Abstract
The present study investigated fibrotic foci (FFs), the grading system for lymph vessel tumor emboli (LVTEs), and the histological characteristics of nodal metastatic tumors that were significantly associated with the outcomes of 115 patients with invasive ductal carcinoma (IDC) who had received neoadjuvant chemotherapy. We compared the outcome predictive power of FFs, the grading system for LVTEs, and the histological characteristics of metastatic tumors in lymph nodes with the well‐known clinicopathological characteristics of tumor recurrence and tumor‐related death in multivariate analyses. The presence of FFs, as assessed by a biopsy performed before neoadjuvant chemotherapy, significantly increased the hazard rates (HRs) for tumor‐related death in all the cases and in cases with nodal metastasis. The grading system for LVTEs, which was assessed using surgical specimens obtained after neoadjuvant chemotherapy, was significantly associated with increasing hazard rates (HRs) for tumor recurrence and tumor‐related death in all the cases and in cases with nodal metastasis. Moderate to severe stroma in nodal metastatic tumors and five or more mitotic figures in nodal metastatic tumors were significantly associated with elevated HRs for tumor recurrence and tumor‐related death among all the cases. These results indicated that FFs, the grading system for LVTEs, and the histological characteristics of tumor cells in lymph nodes play important roles in predicting the tumor progression of IDCs of the breast in patients treated with neoadjuvant chemotherapy. (Cancer Sci 2009; 100: 1823–1833)
Traditionally, neoadjuvant chemotherapy has been used for the treatment of locally advanced or inoperable breast cancer.( 1 , 2 ) More recently, neoadjuvant chemotherapy has been used for the treatment of patients with smaller tumors that would have previously been considered operable at the patient's initial presentation.( 3 ) The purpose of neoadjuvant chemotherapy is to reduce the size of the primary tumor in the breast, so as to facilitate breast conservation surgery, and also to abolish or reduce the disease burden associated with micro‐metastatic disease with the intention of prolonging the patient's overall survival.
Gene or protein expression profiles have recently been reported to be significant predictors of the outcome of patients receiving neoadjuvant chemotherapy.( 4 , 5 , 6 ) However, identifying histological predictors of prognosis is very important because histopathological examinations of invasive ductal carcinomas (IDCs) can be routinely performed at any hospital and also are a very useful method for following IDC patients who received neoadjuvant chemotherapy clinically. Clinicopathological factors including age, residual invasive tumor size, histologic grade of the primary invasive tumors, axillary node status, and pathological response have been reported to be good predictors of prognosis among patients with IDC who have received neoadjuvant chemotherapy,( 7 , 8 , 9 , 10 ) and we recently demonstrated that a grading system for lymph vessel tumor emboli (LVTEs) and the histological characteristics of tumor cells in lymph nodes are very important histological predictors of prognosis among IDC patients who did not receive neoadjuvant therapy.( 11 , 12 ) These findings strongly suggest that a grading system for LVTEs or the histological characteristics of tumor cells in lymph node might also be very important histological predictors of prognosis among IDC patients who received neoadjuvant chemotherapy.
The purpose of this study was to investigate the histological characteristics of primary invasive tumors, the grading system for LVTEs, and the histological characteristics of nodal metastatic tumors that were significantly associated with the outcomes of IDC patients who received neoadjuvant chemotherapy. We found that the presence of fibrotic foci (FFs), as assessed using biopsy materials obtained before neoadjuvant chemotherapy; the grading system for LVTEs, as assessed using surgical specimens obtained after neoadjuvant chemotherapy; and several histological characteristics of tumor cells in lymph nodes, as assessed using surgical specimens obtained after neoadjuvant chemotherapy, had significant effects on outcome among IDC patients who received neoadjuvant chemotherapy.
Materials and Methods
Patients. The subjects of this study comprised 115 consecutive patients with IDC of the breast who had been surgically treated at the National Cancer Center Hospital between January 1997 and December 2002. The IDCs were diagnosed preoperatively by aspiration cytology, mammography, or ultrasonography. Clinical information was obtained from the patients’ medical records after a complete histological examination of all the IDCs. All the patients were Japanese women, ranging in age from 30 to 71 years (median, 50 years). All the patients had a solitary lesion; 49 patients were premenopausal, and 57 were postmenopausal. A partial mastectomy had been performed in 14 patients, and a modified radical mastectomy had been performed in 101. A level I and II axillary lymph node dissection had been performed in all the patients, and a level III axillary lymph node dissection had been performed in some of the IDC patients.
Of the 115 patients, 17 (12%) had only residual ductal carcinoma in situ, while 98 (88%) patients had residual IDC; none of the patients exhibited a pathological complete response (no tumor) to neoadjuvant chemotherapy. All the neoadjuvant chemotherapy regimens were anthracycline‐based with or without taxane. No cases of inflammatory breast cancer were encountered in this series. All the tumors were classified according to the pathological International Union Against Cancer (UICC)‐TNM (pTNM) classification.( 13 )
For the pathological examination, biopsy specimens obtained before neoadjuvant chemotherapy and surgically resected specimens obtained after neoadjuvant chemotherapy were fixed in 10% formalin and subsequently examined. The size and gross appearance of the surgically resected tumor specimens were recorded as the residual invasive tumor size. The tumor size of the surgically resected specimens was confirmed by comparison with the tumor size on histological slides; if more than one invasive focus was present, the size of the largest invasive focus was recorded as the residual invasive tumor size in this study.
Histological examination. Serial sections of the biopsy materials obtained before neoadjuvant chemotherapy, and serial sections of the tumor area in the surgically resected materials obtained after neoadjuvant chemotherapy were cut from paraffin blocks. One section of each biopsy or surgical specimen was stained with hematoxylin and eosin (H&E) and examined histologically to confirm the diagnosis, and another section was subjected to immunohistochemistry. The following eight histological features of the primary invasive tumors were evaluated in the biopsy materials obtained before neoadjuvant chemotherapy and the surgical materials obtained after neoadjuvant chemotherapy: (1) clinical invasive tumor size or residual invasive tumor size (≤20, >20 to ≤0, >50 mm); (2) histologic grade (1, 2, 3);( 14 ) (3) tumor necrosis (absent, present);( 15 ) (4) FF (absent, present) (Fig. 1a,b);( 16 , 17 ) (5) blood vessel invasion (absent, present); (6) adipose tissue invasion (absent, present); and (7) skin invasion (absent, present). We also evaluated a grading system for LVTEs, as assessed using biopsy materials obtained before neoadjuvant chemotherapy and surgical materials obtained after neoadjuvant chemotherapy (Table 1, Fig. 1c,d).( 11 ) Briefly, the number of tumor cell mitotic figures and the number of apoptotic figures in the lymph vessels were counted in 20 high‐power fields of the surgical materials. In practice, for the surgical materials, we first examined all the slides of the IDCs containing both tumor areas and non‐tumor areas to identify the LVTEs. Next, we selected the LVTEs, e.g. large LVTEs located far from the stroma–invasive tumor margin, and recorded the number of mitotic figures and the number of apoptotic figures in the tumor cells composing the LVTEs of the IDC. The mitotic and apoptotic figures were counted under a high‐power field, and the largest number of mitotic figures and/or the largest number of apoptotic figures were recorded as the number of mitotic figures and apoptotic figures in the LVTEs of the IDC, respectively. The cumulative numbers of tumor cell mitotic figures and apoptotic figures in the LVTEs in all 20 high‐power fields were not used. In IDCs containing a small number of LVTEs, the mitotic figures and apoptotic figures were counted in less than 20 high‐power fields. For the biopsy materials, we examined the presence or absence of LVTE or LVTEs; when LVTE or LVTEs were observed in the biopsy material, an assessment similar to that described above was performed. We also evaluated the prognostic predictive power of the location of lymph vessel invasion,( 18 ) the Fisher's neoadjuvant‐chemotherapy‐effect classification,( 19 , 20 ) and the Japanese Breast Cancer Society (JBCS) neoadjuvant‐chemotherapy‐effect classification for surgical materials obtained after neoadjuvant chemotherapy.( 21 ) Cases with non‐invasive ductal carcinoma (NIDC) after neoadjuvant chemotherapy were classified as belonging to grade 3 of the JBCS neoadjuvant‐chemotherapy effect classification.( 21 ) None of the IDC cases exhibited the disappearance of all the tumor cells (invasive tumor cells and non‐invasive tumor cells) after neoadjuvant chemotherapy in this series.
