closely with poor PFS (median 8.6 vs 29.5 months, P < 0.001) and poor DSS (median 13.2 vs 52.1 months, P < 0.001) (Fig. 2, Table 2). In stratified survival analysis, AIB1 expression could differentiate PFS and DSS of the patients in stages T2–3, T4, N1, and M0, as well as stratify the outcome of patients in the CRT effective group (Fig. 3, Table 2). Kaplan–Meier analysis also demonstrated a significant impact of certain clinicopathological prognostic parameters such as CRT response (P < 0.001) on PFS; and N status (P = 0.033), M‐lym status (P = 0.005), CRT response (P < 0.001), and tumor location (P = 0.017) on DSS. No significant association was found between survival and other clinicopathological variables, including radiotherapy dose and receiving adjuvant chemotherapy or not (P > 0.05). The parameters that were significant in univariate analysis were further examined in multivariate analysis. The results showed that AIB1 expression together with CRT response were independent predictors of PFS and DSS (Table 3).
Figure 2.
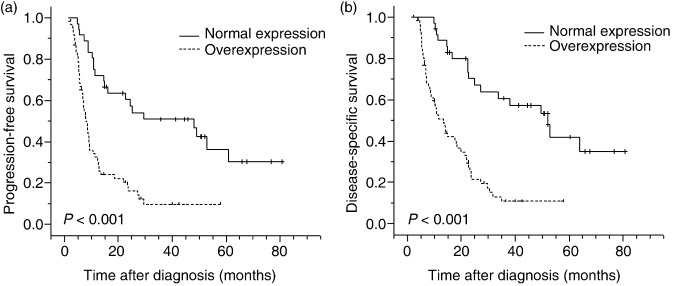
Progression‐free survival and disease‐specific survival curves for esophageal squamous cell carcinoma patients according to AIB1 (amplified in breast cancer 1) expression status. (a) Progression‐free survival curve and (b) disease‐specific survival curve: patients with normal expression (n = 35) or overexpression (n = 63) of AIB1.
Table 2.
Prognostic value of AIB1 expression in subsets of 98 ESCC patients by univariate survival analysis (log‐rank test)
| Variable | Case | PFS (months) | DSS (months) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | P‐values | Mean | Median | P‐values | ||
| Total | <0.001 | <0.001 | |||||
| Normal expression | 35 | 41.4 | 29.5 | 48.6 | 52.1 | ||
| Overexpression | 63 | 15.4 | 8.6 | 19.4 | 13.2 | ||
| T status | |||||||
| T2–3 | 0.002 | <0.001 | |||||
| Normal expression | 20 | 44.9 | 48.1 | 51.9 | 52.8 | ||
| Overexpression | 27 | 17.8 | 9.4 | 20.3 | 14.0 | ||
| T4 | 0.006 | 0.004 | |||||
| Normal expression | 15 | 35.8 | 25.2 | 44.1 | 38.0 | ||
| Overexpression | 36 | 13.6 | 7.9 | 18.5 | 11.6 | ||
| N status | |||||||
| N0 | 0.297 | 0.163 | |||||
| Normal expression | 8 | 50.9 | NR | 60.5 | NR | ||
| Overexpression | 8 | 23.9 | 18.7 | 30.2 | 27.2 | ||
| N1 | <0.001 | <0.001 | |||||
| Normal expression | 27 | 36.7 | 26.0 | 44.2 | 49.7 | ||
| Overexpression | 55 | 13.9 | 9.7 | 17.6 | 10.8 | ||
| M status | |||||||
| M0 | <0.001 | <0.001 | |||||
| Normal expression | 27 | 44.8 | 48.1 | 53.7 | 63.8 | ||
| Overexpression | 32 | 15.9 | 7.8 | 21.0 | 11.6 | ||
| M1‐lym † | 0.086 | 0.148 | |||||
| Normal expression | 8 | 25.2 | 24.5 | 28.2 | 27.1 | ||
| Overexpression | 31 | 14.7 | 8.6 | 17.8 | 13.5 | ||
| CRT‐effective ‡ | 0.012 | 0.004 | |||||
| Normal expression | 30 | 41.3 | 29.5 | 49.4 | 52.1 | ||
| Overexpression | 31 | 20.5 | 9.4 | 25.2 | 22.7 | ||
Distant lymph node metastases;
‡complete response and partial response.
