Abstract
Tumor‐cell hypoxia is one of the main factors inducing radioresistance. Enhanced tumor oxygenation has previously been achieved in an animal model using the synthetic heme‐based oxygen carrier ‘albumin‐heme’ (recombinant human serum albumin‐Fe cyclohexanoil heme; rHSA‐FeP). The present study was done to determine whether rHSA‐FeP enhances the radiation response in an experimental tumor model. Male Donryu rats and LY80, a variant of the syngenic liver ascites tumor, were used. A total of 1 × 106 cells were injected into the subfascial tissue of the right thigh. The rats were divided randomly into five groups: sham (tumor implantation and sham operation); rHSA‐FeP; irradiation; rHSA + irradiation; and rHSA‐FeP + irradiation. Six days after, under general anesthesia, intra‐arterial administration of 10 mL/kg of either 5% rHSA solution or oxygenated rHSA‐FeP solution at 2.5 mL/min was done and a dose of 20 Gy was given. There were significant differences in tumor growth between the sham and irradiation groups, and between the sham and rHSA‐FeP + irradiation groups. Tumor growth delay was observed and differences were significant between the sham and irradiation groups, and between the irradiation and rHSA‐FeP + irradiation groups. In the present study, rHSA‐FeP itself had a slight effect on tumor growth without irradiation. Enhancing the effect of rHSA‐FeP on the radiation response is responsible in part for the oxygen‐carrying property of rHSA‐FeP. In conclusion, rHSA‐FeP is a candidate radiation‐enhancing drug. Arterial infusion of rHSA‐FeP may serve as a local oxygenation method that enhances the radiation effect. (Cancer Sci 2008; 99: 1274–1278)
Resistance to radiation therapy is observed in many types of tumors and can be due to several causes.( 1 , 2 ) Tumor cell hypoxia and tumor cell repopulation are the main factors causing radioresistance. Oxygen mediates the majority of biological effects of sparsely ionizing radiation, and the response of cells to radiation depends strongly on the availability of oxygen.( 3 ) Various methods to deliver oxygen to cancer tissue have been studied, including inhalation of high oxygen‐content gas,( 4 ) the use of an artificial oxygen carrier,( 5 , 6 , 7 ) and the use of agents that manipulate tumor blood flow.( 8 ) Some of these methods have been studied in the clinical setting.( 9 , 10 )
Tumor circulation is characterized by tortuous capillaries, compressed vessels, a scant capillary network, arterio‐venous junctions, and plasma channels. It is difficult to deliver adequate oxygen to hypoxic cancer tissue as the tortuous circulation causes limited flow of red blood cells. We developed a synthetic heme‐based molecular oxygen carrier, albumin‐heme (rHSA‐FeP), which is recombinant human serum albumin (rHSA) incorporating a Fe (II)tetra(phenyl)porphyrin derivative (FeP) (Fig. 1). We found that this molecule has the potential to transport oxygen in vivo.( 11 ) As for the tumor tissue oxygen status, a hypoxic environment is usual in experimental tumor models as well as in clinical cases. Thus, we analyzed the tissue oxygen tension in the transplanted tumor in a previous report.( 12 ) LY80 was evaluated using the Pd coproporphyrin phosphorescence decay method and we found that intra‐arterial injection of rHSA‐FeP can increase tissue oxygen tension 2.4 times higher than rHSA solution.
Figure 1.
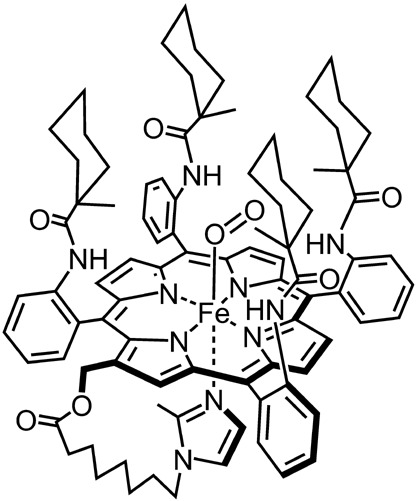
The chemical structure of FeP: 2‐(8‐[N‐{2‐methylimidazolyl}] octanoyloxymethyl)‐5,10,15,20‐terakis(α,α,α,α‐o‐[1‐methylcyclohexanamido] phenyl)porphinatoiron(II).