Figure 1.
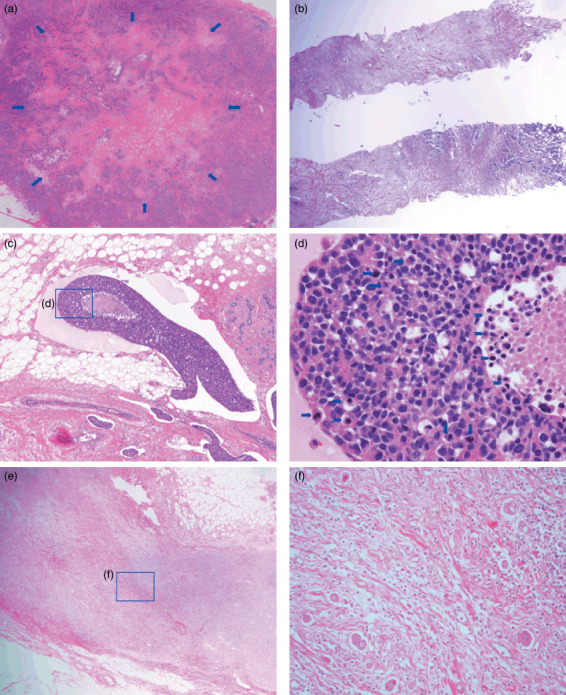
Histological characteristics of fibrotic foci (FFs), lymph vessel tumor emboli, and nodal metastatic tumors. (a) An FF measuring 8.4 × 6.2 mm is visible within the tumor (arrows) in a surgical specimen. The FF has the appearance of a scar‐like feature, and it is surrounded by invasive ductal carcinoma cells. The FF area consists of fibroblasts and collagen fibers arranged in a storiform pattern with tumor cell nests. (b) A core‐needle biopsy specimen shows an FF consisting of fibroblasts and collagen fibers in a storiform arrangement intermingled with invasive tumor cells (fibrosis grade 3). (c) One large lymph vessel tumor embolus and five lymph vessel tumor emboli are shown. A necrotic tumor focus is visible in the large lymph vessel tumor embolus. (d) Several apoptotic bodies and apoptotic tumor cells are visible (arrowheads), and six mitotic tumor cells (arrows) can be seen in the lymph vessel tumor embolus. The apoptotic bodies are small, variously shaped pyknotic bodies that resemble sesame seeds, and the apoptotic tumor cells were identified as tumor cells containing eosinophilic or amphophilic cytoplasm and irregularly shaped pyknotic nuclei. (e) Metastatic tumor in the lymph node exhibiting dense stromal fibrosis. (f) Tumor cells with light eosinophilic cytoplasm and irregularly shaped nuclei exhibiting scattered growth in dense fibrous stroma of a metastatic tumor in a lymph node.
Table 1.
Criteria used in the grading systems for lymph vessel tumor emboli in invasive ductal carcinoma (IDC)
| Grading system for lymph vessel tumor emboli according to the number of mitotic and apoptotic figures in tumor cells of lymph vessel tumor emboli | ||
|---|---|---|
| Grade 0 | IDCs with no lymph vessel tumor emboli | |
| Grades 1, 2, and 3 | IDCs with one or more lymph vessel tumor emboli | |
| No. of mitotic figures | No. of apoptotic figures | |
| Grade 1 | Low‐proliferative type | |
| 1a | 0 | 0 |
| 1b | 0 | >0 |
| 1c | >0 | 0 |
| Grade 2 | Intermediate‐proliferative type | |
| 2a | 1 to 4 | >0 |
| 2b | >0 | 1 to 6 |
| Grade 3 | High‐proliferative type | |
| 3a | >4 | >6 |
The following histological features of metastatic tumors in lymph nodes dissected at the time of surgery (after neoadjuvant chemotherapy) were examined:( 12 ) (1) the maximum dimension of nodal metastatic tumors; (2) lymph nodes with extra‐nodal invasion (absent, present); (3) extra‐nodal blood vessel tumor emboli (absent, present); (4) number of mitotic figures in tumors in the lymph node (≤5, >5); (5) histologic grade of tumors in the lymph node (1, 2, 3); and (6) grade of stromal fibrosis of tumors in the lymph node (none, mild, moderate, severe) (Fig. 1e,f). Extra‐nodal invasion was defined as the extension of tumor cells through the capsule of at least one lymph node into the perinodal adipose tissue. Nuclear atypia, structural atypia, and the number of mitotic figures were evaluated in the same manner as for the primary invasive tumors. The presence of metastases in the lymph nodes was evaluated using single sections of each node or half of each node stained with H&E.
Immunohistochemistry. Immunohistochemical staining for estrogen receptors (ERs), progesterone receptors (PRs), and HER2 products was performed using an autoimmunostainer (Optimax Plus; BioGenex, San Ramon, CA, USA). The antigen retrieval device of the Optimax Plus was autoclaved, and each specimen was immersed in citrate buffer and incubated at 121°C for 10 min. Immunoperoxidase staining was performed using a labeled streptavidin biotin staining kit (BioGenex) according to the manufacturer's instructions. The antibodies used were an anti‐ER mouse monoclonal antibody (mAb), ER88 (BioGenex); an anti‐PR mAb, PR88 (BioGenex); and an anti‐HER2 mAb, CB11 (BioGnex). ER88, PR88, and CB11 were already diluted. After immunostaining, the sections were counterstained with hematoxylin. Sections of IDCs positive for ER, PR, and HER2 were used each time as a positive control. As a negative control, the primary antibody was replaced with normal mouse immunoglobin. An IDC with nuclear staining for ER or PR in 10% or more of its tumor cells was assessed as ER‐positive or PR‐positive. The HER2 status of the tumor cells was semi‐quantitatively scored on a 0 to 3 scale according to the level of HER2 protein expression.( 22 )
One author (N.T.) assessed all the characteristics of the primary tumors, the tumors in the lymph vessels, and the nodal metastatic tumors as well as the immunohistochemical parameters of the biopsy and surgical materials, and another author (T.H.) identified the characteristics of all the IDCs or the immunohistochemical parameters to confirm the tumor cell characteristics in these tumor components and the immunohistochemical characteristics recorded by N.T. Whenever a discrepancy occurred, the authors re‐examined the slides to reach a consensus.
Patient outcome and statistical analysis. Survival was evaluated using a median follow‐up period of 52.3 months (range, 4.9 to 84.6 months) until February 2007. At that time, 83 of the 115 patients who had received neoadjuvant chemotherapy were alive and well, 32 had developed tumor recurrences, and 16 had died of their disease. The recurrence‐free and overall survival periods were determined beginning at the time of surgery. Tumor relapse was considered to have occurred whenever evidence of metastasis was first observed.