AIB1, amplified in breast cancer 1; CRT, chemoradiotherapy; DSS, disease‐specific survival; ESCC, esophageal squamous cell carcinoma; NR, not reached; PFS, progression‐free survival.
Figure 3.
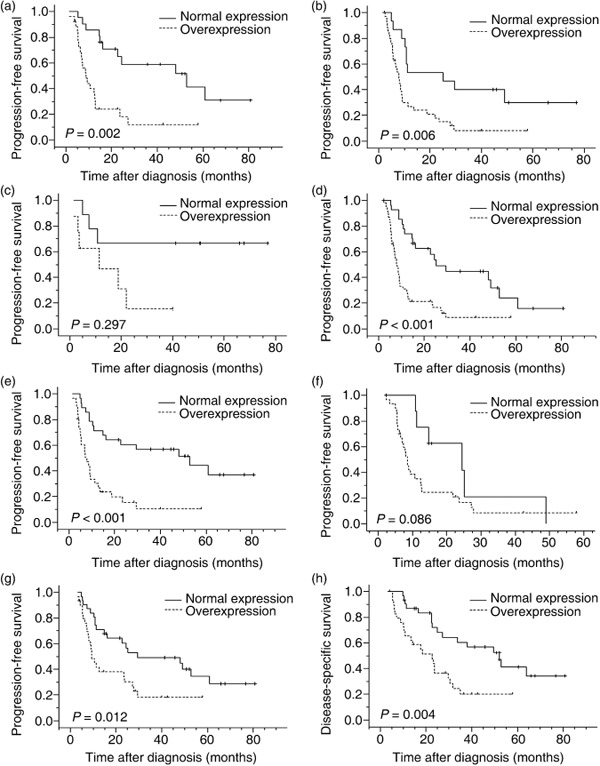
Survival curves for different subsets of esophageal squamous cell carcinoma patients according to AIB1 (amplified in breast cancer 1) expression status. Progression‐free survival curves for (a) T2–3 subset: normal expression (solid line), n = 20; overexpression (dashed line), n = 27. (b) T4 subset: normal expression (solid line), n = 15; overexpression (dashed line), n = 36. (c) N0 subset: normal expression (solid line), n = 8; overexpression (dashed line), n = 8. (d) N1 subset: normal expression (solid line), n = 27; overexpression (dashed line), n = 55. (e) M0 subset: normal expression (solid line), n = 27; overexpression (dashed line), n = 32. (f) M1‐lym (distant lymph node metastases) subset: normal expression (solid line), n = 8; overexpression (dashed line), n = 31. (g) Progression‐free survival curve and (h) disease‐specific survival curve for chemoradiotherapy‐effective subset: normal expression (solid line), n = 30; overexpression (dashed line), n = 31.
Table 3.
Multivariate Cox regression analysis for MFS, PFS, and DSS
| Variable | PFS | DSS | ||||
|---|---|---|---|---|---|---|
| P‐values | Hazard ratios | 95% CI | P‐values | Hazard ratios | 95% CI | |
| AIB1 expression † | <0.001 | 2.939 | 1.701–5.076 | 0.001 | 2.841 | 1.521–5.307 |
| N status ‡ | – | – | – | 0.084 | 1.969 | 0.913–4.246 |
| M status§ | – | – | – | 0.587 | 1.161 | 0.677–1.992 |
| CRT response ¶ | 0.005 | 2.026 | 1.231–3.333 | 0.001 | 2.569 | 1.507–4.381 |
| Tumor location* | – | – | – | 0.393 | 1.359 | 0.672–2.745 |
† Normal expression versus overexpression; ‡N0 versus N1; §M0 versus M1‐lym; ¶effective versus resistant; *cervical versus thoracic.