In the present study, we studied whether rHSA‐FeP could enhance the response to radiation therapy in an experimental tumor model.
Materials and Methods
Animal and tumor. Five‐week‐old male Donryu rats (Crj‐Donryu; Nippon Charles‐River, Yokohama, Japan), with an average weight of 120–150 g, were used in the present study. The rats (two to three per cage) were maintained on a bed of pulp paper in a ventilated, temperature‐controlled (23 ± 1°C), specific pathogen‐free environment with a 12:12 h L:D cycle and access to food and water ad libitum.
The LY80 tumor cell, a variant of the Yoshida sarcoma, was used for this study. LY80 was established in 1966 by Hiroshi Satoh and has been maintained by successive intraperitoneal transplantation. This tumor was kindly donated by Dr Katsuyoshi Hori of Tohoku University. The characteristics of this tumor have been described by Tanda et al.( 13 ) Briefly, this tumor was a subline of ascites hepatoma. Once transplanted intraperitoneally, tumor cells grew in ascites and caused peritoneal dissemination as well as systemic spread. Solid tumors may be obtained by subcutaneous transplantation of tumor cells.
All experimental protocols were reviewed by the Committee on the Ethics of Animal Experiments at our university and were carried out in accordance with the Guidelines for Animal Experiments issued by Keio University School of Medicine, Experimental Animal Center, and law no. 105 and notification no. 6 issued by the Japanese Government. The ethical guidelines that were followed meet the guidelines for animal handling issued by the United Kingdom Co‐ordinating Committee on Cancer Research, 1998.
Tumor cell implantation. LY80 cells growing in the peritoneal effusion of a donor rat were collected, suspended in phosphate‐buffered saline, and adjusted to a concentration of 107 cells/mL. Recipient rats were anesthetized with diethyl ether (Wako Pure Chemical Industries, Osaka, Japan). A small skin incision was made on the lateral side of the inguinal ligament of the right thigh. The tumor cell suspension was drawn into a 1‐mL graduated syringe (Nipro, Osaka, Japan), and a 30‐gauge syringe needle (Becton Dickinson, Franklin Lakes, NJ, USA) was used to inject 100 µL of the cell suspension directly under the subfascial tissue of the biceps muscle, avoiding the femoral artery and vein. The incision wound was then closed with one layer of thin silk thread, and the animals were allowed to recover.
Artificial oxygen carrier. rHSA‐FeP is an artificial oxygen carrier composed of rHSA incorporating FeP (Fig. 1).( 14 , 15 ) Its characteristics have been described previously.( 14 ) Briefly, rHSA was obtained from Nipro (Osaka, Japan), and FeP was synthesized according to our previous reported procedure in Tsuchida's laboratory.( 15 ) We named this material ‘albumin‐heme’. The physical, chemical, and solution properties of rHSA‐FeP used in the present study are shown in Table 1.
Table 1.
Characteristics of albumin‐heme
| Characteristic | rHSA‐FeP |
|---|---|
| Osmotic pressure ratio | 0.97 |
| Chlorine (mM) | 141.30 |
| pH | 7.22 |
| Viscosity (cP) | 1.18 |
| Met ratio (%) | 0.0 |
| Endotoxin (EU/mL) | <0.6 |
| P 1/2 (Torr) (37°C) | 34.0 |
| FeP (mM) | 2.94 |
| rHSA (%) | 4.79 |
EU, endotoxin unit; P 1/2, half saturation tension at pH7.4; rHSA, recombinant human serum albumin.