We analyzed the outcome predictive power of a grading system for LVTEs assessed using biopsy or surgical materials, the seven histological factors of primary invasive tumors assessed using biopsy or surgical materials, six histological factors of metastatic tumors in lymph nodes assessed using surgical materials, ER and PR expression in primary invasive tumor cells assessed using biopsy or surgical materials, the category of HER2 expression in primary invasive tumor cells using biopsy or surgical materials, the Fisher's classification for neoadjuvant chemotherapy,( 19 , 20 ) the classification of the JBCS for neoadjuvant chemotherapy,( 21 ) age (≤39 years and >39 years), the UICC‐pathological nodal status (UICC pN: no nodal metastasis, N0; 1 to 3 nodal metastases, N1; 4 to 9 nodal metastases, N2; and 10 or more nodal metastases, N3), the UICC‐pTNM stage classification( 13 ) for tumor recurrence, and tumor‐related death using univariate analyses with the Cox proportional hazard regression model.( 23 ) Factors significantly associated with outcome in the univariate analyses were then entered together into the multivariate analyses using the Cox proportional hazard regression model( 23 ) according to nodal status. The step‐down method was applied until all of the remaining factors were significant at a P‐value of less than 0.05. Since the following factors were examined using both biopsy materials obtained before neoadjuvant therapy and surgical materials obtained after neoadjuvant chemotherapy, to be able to accurately assess the prognostic value of each of these factors using multivariate analyses, their mutual influence on the outcome was avoided by analyzing the prognostic predictive power of the biopsy materials obtained before neoadjuvant chemotherapy and that of the surgical materials obtained after neoadjuvant chemotherapy separately (model 1, factors examined using biopsy materials; model 2, factors examined using surgical materials): (1) invasive tumor size; (2) histologic grade; (3) FF; (4) tumor necrosis; (5) grading system for LVTEs; (6) blood vessel invasion; (7) ER and PR status; and (8) HER2 status. In IDC patients without nodal metastasis, since tumor recurrence was observed in three patients, and tumor‐related death was observed in only two patients, we were unable to perform multivariate analyses for tumor recurrence or tumor‐related death. The survival curves were drawn using the Kaplan–Meier method.( 24 ) All analyses were performed with Statistica/Windows software (StatSoft, Tulsa, OK, USA).
Results
Factors significantly associated with tumor recurrence and tumor‐related death. The univariate analyses of data for biopsy materials obtained before neoadjuvant chemotherapy showed that the clinical invasive tumor size and skin invasions were significantly associated with tumor recurrence, while the presence of FF (Fig. 2a) and the grading system for LVTEs were significantly associated with tumor‐related death (Table 2). None of the biopsy materials obtained before neoadjuvant chemotherapy exhibited blood vessel invasion.
Figure 2.
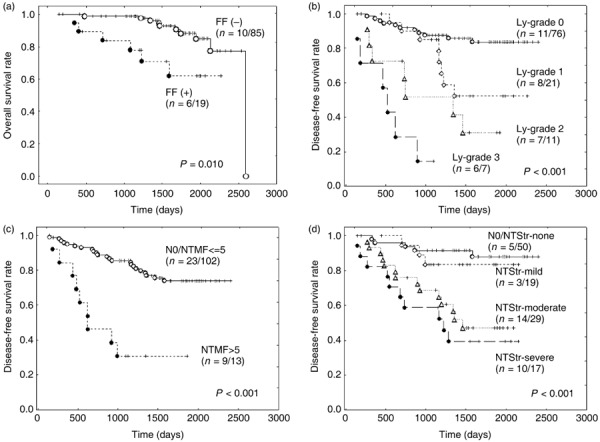
(a–d) Overall survival curves and disease‐free survival curves of invasive ductal carcinoma (IDC) patients who received neoadjuvant chemotherapy. (a) Patients with IDCs exhibiting fibrotic foci (FFs) assessed using biopsy specimens obtained before neoadjuvant chemotherapy have a significantly shorter overall survival period than patients with IDCs that do not exhibit FFs, as assessed using biopsy specimens obtained before neoadjuvant chemotherapy. (b) The disease‐free survival of IDC patients classified according to a grading system for lymph vessel tumor emboli assessed using surgical materials obtained after neoadjuvant chemotherapy decreases significantly according to the grade. Ly, lymph vessel tumor embolus or emboli. (c) The disease‐free survival of IDC patients with nodal metastatic tumors containing five or more mitotic figures is significantly shorter than that of IDC patients without nodal metastasis or those with nodal metastatic tumors containing less than five mitotic figures. N0, no nodal metastasis; NTMF, mitotic figures in nodal metastatic tumor. (d) The disease‐free survival of IDC patients classified according to the tumor stroma of nodal metastatic tumors decreases significantly according to the degree of fibrosis in the nodal metastatic tumors. NTStr, nodal metastatic tumor stroma.
Table 2.
Association of clinicopathological factors assessed using biopsy materials obtained before neoadjuvant chemotherapy with tumor recurrence and tumor‐related death in all patients with invasive ductal carcinoma who received neoadjuvant chemotherapy
| Factors | Cases 115 | Number of patients (%) | |||
|---|---|---|---|---|---|
| Tumor recurrence | Tumor‐related death | ||||
| Present (n = 32) | P‐values | Present (n = 16) | P‐values | ||
| Clinical invasive tumor size (mm) | |||||
| ≤20 | 0 | 0.004 | 0.088 | ||
| >20 to ≤50 | 72 | 14 (19) | 8 (11) | ||
| >50 | 43 | 18 (42) | 8 (19) | ||
| Histologic grade of primary invasive tumor | |||||
| 1 | 25 | 8 (32) | 0.352 | 2 (8) | 0.890 |
| 2 | 70 | 21 (30) | 13 (19) | ||
| 3 | 20 | 3 (15) | 1 (5) | ||
| Fibrotic focus | |||||
| Absent | 96 | 25 (26) | 0.285 | 10 (11) | 0.010 |
| Present | 19 | 7 (37) | 6 (32) | ||
| Tumor necrosis | |||||
| Absent | 80 | 23 (29) | 0.769 | 10 (13) | 0.499 |
| Present | 35 | 9 (26) | 6 (17) | ||
| Grading system for lymph vessel tumor emboli | |||||
| Grade 0 | 106 | 28 (26) | 0.079 | 12 (11) | 0.009 |
| Grade 1 | 5 | 1 (20) | 1 (20) | ||
| Grade 2 | 4 | 3 (75) | 3 (75) | ||
| Grade 3 | 0 | ||||
| ER and PR status (n = 106) | |||||
| Negative | 42 | 12 (29) | 0.707 | 7 (17) | 0.590 |
| Positive | 64 | 17 (27) | 7 (11) | ||
| HER2 status (n = 109) | |||||
| 0 to 2 | 82 | 21 (26) | 0.422 | 8 (10) | 0.155 |
| 3 | 27 | 9 (33) | 7 (26) | ||
ER and PR status negative, ER and PR both negative; ER and/or PR status positive, ER positive or PR positive, or both positive. ER, estrogen receptor; PR, progesterone receptor.