AIB1, amplified in breast cancer 1; CI, confidence interval; CRT, chemoradiotherapy; DSS, disease‐specific survival; PFS, progression‐free survival.
Discussion
Our previous study demonstrated that the putative oncogene, AIB1, is frequently overexpressed in ESCC, and it is associated closely with increased cell proliferation and advanced T stage.( 26 ) Because the majority of ESCC patients were in early clinical stages (stage I–II, 76.1%), and the survival data was not available, we could not investigate the effect of AIB1 expression on patient prognosis. Thus, we felt great interest in performing the present study to determine the significance of AIB1 expression in CRT sensitivity and its effect on the prognosis of ESCC patients treated with definite CRT.
In this study, our results showed that expression of AIB1 in all of the normal esophageal mucosa specimens was absent or at low levels. In many of our ESCC specimens, in contrast, an overexpression of AIB1 was frequently detected and the frequency of AIB1 overexpression increased with the ascending clinical stage of the patients. Moreover, M‐lym status was the only independent factor correlated with AIB1 expression in ESCCs. Consistent with our previous finding,( 26 ) no significant association was observed between AIB1 expression and the N status of the tumors. These results suggest that up‐regulated expression of AIB1 may provide a selective advantage in the extra‐regional metastasis of lymph node in ESCC. Further analysis showed that in the M0 subgroup, AIB1 overexpression was more frequently observed in ESCCs in stage T4 than in stages T2–3. Taken together, these data provide evidence that the expression levels of AIB1 increase steadily from normal epithelium to earlier invasion, advanced invasion, and extra‐regional lymph node metastasis, suggesting that AIB1 may play an important role in the tumorigenic process of ESCC. Similar results were also observed in other human cancers, such as prostate, gastric, colorectal, and nasopharyngeal cancers,( 18 , 19 , 21 , 24 ) in which the increased expression of AIB1 was shown to associate closely with an ascending clinical stage and/or poor prognosis of these solid tumors.
As we know, definitive CRT could be considered as the standard treatment for patients with locally advanced ESCC, and CRT response was recognized as the most important prognostic factor.( 6 , 27 ) However, individual tumors can exhibit widely differing susceptibility to CRT. Therefore, if predictive factors of the effectiveness of CRT could be found, more appropriate design of treatment for each patient may become possible, leading to an improvement in the outcome. In our present study, we observed that overexpression of AIB1 was the only significant predictor of CRT response. These findings suggest a potential impact of AIB1 on the cellular responses to ionizing radiation and cytotoxic drugs in ESCC. With regards to the molecular mechanism of AIB1, it is known to possibly interact with a broad spectrum of transcription factors in which the phosphoinositide‐3‐kinase (PI3K)/v‐akt murine thymomaviral oncogene homolog (AKT) signal pathway has been identified as a predominant downstream pathway of AIB1.( 16 , 28 ) Recently, the PI3K/AKT pathway has been reported to correlate with tumor therapeutic resistance,( 29 , 30 ) and Hildebrandt et al.( 31 ) reported that genetic variations in the pathway were associated with clinical outcomes in ESCC patients treated with CRT. Thus, we suppose that the PI3 K/AKT pathway is likely to be one of the mechanisms involved in the possible CRT‐resistant role of AIB1. Clearly, further work needs to be done to precisely understand the potential oncogenic function of AIB1 in human ESCC pathogenesis and which signaling pathway is involved in the sensitivity of ESCC to CRT.