Irradiation of tumor‐bearing rats. Radiation was delivered to the tumor‐bearing rats using a radiation unit (MBR‐1520R‐3; Hitachi Medicotechnology, Hitachi, Japan). A specially designed lead cage was used to deliver radiation only to the area with the tumor as the rest of the body was shielded. The radiation power output was 150 kV at 20 mAmp. A 1.0‐mm aluminum filter was used to filter forward‐scattered radiation. Dose rate was measured using a Fricke dosimeter. The dosimeter was placed inside the shield at the spot where the tumor was to be located.
The radiation dosage was determined based on the results of a preliminary experiment. Tumor‐bearing rats were given 5, 10, 20, or 40 Gy radiation, and tumor growth was observed. The growth rate of tumors given 5 or 10 Gy was not significantly decreased compared to the sham group. However, 20 Gy radiation decreased the tumor growth rate significantly compared to the sham group, and 40 Gy irradiation eradicated the tumors. No dermal reaction was observed among any animal that was given less than 20 Gy irradiation. Therefore, 20 Gy was the appropriate dosage to use in the present study. It took around 8 min to irradiate a tumor.
Six days after tumor cell inoculation, when the tumor grew to a diameter of 10 mm (estimated weight 0.4 g), the rats were divided randomly into five groups: sham group (tumor implantation and sham operation); rHSA‐FeP group; irradiation group; rHSA + irradiation group; and rHSA‐FeP + irradiation group.
The animals were anesthetized by intraperitoneal injection of pentobarbital (75 mg/kg; DaiNippon Pharmaceutical, Osaka, Japan) prior to catheterization. Intra‐arterial catheterization was done using the following technique. A small incision was made on the neck, and the left carotid artery was dissected. A polyethylene catheter (SP31, 0.5 mmID; Natsume Seisakusho, Tokyo, Japan) filled with saline was inserted to a length of 9 cm; in preliminary studies, we had confirmed that the end of the catheter was located approximately 1.0 cm upward of the bifurcation of the descending aorta.
After the polyethylene catheter was inserted, the rat was placed in the irradiation cage, which was then properly positioned with respect to the radiation source and with the shield. The rHSA‐FeP or rHSA solution was injected at a rate of 2.5 mL/kg/min for 4 min. Immediately after administration of the material, the irradiation chamber was closed and 20 Gy irradiation was given.
After irradiation the carotid catheter was removed, the left carotid artery was ligated, the wound was sutured, and the animals were allowed to recover. After full recovery, animals were returned to their cages. Animals in the sham operation group were catheterized but received neither materials nor irradiation. They were returned to the cage after removal of the carotid catheter.
Monitoring of animal. Animals were monitored every day. Tumor size and bodyweight were measured and recorded. Movement was also observed. Tumor weight was calculated according to following formula:( 16 )
| estimated tumor weight (g) = A × B 2/2/1000, |
where A is the longer diameter of the tumor (mm) and B is the shorter diameter of the tumor (mm).
When the estimated tumor weight reached 30 g (below 12% of the animal's weight), the animal was removed from the study and killed with an excess volume of pentobarbital sodium injected intraperitoneally. The date of removal from the study was recorded.
Tumor suppression rate. Tumor size was measured six days a week, and the tumor weight was calculated. The tumor suppression rate (TSR) was calculated according to the following formula:
| TSR n (%) = Tn /Cn × 100, |
where Tn is the mean tumor weight at n days after tumor implantation in the radiation, rHSA‐FeP, rHSA + radiation, and rHSA‐FeP + radiation groups and Cn is the mean tumor weight in the sham group at n days after tumor implantation.
Statistics. The results are expressed as mean ± standard difference. Differences between the groups were assessed using the Tukey–Kramer test. The Kaplan–Meier method was applied to access tumor growth delay, and differences between groups were analyzed using the Log rank test.
Results
Change of bodyweight. Bodyweight is a parameter that can indicate the severity of disease. The sham group stopped gaining weight 10 days after tumor inoculation; bodyweight decreased thereafter. Although gain of weight ceased, food intake didn't decrease and animals didn't seem distressed. In contrast, animals that received treatment continued to gain bodyweight, except for a temporary 3‐day decrease after irradiation (Fig. 2).
Figure 2.