The univariate analyses of data for surgical materials obtained after neoadjuvant chemotherapy showed that skin invasion, the histologic grade of the primary invasive tumors, tumor necrosis, the grading system for LVTEs (Fig. 2b), the UICC pN category, nodal metastatic tumor stroma (Fig. 2d), five or more mitotic figures in nodal metastatic tumors (Fig. 2c), the histologic grade of the nodal metastatic tumors, the presence of a node with extranodal blood vessel tumor emboli, the presence of a node with extranodal invasion, and the UICC pTNM stage classification were significantly associated with tumor recurrence and tumor‐related death (Table 3). Residual invasive tumor size, the presence of lymph vessel tumor emboli in the advanced area of primary invasive tumors, and the presence of lymph vessel tumor emboli in the non‐tumor areas of primary invasive tumors were significantly associated with tumor recurrence but not tumor‐related death, while the other factors were not significantly associated with tumor recurrence or tumor‐related death in the univariate analyses (Table 3).
Table 3.
Association of clinicopathological factors using surgical materials obtained after neoadjuvant therapy with tumor recurrence and tumor‐related death in all patients with invasive ductal carcinoma (IDC) who received neoadjuvant chemotherapy
| Factors | Cases 115 | Number of patients (%) | |||
|---|---|---|---|---|---|
| Tumor recurrence | Tumor‐related death | ||||
| Present (n = 32) | P‐values | Present (n = 16) | P‐values | ||
| Age, years | |||||
| ≤39 | 20 | 4 (20) | 0.332 | 2 (10) | 0.622 |
| >39 | 95 | 28 (30) | 14 (15) | ||
| Adjuvant therapy | |||||
| No | 10 | 1 (10) | 0.234 | 0 | 0.195 |
| Yes | 105 | 31 (30) | 16 (15) | ||
| Fisher's classification | |||||
| NIDC cases | 17 | 2 (12) | 0.095 | 1 (6) | 0.162 |
| IDC cases | 98 | 30 (31) | 15 (15) | ||
| Grade classification of neoadjuvant chemotherapy according to the Japan Breast Cancer Society classification | |||||
| Grade 0 | 3 | 0 | 0.364 | 0 | 0.368 |
| Grade 1a | 48 | 14 (29) | 6 (13) | ||
| Grade 1b | 31 | 10 (32) | 6 (19) | ||
| Grade 2 | 14 | 4 (29) | 3 (21) | ||
| Grade 3 | 17 | 2 (12) | 1 (6) | ||
| Residual invasive tumor size (mm) | |||||
| NIDC cases | 17 | 2 (12) | 0.014 | 1 (6) | 0.163 |
| ≤20 | 33 | 9 (27) | 7 (21) | ||
| >20 to ≤50 | 45 | 11 (24) | 4 (9) | ||
| >50 | 20 | 10 (50) | 4 (20) | ||
| Skin invasion | |||||
| Absent | 86 | 20 (24) | 0.030 | 8 (9) | 0.006 |
| Present | 29 | 12 (41) | 8 (28) | ||
| Histologic grade of primary invasive tumor | |||||
| NIDC cases | 17 | 2 (12) | 0.012 | 1 (6) | 0.003 |
| 1 | 27 | 5 (19) | 0 | ||
| 2 | 48 | 17 (35) | 11 (23) | ||
| 3 | 23 | 8 (35) | 4 (17) | ||
| Fibrotic focus | |||||
| Absent | 88 | 23 (26) | 0.417 | 12 (14) | 0.687 |
| Present | 27 | 9 (33) | 4 (15) | ||
| Tumor necrosis | |||||
| Absent | 88 | 20 (23) | 0.010 | 10 (11) | 0.038 |
| Present | 27 | 12 (44) | 6 (22) | ||
| Grading system for lymph vessel tumor emboli | |||||
| Grade 0 | 76 | 11 (14) | <0.001 | 7 (9) | <0.001 |
| Grade 1 | 21 | 8 (38) | 2 (10) | ||
| Grade 2 | 11 | 7 (64) | 5 (45) | ||
| Grade 3 | 7 | 6 (86) | 2 (30) | ||
| Lymph vessel tumor emboli in the advance area | |||||
| Absent | 91 | 20 (22) | 0.003 | 10 (11) | 0.328 |
| Present | 24 | 12 (50) | 6 (30) | ||
| Lymph vessel tumor emboli in the non‐tumor stroma area | |||||
| Absent | 88 | 18 (20) | <0.001 | 10 (11) | 0.051 |
| Present | 27 | 14 (52) | 6 (22) | ||
| Blood vessel invasion | |||||
| Absent | 6 | 1 (17) | 0.510 | 1 (17) | 0.757 |
| Present | 109 | 31 (28) | 15 (14) | ||
| UICC pN category | |||||
| N0 | 41 | 3 (7) | <0.001 | 2 (5) | 0.032 |
| N1 | 39 | 11 (28) | 6 (15) | ||
| N2 | 24 | 10 (42) | 6 (25) | ||
| N3 | 11 | 8 (73) | 2 (18) | ||
| Nodal metastatic tumor stroma | |||||
| N0/none | 50 | 5 (10) | <0.001 | 4 (8) | 0.009 |
| Mild | 19 | 3 (16) | 1 (5) | ||
| Moderate | 29 | 14 (48) | 6 (21) | ||
| Severe | 17 | 10 (59) | 5 (29) | ||
| Number of mitotic figures in nodal metastatic tumor (/1‐high power field) | |||||
| N0/≤5 | 102 | 23 (23) | <0.001 | 12 (12) | 0.002 |
| >5 | 13 | 9 (69) | 4 (31) | ||
| Histologic grade of nodal metastatic tumor | |||||
| N0 | 41 | 3 (7) | <0.001 | 2 (5) | 0.022 |
| 1 | 10 | 5 (50) | 1 (10) | ||
| 2 | 45 | 15 (33) | 10 (22) | ||
| 3 | 19 | 9 (47) | 3 (16) | ||
| Nodes with extranodal blood vessel invasion | |||||
| N0 | 41 | 3 (7) | <0.001 | 2 (5) | 0.004 |
| Absent | 45 | 13 (29) | 5 (11) | ||
| Present | 29 | 16 (55) | 9 (31) | ||
| Nodes with extranodal invasion | |||||
| N0 | 41 | 3 (7) | <0.001 | 2 (5) | 0.039 |
| Absent | 30 | 8 (27) | 6 (20) | ||
| Present | 44 | 21 (48) | 8 (18) | ||
| UICC pTNM stage classification | |||||
| 0 | 15 | 0 | <0.001 | 0 | 0.014 |
| I | 1 | 0 | 0 | ||
| IIA | 19 | 5 (26) | 3 (16) | ||
| IIB | 30 | 4 (13) | 3 (10) | ||
| IIIA | 23 | 9 (39) | 4 (17) | ||
| IIIB | 16 | 6 (38) | 4 (25) | ||
| IIIC | 11 | 8 (73) | 2 (18) | ||
| ER and PR status (n = 107) | |||||
| Negative | 42 | 12 (29) | 0.667 | 7 (17) | 0.549 |
| Positive | 65 | 17 (26) | 7 (11) | ||
| HER2 status (n = 101) | |||||
| 0 to 2 | 84 | 24 (29) | 0.923 | 10 (11) | 0.087 |
| 3 | 17 | 6 (35) | 5 (29) | ||
| ER and PR status negative, ER and PR both negative; ER and PR status positive, ER positive or PR positive, or both positive. N0, no nodal metastasis; N1, one to three nodal metastases; N2, four to nine nodal metastases; N3, 10 or more nodal metastases. ER, estrogen receptor; NIDC, non‐invasive ductal carcinoma; pN, pathological regional lymph node; PR, progesterone receptor; UICC, International Union Against Cancer. | |||||
Overall, five or more mitotic figures in nodal metastatic tumors, and nodes with extranodal invasion were significantly associated with elevated hazard rates (HRs) for tumor recurrence and tumor‐related death (Table 4, model 1). Clinical invasive tumor size, the presence of tumor necrosis (assessed using surgical materials), and severe nodal metastatic tumor stroma were significantly associated with elevated HRs for tumor recurrence (Table 4, model 1). Grade 2 LVTEs (assessed using biopsy materials) and the presence of FF (assessed using biopsy materials) were significantly associated with elevated HRs for tumor‐related death in the multivariate analyses (Table 4, model 1). In model 2, the grading system for LVTEs (assessed using surgical materials), five or more mitotic figures in nodal metastatic tumors, moderate to severe stroma in nodal metastatic tumors, and the presence of tumor necrosis (assessed using surgical materials) were significantly associated with elevated HRs for tumor recurrence; among these factors, grade 2 LVTEs (assessed using surgical materials), five or more mitotic figures in nodal metastatic tumors, and severe stroma in nodal metastatic tumors were also significantly associated with elevated HRs for tumor‐related death in the multivariate analyses (Table 4).