The most important finding of the current study was the prognostic significance of AIB1 expression in ESCC. Overexpression of AIB1 was demonstrated to be the most significant predictor of poor PFS and DSS in multivariate analysis surpassing the CRT resistance. ESCC patients with AIB1 overexpression, who were likely to be resistant to CRT, were also at high risk of suffering from tumor progression. The poor survival of ESCC patients with AIB1 overexpression may be attributable to the intrinsic aggressiveness of the tumor, considering the key role of AIB1 in many biological processes, such as cell proliferation, apoptosis, and migration. Involved in the invasive and metastasis processes of ESCC, overexpression of AIB1 may correlate with a high risk of recurrence and/or distant metastasis. Alternatively, poor response to CRT with overexpression of AIB1 could ultimately lead to poor survival in ESCC patients. In addition, AIB1 expression can stratify PFS and DSS in different subsets of the patients, including the CRT effective group, which may help us to understand the heterogeneity in the prognosis of ESCC patients, even with a good response to CRT. These findings further underscore a potentially important role of AIB1 as an underlying biological mechanism in the progression of ESCC. The examination of AIB1 expression by IHC, therefore, could be used as an additional tool to identify those ESCC patients at risk of progression; and AIB1 expression analysis may also be useful in optimizing individual ESCC therapy management.
In summary, in our study, we describe, for the first time, the role of AIB1 in CRT sensitivity and its effect on the prognosis of ESCC patients treated with definite CRT. Our results provide some evidence for the concept that AIB1 overexpression may be important in the acquisition of an aggressive and/or progression phenotype, and meanwhile, the expression of AIB1, as detected by IHC, may be a useful predictor of CRT resistance and an independent molecular marker for poor prognosis of ESCC patients treated with definite CRT.
Acknowledgments
This study was supported by grants from the Major State Basic Research Program of China (no. 2006CB910104) and the 863 Project of China (no. 2007AA021901).
References
- 1. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241–52. [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Stahl M, Stuschke M, Lehmann N et al . Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005; 23: 2310–7. [DOI] [PubMed] [Google Scholar]
- 4. Brenner B, Ilson DH, Minsky BD. Treatment of localized esophageal cancer. Semin Oncol 2004; 31: 554–65. [DOI] [PubMed] [Google Scholar]
- 5. Rohatgi PR, Swisher SG, Correa AM et al . Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer 2005; 104: 1349–55. [DOI] [PubMed] [Google Scholar]
- 6. Di Fiore F, Lecleire S, Rigal O et al . Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol 2006; 12: 4185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luthra R, Wu TT, Luthra MG et al . Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol 2006; 24: 259–67. [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Gu J, Wu TT et al . Genetic variations in radiation and chemotherapy drug action pathways predict clinical outcomes in esophageal cancer. J Clin Oncol 2006; 24: 3789–98. [DOI] [PubMed] [Google Scholar]
- 9. Akimoto T, Nonaka T, Harashima K, Ishikawa H, Sakurai H, Mitsuhashi N. Selective inhibition of survival signal transduction pathways enhanced radiosensitivity in human esophageal cancer cell lines in vitro. Anticancer Res 2004; 24: 811–9. [PubMed] [Google Scholar]
- 10. Kim R, Inoue H, Toge T. Bax is an important determinant for radiation sensitivity in esophageal carcinoma cells. Int J Mol Med 2004; 14: 697–706. [PubMed] [Google Scholar]
- 11. Okumura H, Natsugoe S, Matsumoto M et al . The predictive value of p53, p53R2, and p21 for the effect of chemoradiation therapy on oesophageal squamous cell carcinoma. Br J Cancer 2005; 92: 284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sur M, Sur RK, Cooper K, Bizos D. Preliminary report on the effect of brachytherapy on expression of p53, bc1–2 and apoptosis in squamous cell carcinoma of the oesophagus. S Afr J Surg 2003; 41: 14–20. [PubMed] [Google Scholar]