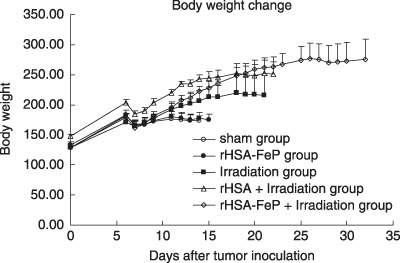
The bodyweights of rats bearing LY80. The rats were inoculated subcutaneously with 1 × 106 tumor cells on day 0. Radiation treatment was given on day 6. Each value represents the mean ± SD of six rats. There were significant differences between the non‐irradiated and irradiated groups after day 12. rHSA, recombinant human serum albumin.
Tumor growth curve and tumor suppression rate. There were significant differences between the sham and irradiation groups after day 9. There were also significant differences between the sham and rHSA‐FeP + irradiation groups after day 7. A significant difference between the irradiation and rHSA‐FeP + irradiation groups was seen after day 19 (Fig. 3).
Figure 3.
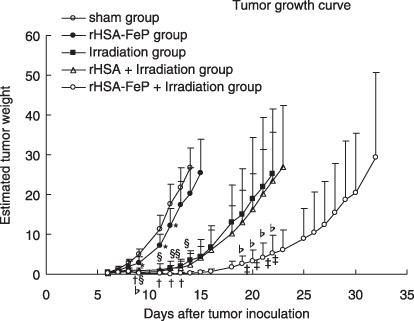
The tumor weights in rats bearing LY80. Tumor cells were transplanted on day 0. Radiation treatment was carried out on day 6. Each value represents the mean ± SD of six rats. There were significant differences between the sham and recombinant human serum albumin (rHSA)‐FeP groups on days 9, 11, and 12 (*P < 0.05), and between the sham and irradiation groups on days 9, 11, 12, 13, and 14 (§
P < 0.01). There were also significant differences between the sham and rHSA‐FeP + irradiation groups on days 7, 9, 11, 12, 13, and 14 (†
P < 0.01). Significant differences between the irradiation and rHSA‐FeP + irradiation groups were seen on days 19, 20, 21, and 22 (‡
P < 0.05), and between the rHSA + irradiation and rHSA‐FeP + irradiation groups on days 20, 21, and 22 ( P < 0.05).
P < 0.05).
We compared the TSR on days 7, 12, and 14. The tumor suppression rate is shown in Table 2. On day 7, the TSR was 65.5% in the rHSA‐FeP group, 64.3% in the irradiation group, 47.9% in the rHSA + irradiation group, and 39.8% in the rHSA‐FeP + irradiation group. We didn't find any significant differences between the groups on day 7. On day 12, the TSR was 69.0% in the rHSA‐FeP group, 8.5% in the irradiation group, 8.0% in the rHSA + irradiation group, and 0.6% in the rHSA‐FeP + irradiation group. There were significant differences between the sham and irradiation groups, the rHSA‐FeP and rHSA‐FeP + irradiation groups, and the irradiation group and the rHSA‐FeP group, whereas there were no significant differences between the sham and rHSA‐FeP groups or the irradiation and rHSA + irradiation groups. On day 14, the TSR was 75.6% in the rHSA‐FeP group, 12.8% in the irradiation group, 8.8% in the rHSA + irradiation group, and 0.93% in the rHSA‐FeP + irradiation group (Table 2). There were significant differences between the sham and irradiation groups, the sham and rHSA‐FeP + irradiation groups, and the rHSA + irradiation and rHSA‐FeP + irradiation groups. No differences were observed between the sham and rHSA‐FeP groups or the irradiation and rHSA + irradiation groups.
Table 2.
Tumor suppression rate
| Group | D7 | D12 | D14 |
|---|---|---|---|
| Sham | 100 | 100 | 100 |
| Albumin‐heme | 65.5 | 69.0 | 75.6 |
| Irradiation | 64.3 | 8.5 | 12.8 |
| rHSA + irradiation | 47.9 | 8.0 | 8.8 |
| Albumin‐heme + irradiation | 39.8 | 0.6 | 0.93 |
rHSA, recombinant human serum albumin.