Table 4.
Multivariate analyses for tumor recurrence and tumor‐related death in all patients with invasive ductal carcinoma (IDC) who received neoadjuvant chemotherapy
| Factors | Tumor recurrence | Tumor‐related death | ||||
|---|---|---|---|---|---|---|
| HRs | 95% CI | P‐values | HRs | 95% CI | P‐values | |
| Model 1 | ||||||
| Clinical invasive tumor size (mm) before neoadjuvant chemotherapy | ||||||
| >20 to ≤50 | Referent | Referent | ||||
| >50 | 2.2 | 1.1–4.4 | 0.034 | – | – | |
| Grading system for lymph vessel tumor emboli assessed using biopsy materials obtained before neoadjuvant chemotherapy | ||||||
| Grade 0 | Referent | Referent | ||||
| Grade 1 | – | – | 0.7 | 0.03–14.2 | 0.796 | |
| Grade 2 | – | – | 5.9 | 1.3–27.9 | 0.025 | |
| Fibrotic focus assessed using biopsy materials obtained before neoadjuvant chemotherapy | ||||||
| Absent | Referent | Referent | ||||
| Present | – | – | 6.2 | 1.9–19.6 | 0.002 | |
| Tumor necrosis assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| Absent | Referent | Referent | ||||
| Present | 2.9 | 1.1–8.0 | 0.034 | 1.2 | 0.2–9.1 | 0.868 |
| No. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| N0 and ≤5 | Referent | Referent | ||||
| >5 | 4.5 | 1.7–11.9 | 0.003 | 7.5 | 1.7–31.5 | 0.006 |
| Nodes with extranodal invasion assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| N0 and absent | Referent | Referent | ||||
| Present | 4.8 | 2.3–10.6 | <0.001 | 5.0 | 1.7–14.7 | 0.003 |
| Nodal metastatic tumor stroma assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| N0 and none | Referent | Referent | ||||
| Mild | 0.7 | 0.1–5.3 | 0.719 | 1.1 | 0.04–28.3 | 0.967 |
| Moderate | 1.3 | 0.2–7.8 | 0.771 | 4.5 | 0.3–76.9 | 0.302 |
| Severe | 3.9 | 1.6–9.2 | 0.002 | 7.6 | 0.3–183.9 | 0.214 |
| Model 2 | ||||||
| Grading system for lymph vessel tumor emboli assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| Grade 0 | Referent | Referent | ||||
| Grade 1 | 3.2 | 1.2–8.6 | 0.020 | 0.2 | 0.01–3.8 | 0.302 |
| Grade 2 | 9.5 | 3.3–27.3 | <0.001 | 5.9 | 1.9–18.8 | 0.002 |
| Grade 3 | 5.5 | 1.7–17.4 | 0.004 | 5.3 | 0.5–61.6 | 0.183 |
| Tumor necrosis assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| Absent | Referent | Referent | ||||
| Present | 3.1 | 1.1–8.8 | 0.038 | 2.4 | 0.8–13.3 | 0.300 |
| No. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| N0 and ≤5 | Referent | Referent | ||||
| >5 | 3.7 | 1.2–11.7 | 0.027 | 12.6 | 3.2–48.5 | <0.001 |
| Nodal metastatic tumor stroma assessed using surgical materials obtained after neoadjuvant chemotherapy | ||||||
| N0 and none | Referent | Referent | ||||
| Mild | 0.4 | 0.05–3.1 | 0.366 | 0.2 | 0.01–6.9 | 0.395 |
| Moderate | 2.9 | 1.2–7.1 | 0.017 | 1.3 | 0.1–17.9 | 0.856 |
| Severe | 10.0 | 3.3–20.9 | <0.001 | 3.5 | 1.1–10.8 | 0.034 |
–/–, not significant in univariate analysis; CI, confidence interval; HR, hazard rate; N0, no nodal metastasis.
In patients with nodal metastasis, five or more mitotic figures in the nodal metastatic tumors was significantly associated with elevated HRs for tumor recurrence and tumor‐related death, while clinical invasive tumor size and the UICC pN3 category were significantly associated with elevated HRs for tumor recurrence (Table 5, model 1). The presence of FF (assessed using biopsy materials) and the presence of nodes with extranodal invasion were significantly associated with elevated HRs for tumor‐related death in the multivariate analyses (Table 5, model 1). In model 2, the grading system for LVTEs (assessed using surgical materials), severe stroma in nodal metastatic tumors, and the presence of tumor necrosis (assessed using surgical materials) were significantly associated with elevated HRs for tumor recurrence in the multivariate analysis (Table 5). Grade 2 LVTEs (assessed using surgical materials) and five or more mitotic figures in nodal metastatic tumors were significantly associated with elevated HRs for tumor‐related death in the multivariate analysis (Table 5).
Table 5.