- 13. Dreilich M, Wanders A, Brattstrom D et al . HER‐2 overexpression (3+) in patients with squamous cell esophageal carcinoma correlates with poorer survival. Dis Esophagus 2006; 19: 224–31. [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi H, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M. Cell‐cycle regulators and the Ki‐67 labeling index can predict the response to chemoradiotherapy and the survival of patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol 2003; 10: 792–800. [DOI] [PubMed] [Google Scholar]
- 15. Natsugoe S, Matsumoto M, Okumura H et al . Bax and Bcl‐X(L) expression are not related to prognosis in patients with advanced esophageal squamous cell carcinoma. Cancer Lett 2001; 174: 91–7. [DOI] [PubMed] [Google Scholar]
- 16. Yan J, Tsai SY, Tsai MJ. SRC‐3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin 2006; 27: 387–94. [DOI] [PubMed] [Google Scholar]
- 17. Ghadimi BM, Schrock E, Walker RL et al . Specific chromosomal aberrations and amplification of the AIB1 nuclear receptor coactivator gene in pancreatic carcinomas. Am J Pathol 1999; 154: 525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakakura C, Hagiwara A, Yasuoka R et al . Amplification and over‐expression of the AIB1 nuclear receptor co‐activator gene in primary gastric cancers. Int J Cancer 2000; 89: 217–23. [PubMed] [Google Scholar]
- 19. Xie D, Sham JS, Zeng WF et al . Correlation of AIB1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum Pathol 2005; 36: 777–83. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c‐myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high‐throughput tissue microarray. Cancer 2002; 95: 2346–52. [DOI] [PubMed] [Google Scholar]
- 21. Liu MZ, Xie D, Mai SJ et al . Overexpression of AIB1 in nasopharyngeal carcinomas correlates closely with advanced tumor stage. Am J Clin Pathol 2008; 129: 728–34. [DOI] [PubMed] [Google Scholar]
- 22. Luo JH, Xie D, Liu MZ et al . Protein expression and amplification of AIB1 in human urothelial carcinoma of the bladder and overexpression of AIB1 is a new independent prognostic marker of patient survival. Int J Cancer 2008; 122: 2554–61. [DOI] [PubMed] [Google Scholar]
- 23. Thorat MA, Turbin D, Morimiya A et al . Amplified in breast cancer 1 expression in breast cancer. Histopathology 2008; 53: 634–41. [DOI] [PubMed] [Google Scholar]
- 24. Zhou HJ, Yan J, Luo W et al . SRC‐3 is required for prostate cancer cell proliferation and survival. Cancer Res 2005; 65: 7976–83. [DOI] [PubMed] [Google Scholar]
- 25. Tanner MM, Grenman S, Koul A et al . Frequent amplification of chromosomal region 20q12‐q13 in ovarian cancer. Clin Cancer Res 2000; 6: 1833–9. [PubMed] [Google Scholar]
- 26. Xu FP, Xie D, Wen JM et al . SRC‐3/AIB1 protein and gene amplification levels in human esophageal squamous cell carcinomas. Cancer Lett 2007; 245: 69–74. [DOI] [PubMed] [Google Scholar]
- 27. Chirieac LR, Swisher SG, Ajani JA et al . Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005; 103: 1347–55. [DOI] [PubMed] [Google Scholar]
- 28. Torres‐Arzayus MI, Font de Mora J, Yuan J et al . High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 2004; 6: 263–74. [DOI] [PubMed] [Google Scholar]
- 29. Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol 2005; 97: 26–34. [DOI] [PubMed] [Google Scholar]
- 30. Horiguchi K, Arai S, Nishihara T, Nishikawa J. AIB1 promotes DNA replication by JNK repression and AKT activation during cellular stress. J Biochem 2006; 140: 409–19. [DOI] [PubMed] [Google Scholar]
- 31. Hildebrandt MA, Yang H, Hung MC et al . Genetic Variations in the PI3K/PTEN/AKT/mTOR Pathway Are Associated With Clinical Outcomes in Esophageal Cancer Patients Treated With Chemoradiotherapy. J Clin Oncol 2009; 27: 857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