Multivariate analyses for tumor recurrence and tumor‐related death in lymph node–metastasis‐positive invasive ductal carcinoma (IDC) patients who received neoadjuvant chemotherapy
| Factors | Cases 74 | Number of patients (%) | |||
|---|---|---|---|---|---|
| Tumor recurrence | Tumor‐related death | ||||
| Present (n = 29) | HRs/95% CI P‐values | Present (n = 14) | HRs/95% CI P‐values | ||
| Model 1 | |||||
| Clinical invasive tumor size (mm) before neoadjuvant chemotherapy | |||||
| >20 to ≤50 | 41 | 11 (27) | Referent | 6 (15) | Referent |
| >50 | 33 | 18 (54) | 2.7/1.2–5.7 | 8 (24) | –/– |
| 0.013 | |||||
| Fibrotic focus assessed using biopsy materials obtained before neoadjuvant chemotherapy | |||||
| Absent | 60 | 22 (37) | Referent | 8 (13) | Referent |
| Present | 13 | 7 (54) | –/– | 6 (46) | 7.0/2.2–22.3 |
| <0.001 | |||||
| UICC pN category | |||||
| N1 | 39 | 11 (28) | Referent | 6 (15) | Referent |
| N2 | 24 | 10 (42) | 2.3/0.6–8.0 | 6 (25) | –/– |
| 0.211 | |||||
| N3 | 11 | 8 (73) | 3.4/1.4–8.1 | 2 (18) | –/– |
| 0.005 | |||||
| No. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant therapy | |||||
| ≤5 | 61 | 20 (33) | Referent | 10 (16) | Referent |
| >5 | 13 | 9 (69) | 3.9/1.6–9.1 | 4 (31) | 8.6/2.0–37.0 |
| 0.002 | 0.004 | ||||
| Nodes with extranodal invasion assessed using surgical materials obtained after neoadjuvant chemotherapy | |||||
| Absent | 45 | 13 (29) | Referent | 5 (11) | Referent |
| Present | 29 | 16 (55) | 2.4/0.7–7.7 | 9 (31) | 5.3/1.5–18.3 |
| 0.143 | 0.007 | ||||
| Model 2 | |||||
| Grading system for lymph vessel tumor emboli assessed using surgical materials obtained after neoadjuvant chemotherapy | |||||
| Grade 0 | 39 | 9 (23) | Referent | 6 (15) | Referent |
| Grade 1 | 19 | 8 (42) | 2.7/1.0–7.4 | 2 (11) | 1.2/0.7–7.0 |
| 0.047 | 0.872 | ||||
| Grade 2 | 9 | 6 (67) | 8.5/2.6–27.8 | 4 (44) | 3.9/1.1–13.7 |
| <0.001 | 0.035 | ||||
| Grade 3 | 7 | 6 (86) | 8.0/2.5–26.0 | 2 (29) | 3.4/0.6–19.2 |
| <0.001 | 0.172 | ||||
| Nodal metastatic tumor stroma assessed using surgical materials obtained after neoadjuvant therapy | |||||
| N0/none | 9 | 2 (22) | Referent | 2 (22) | Referent |
| Mild | 19 | 3 (16) | 0.8/0.1–6.9 | 1 (5) | –/– |
| 0.826 | |||||
| Moderate | 29 | 14 (48) | 3.7/0.6–23.9 | 6 (21) | –/– |
| 0.168 | |||||
| Severe | 17 | 10 (59) | 5.3/2.0–14.2 | 5 (29) | –/– |
| <0.001 | |||||
| Tumor necrosis assessed using surgical materials obtained after neoadjuvant chemotherapy | |||||
| Absent | 54 | 18 (33) | Referent | 9 (17) | Referent |
| Present | 20 | 11 (55) | 5.3/1.7–16.4 | 5 (25) | –/– |
| 0.004 | |||||
| No. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy | |||||
| ≤5 | 61 | 20 (33) | Referent | 10 (16) | Referent |
| >5 | 13 | 9 (69) | 2.0/0.4–9.5 | 4 (31) | 6.1/1.6–22.9 |
| 0.376 | 0.008 | ||||
–/–, not significant in univariate analysis; CI, confidence interval; HR, hazard rate; NIDC, non‐invasive ductal carcinoma; pN, pathological regional lymph node; UICC, International Union Against Cancer.
Model 1
Tumor recurrence: adjusted for clinical invasive tumor size before neoadjuvant chemotherapy, tumor necrosis assessed using surgical materials obtained after neoadjuvant chemotherapy, nodal metastatic tumor stroma assessed using surgical materials obtained after neoadjuvant chemotherapy, no. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy, UICC pTNM‐pN category assessed using surgical materials obtained after neoadjuvant chemotherapy, nodes with extranodal invasion assessed using surgical materials obtained after neoadjuvant chemotherapy, and histologic grade of primary invasive tumors assessed using surgical materials obtained after neoadjuvant chemotherapy.
Tumor‐related death: adjusted for fibrotic focus assessed using biopsy materials obtained before neoadjuvant chemotherapy, no. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy, and nodes with extranodal blood vessel invasion assessed using surgical materials obtained after neoadjuvant chemotherapy.
Model 2
Tumor recurrence: adjusted for grading system for lymph vessel tumor emboli assessed using surgical materials obtained after neoadjuvant chemotherapy, tumor necrosis assessed using surgical materials obtained after neoadjuvant chemotherapy, nodal metastatic tumor stroma assessed using surgical materials obtained after neoadjuvant chemotherapy, no. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy, UICC pTNM‐pN category assessed using surgical materials obtained after neoadjuvant chemotherapy, nodes with extranodal invasion assessed using surgical materials obtained after neoadjuvant chemotherapy, and histologic grade of nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy.
Tumor‐related death: adjusted for grading system for lymph vessel tumor emboli assessed using surgical materials obtained after neoadjuvant chemotherapy, no. of mitotic figures in nodal metastatic tumors assessed using surgical materials obtained after neoadjuvant chemotherapy, and nodes with extranodal blood vessel invasion assessed using surgical materials obtained after neoadjuvant chemotherapy.
Discussion
The results of this study clearly showed that a grading system for LVTEs (assessed using surgical materials) can be used to classify IDC patients with lymph vessel invasion who received neoadjuvant chemotherapy into low‐risk, intermediate‐risk, and high‐risk groups; furthermore, this grading system for LVTEs was significantly associated with the HRs for tumor recurrence and tumor‐related death in patients with IDC both overall and in patients with nodal metastasis, and the outcome predictive power of the grading system for LVTEs assessed using surgical materials was superior to that of the grading system for LVTE assessed using biopsy materials obtained before neoadjuvant chemotherapy. Although there have been many studies showing the prognostic usefulness of the presence of lymphatic invasion,( 25 , 26 , 27 ) we previously demonstrated that the biological histological characteristics, especially mitotic figures and/or apoptotic figures, of tumor cells in lymph vessels are a more significant outcome predictor than the presence or absence of lymph vessel invasion or the number of lymph vessels that have been invaded.( 28 ) We have also demonstrated that the location of lymph vessel invasion is an important outcome predictor for IDC patients,( 18 ) but the result of this study clearly demonstrated that the grading system for LVTEs assessed using surgical materials is significantly superior to the location of lymph vessel invasion for accurately predicting the outcomes of IDC patients who have received neoadjuvant chemotherapy. Thus, this grading system for LVTEs assessed using surgical materials, but not biopsy materials, appears to be an excellent histological system for accurately predicting the outcome of IDC patients who do or do not receive neoadjuvant chemotherapy. Although we could not examine the outcome predictive power of the grading system for LVTEs in IDC patients without nodal metastasis in this study, we previously reported that this grading system for LVTEs assessed using surgical materials was a very important histological predictor of the prognosis of patients with IDC who did not receive neoadjuvant therapy independent of their nodal status.( 11 ) Thus, the grading system for LVTE might be an important outcome predictor for IDC patients who have received neoadjuvant chemotherapy and do not have nodal metastasis, although the outcome predictive power of the grading system for LVTEs should be investigated in this patient population. Since the presently described grading system for LVTEs is based on assessments of mitotic figures and apoptotic figures in tumor cells located in lymph vessels, tumor cells with a high turnover rate in lymph vessels are more likely to be capable of spreading tumor nests throughout the lymph vessels than tumor cells with a low turnover rate. Thus, factors that accelerate the turnover rate of tumor cells in lymph vessels are probably very important for explaining the significant outcome of the predictive power of this grading system for LVTEs.
The histological characteristics of the nodal metastatic tumors were also significantly associated with tumor recurrence or tumor‐related death in the patients with IDCs who received neoadjuvant chemotherapy in the current study. Among these histological characteristics, the degree of nodal tumor stroma and the number of mitotic figures in the nodal metastatic tumors were the most accurate predictors of outcome among the IDC patients who received neoadjuvant chemotherapy. We previously reported that severe tumor stroma and the number of mitotic figures in nodal metastatic tumors are significant predictors of outcome among IDC patients with nodal metastasis who did not receive neoadjuvant chemotherapy.( 12 , 29 ) Thus, this study clearly confirmed that these two factors are also significant histological predictors of outcome among IDC patients with nodal metastasis who received neoadjuvant chemotherapy. We previously reported that the proliferative activity of tumor–stromal fibroblasts plays a very important role in nodal metastasis and distant organ metastasis by IDCs,( 30 , 31 ) and that growth factors produced by tumor cells and tumor stromal cells play a very important role in tumor progression by IDC.( 32 ) These findings strongly suggest that the tumor stroma plays a significant role in tumor progression in IDC. Furthermore, the gene expression profile and the protein expression profile of the tumor stroma have recently been reported to play a very important roles in tumor progression in carcinoma,( 33 , 34 , 35 ) and the interaction between tumor cells and stromal cells also plays a very important role in tumor progression in carcinoma.( 36 , 37 , 38 ) Thus, tumor cell–stromal cell interactions probably heighten the malignant potential of nodal metastatic tumors with moderate to severe tumor stroma. Furthermore, in previous studies we and others have reported that a characteristic histological feature of the tumor stroma in primary invasive tumors, an FF, is a very useful prognostic histological tumor–stromal indicator for accurately predicting the outcome of IDC patients who did not receive neoadjuvant therapy;( 16 , 17 , 39 , 40 ) the present study clearly demonstrated that the presence of FFs (assessed using biopsy materials obtained before neoadjuvant chemotherapy, but not using surgical materials obtained after neoadjuvant chemotherapy) was a significant tumor‐death‐related factor. Thus, tumor cell–stromal cell interactions in nodal metastatic tumors as well as in primary invasive tumors probably play very important roles in the progression of IDCs that have been treated with neoadjuvant chemotherapy, and in IDC patients who have received neoadjuvant chemotherapy, the outcome predictive power of FFs should be assessed using biopsy materials obtained before neoadjuvant chemotherapy.
The grading system for LVTEs assessed using surgical materials and the histological features of the nodal metastatic tumors mentioned above were superior to Fisher's classification or the classification of the JBCS for neoadjuvant chemotherapy for predicting the outcome of IDC patients who had received neoadjuvant chemotherapy in this study. The classification of the JBCS for neoadjuvant chemotherapy assesses the degree of fibrosis or the presence or absence of tumor necrosis in primary invasive tumors and tumors metastasizing to the lymph node, and a severe degree of fibrosis and the presence of tumor necrosis are considered as histological findings predicting a good response to neoadjuvant chemotherapy.( 21 ) In the classification of JBCS for neoadjuvant chemotherapy, a complete response (grade 3) is regarded as necrosis or the disappearance of all tumor cells, with all carcinoma cells being replaced by granuloma‐like and/or fibrous tissue. However, this study clearly demonstrated that the presence of tumor necrosis in primary invasive tumors and a moderate to severe degree of fibrosis in nodal metastatic tumors were important histological predictors of a poor prognosis among IDC patients who have received neoadjuvant chemotherapy. Therefore, determining whether the presence of tumor necrosis or the presence of tumor–stromal dense fibrosis in IDCs treated with neoadjuvant chemotherapy have truly been produced by neoadjuvant chemotherapy or not is of great importance, and the latter finding strongly suggests that the presence of tumor necrosis or the presence of tumor–stromal dense fibrosis may reflect biological tumor characteristics that are closely associated with a poor outcome among patients with IDCs. The tumor‐related predictive ability of the presence of FF assessed using biopsy materials obtained before neoadjuvant chemotherapy was lost when the presence of FF was assessed using surgical materials obtained after neoadjuvant chemotherapy. This strongly suggests that FF‐like stromal changes produced by neoadjuvant chemotherapy probably occurred in the IDCs treated with neoadjuvant chemotherapy, and the true FFs could not be differentiated from the FF‐like stromal changes in IDCs. Thus, when the presence of tumor necrosis in primary invasive tumors or the presence of moderate to severe fibrosis in nodal metastatic tumors is observed during the pathological examination of IDCs treated with neoadjuvant therapy, the pathological assessment of the response to neoadjuvant chemotherapy should be carefully assessed as to whether the presence of tumor necrosis in primary invasive tumors or moderate to severe fibrosis in nodal metastatic tumors truly demonstrates a response to neoadjuvant chemotherapy. Although the outcome predictive power of FFs among patients with IDC was lost after neoadjuvant chemotherapy, the histological factors maintained their significant outcome predictive power among IDC patients who received neoadjuvant chemotherapy. Thus, pathologists carefully assess the response to neoadjuvant chemotherapy based on the presence of tumor necrosis in primary invasive tumors or the degree of fibrosis in nodal metastatic tumors, since pathologists might misjudge IDC patients who have received neoadjuvant chemotherapy and whose primary invasive tumors exhibited tumor necrosis or whose nodal metastatic tumors exhibited dense fibrosis as having attained a good response to neoadjuvant chemotherapy.
The results of this study clearly demonstrated that many histological factors of tumors assessed using biopsy materials, such as histologic grade and tumor necrosis, failed to show a significant association with tumor recurrence or tumor‐related death. These findings strongly suggest that biopsy materials containing small amounts of primary invasive tumors do not accurately reflect the true biological malignant potential of IDCs. Thus, with the exception of evaluating the presence of FF, histological evaluations of the malignant potential of IDCs treated using neoadjuvant chemotherapy should be performed using surgical materials obtained after neoadjuvant chemotherapy.
In conclusion, this is the first study to clearly demonstrate that the presence of FF in biopsy materials obtained before neoadjuvant chemotherapy, the grading system for LVTEs in surgical materials obtained after neoadjuvant chemotherapy, and the histological characteristics of nodal metastatic tumors in surgical materials obtained after neoadjuvant chemotherapy were strongly associated with the outcome of IDC patients who received neoadjuvant chemotherapy. In the future, the following topics should be examined to clarify the tumor progression of IDCs treated with neoadjuvant chemotherapy based on the data in this study: (1) the functions of tumor cells in lymph vessels and nodal metastatic tumor cells should be determined; (2) the factors that accelerate the proliferative activity of tumor cells in lymph vessels or lymph nodes should be identified; and (3) the factors that accelerate tumor cell–stromal cell interactions in nodal metastatic tumors should be discerned.
Acknowledgments
This study was supported by a Grant‐in‐Aid for Scientific Research (KAKENHI) (C) (nos. 19590378, 21590393) and was supported in part by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare (20–16) of Japan.
References
- 1. Ragaz J, Baird R, Rebbeck P, Goldie A, Coldman A, Spinelli J. Preoperative adjuvant chemotherapy (neoadjuvant) for carcinoma of the breast: rationale and safety report. Recent Results Cancer Res 1985; 98: 99–105. [DOI] [PubMed] [Google Scholar]
- 2. Ragaz J. Preoperative (neoadjuvant) chemotherapy for breast cancer: outline of the British Columbia Trial. Recent Results Cancer Res 1986; 103: 85–94. [DOI] [PubMed] [Google Scholar]
- 3. Ferriere JP, Assier I, Cure H et al . Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol 1998; 21: 117–20. [DOI] [PubMed] [Google Scholar]
- 4. Daidone MG, Silvestrini R, Luisi A et al . Changes in biological markers after primary chemotherapy for breast cancers. Int J Cancer 1995; 61: 301–5. [DOI] [PubMed] [Google Scholar]
- 5. Cavailles V, Gompel A, Portois MC et al . Comparative activity of pulsed or continuous estradiol exposure on gene expression and proliferation of normal and tumoral human breast cells. J Mol Endocrinol 2002; 28: 165–75. [DOI] [PubMed] [Google Scholar]
- 6. Koukourakis MI, Simopoulos C, Polychronidis A et al . The effect of trastuzumab/docatexel combination on breast cancer angiogenesis: dichotomus effect predictable by the HIFI alpha/VEGF pre‐treatment status? Anticancer Res 2003; 23: 1673–80. [PubMed] [Google Scholar]
- 7. Petit T, Borel C, Ghnassia JP et al . Chemotherapy response of breast cancer depends on HER‐2 status and anthracycline dose intensity in the neoadjuvant setting. Clin Cancer Res 2001; 7: 1577–81. [PubMed] [Google Scholar]
- 8. Chollet P, Amat S, Cure H et al . Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 2002; 86: 1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penault‐Llorca F, Vincent‐Salomon A. Roles of the pathologist in neoadjuvant chemotherapy: evaluation of response, prognostic and predictive factors. Ann Pathol 2003; 23: 555–63. [PubMed] [Google Scholar]
- 10. Bollet MA, Sigal‐Zafrani B, Gambotti L et al . Pathological response to preoperative concurrent chemo‐radiotherapy for breast cancer: results of a phase II study. Eur J Cancer 2006; 42: 2286–95. [DOI] [PubMed] [Google Scholar]
- 11. Hasebe T, Yamauchi C, Iwasaki M et al . Grading system for lymph vessel tumor emboli for prediction of the outcome of invasive ductal carcinoma of the breast. Hum Pathol 2008; 39: 427–36. [DOI] [PubMed] [Google Scholar]
- 12. Hasebe T, Sasaki S, Imoto S et al . Histological characteristics of tumor in vessels and lymph nodes are significant predictors of progression of invasive ductal carcinoma of the breast: a prospective study. Hum Pathol 2004; 35: 298–308. [DOI] [PubMed] [Google Scholar]
- 13. Sobin LH, Wittekind CH, eds. TNM Classification of Malignant Tumors, Geneva: Wiley‐Liss, 2002; 131–141. [Google Scholar]
- 14. Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer 1957; 11: 359–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilchrist KW, Gray R, Fowble B et al . Tumor necrosis is a prognostic predictor for early recurrence and death in lymph node‐positive breast cancer: a 10‐year follow‐up study of 728 eastern cooperative oncology group patients. J Clin Oncol 1993; 11: 1929–35. [DOI] [PubMed] [Google Scholar]
- 16. Hasebe T, Tsuda H, Hirohashi S et al . Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long‐term survival of the patients. Breast Cancer Res Treat 1998; 49: 195–208. [DOI] [PubMed] [Google Scholar]
- 17. Hasebe T, Sasaki S, Imoto S et al . Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol 2002; 15: 502–16. [DOI] [PubMed] [Google Scholar]
- 18. Yamauchi C, Hasebe T, Iwasaki M et al . Accurate assessment of lymph vessel tumor emboli in invasive ductal carcinoma of the breast according to tumor areas, and their prognostic significance. Hum Pathol 2007; 38: 247–59. [DOI] [PubMed] [Google Scholar]
- 19. Fisher B. Biological and clinical considerations regarding the use of surgery and chemotherapy in the treatment of primary breast cancer. Cancer 1977; 40: 574–87. [DOI] [PubMed] [Google Scholar]
- 20. Fisher B. Adjuvant chemotherapy in the primary management of breast cancer. Med Clin North Am 1977; 61: 953–65. [DOI] [PubMed] [Google Scholar]
- 21. Kurosumi M. Significance of histopathological evaluation in primary therapy for breast cancer – recent trends in primary modality with pathological complete response (pCR) as endpoint. Breast Cancer 2004; 11: 139–47. [DOI] [PubMed] [Google Scholar]
- 22. Wolff AC, Hammond ME, Schwartz JN et al . American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007; 131: 18–43. [DOI] [PubMed] [Google Scholar]
- 23. Cox DR. Regression models and life‐tables. J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- 24. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 25. Lee AHS, Pinder SE, Macmillan RD et al . Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006; 42: 357–62. [DOI] [PubMed] [Google Scholar]
- 26. EI‐Gohary YM, Metwally G, Saad RS et al . Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am J Clin Pathol 2008; 129: 578–86. [DOI] [PubMed] [Google Scholar]
- 27. Amaout‐Alkarain A, Kahn HJ, Narod SA et al . Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2‐40 in node negative breast cancer. Mod Pathol 2007; 20: 183–91. [DOI] [PubMed] [Google Scholar]
- 28. Hasebe T, Sasaki S, Imoto S et al . Characteristics of tumors in lymph vessels play an important role in the tumor progression of invasive ductal carcinoma of the breast: a prospective study. Mod Pathol 2002; 15: 904–13. [DOI] [PubMed] [Google Scholar]
- 29. Hasebe T, Sasaki S, Imoto S et al . Significance of nodal metastatic tumor characteristics in nodal metastasis and prognosis of patients with invasive ductal carcinoma of the breast. Cancer Sci 2003; 94: 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasebe T, Sasaki S, Imoto S et al . Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am J Pathol 2000; 156: 1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hasebe T, Sasaki S, Imoto S et al . Highly proliferative fibroblasts forming fibrotic focus govern metastasis of invasive ductal carcinoma of the breast. Mod Pathol 2001; 14: 325–37. [DOI] [PubMed] [Google Scholar]
- 32. Hasebe T, Imoto S, Ogura T et al . Significance of basic fibroblast growth factor and fibroblast growth factor receptor protein expression in the formation of fibrotic focus in invasive ductal carcinoma of the breast. Jpn J Cancer Res 1997; 88: 877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finak G, Bertos N, Pepin F et al . Stromal gene expression predicts clinical outcome in breast cancer. Nature Med 2008; 14: 518–27. [DOI] [PubMed] [Google Scholar]
- 34. Singer CF, Gschwantler‐Kaulich D, Fink‐Retter A et al . Differential gene expression profile in breast cancer‐derived stromal fibroblasts. Breast Cancer Res Treat 2008; 110: 273–81. [DOI] [PubMed] [Google Scholar]
- 35. Sheehan KM, Gulmann C, Eichler GS et al . Signal pathway profiling of epithelial and stromal compartments of colonic carcinoma reveals epithelial‐mesenchymal transition. Oncogene 2008; 27: 323–31. [DOI] [PubMed] [Google Scholar]
- 36. Hasegawa M, Furuya M, Kasuya Y et al . CD151 dyanmicx in carcinoma‐stroma interaction: integrin expression, adhesion strength and proteolytic activity. Lab Invest 2007; 87: 882–92. [DOI] [PubMed] [Google Scholar]
- 37. Loussouarn D, Campion L, Sagan C et al . Prognostic impact of syndecan‐1 expression in invasive ductal carcinomas. Br J Cancer 2008; 98: 1993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Studebaker AW, Storci G, Werbeck JL et al . Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin‐6‐dependent manner. Cancer Res 2008; 68: 9087–95. [DOI] [PubMed] [Google Scholar]
- 39. Baak JP, Colpaert CG, Van Diest PJ et al . Multivariate prognostic evaluation of the mitotic activity index and fibrotic focus in node‐negative invasive breast cancers. Eur J Cancer 2005; 41: 2093–101. [DOI] [PubMed] [Google Scholar]
- 40. Colpaert C, Vermeulen PB, Van Beest P et al . Intratumoral hypoxia resulting in the presence of a fibrotic focus is an independent predictor of early distant relapse in lymph node‐negative breast cancer patients. Histopathology 2001; 39: 416–25. [DOI] [PubMed] [Google Scholar]