Tumor growth delay. The numbers of animals remaining in the study was recorded and analyzed by the Kaplan–Meier method. Differences between groups were analyzed using the log‐rank test. There were significant differences between the sham and irradiation groups (P < 0.01) and the irradiation and rHSA‐FeP + irradiation groups (P < 0.03). There was no difference between the irradiation and rHSA + irradiation groups (Fig. 4).
Figure 4.
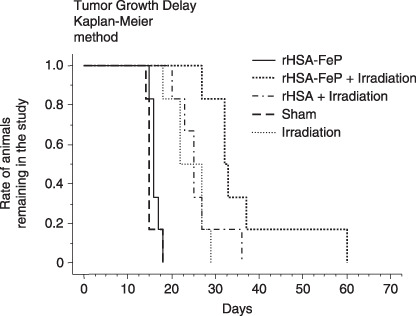
Tumor growth delay was analyzed using the Kaplan–Meier method. When the estimated tumor weight reached 30 g (approximately 12% of bodyweight), animals were removed from the study. The date of removal was recorded and analyzed. Significant differences between the sham and irradiation groups and the irradiation and recombinant human serum albumin (rHSA)‐FeP + irradiation groups were observed (P < 0.03). There were no significant differences between the sham and rHSA‐FeP groups, or between the irradiation and rHSA + irradiation groups.
Discussion
Radiotherapy is a very potent cancer treatment. However, solid tumors may develop radioresistance by cell repopulation, hypoxia, DNA repair, and production of cytoprotective cytokines. Of these factors, hypoxia is the major cause of radiation resistance; thus, releasing hypoxia is a strategy to enhance the effect of radiation.
Several studies have shown that hypoxic tumors may be radioresistant.( 10 , 17 , 18 ) Experimental and clinical studies have proven that oxygenation of tumor tissue increases radiosensitivity.( 19 , 20 ) Currently, oxygen inhalation is one of the main methods of increasing tumor tissue oxygen tension; mixed‐gas inhalation, such as carbogen, is also used. The concept of using an artificial oxygen carrier has long been discussed. Several such products were found to increase tumor tissue oxygen tension (PtO2).( 5 , 21 ) Several reports have suggested that artificial oxygen carriers act as radiosensitizers.( 22 , 23 ) However, in previous papers either oxygen or carbogen inhalation was necessary to enhance radiosensitivity.
In the present study, we used rHSA‐FeP, a unique heme‐based artificial oxygen carrier, to enhance the effect of radiation on the tumor by increasing the oxygen tension in the tumor tissue. rHSA‐FeP has unique characteristics in that the solution's isoelectric point and osmolarity are comparable to native albumin,( 15 ) and it has the potentiality to carry oxygen.( 11 ) rHSA‐FeP is synthesized by the association of totally synthetic heme and recombinant human albumin.( 15 ) Recently, rHSA has been developed and manufactured on an industrial scale. rHSA‐FeP was reported to be metabolized like the albumin molecule, and the heme component is metabolized in the liver.( 11 ) With rHSA‐FeP comprising up to 20% of the blood volume, no toxic effects were noted in an animal study.( 24 ) Thus, rHSA‐FeP could be a useful fluid for resuscitation.( 15 ) Because rHSA‐FeP is dissolved in the plasma fraction, it can pass through the tortuous vascular system of tumors, whereas the cellular component of blood has difficulty passing through. Therefore, rHSA‐FeP is considered to be useful for the delivery of oxygen to hypoxic tumor areas.
In a previously published paper, we reported that rHSA‐FeP can increase PtO2;( 12 ) however, the increase of PtO2 in the tumor was observed for 300 s, followed by a gradual decrease throughout the monitoring period. This solid tumor showed consistent hypoxia throughout the tumor, therefore we thought that the hypoxic fraction in this tumor is composed of chronically (diffusion‐limited) hypoxic fractions.
In the present study, a syngenic tumor model was applied. It is believed that solid tumor growth is orchestrated by various environmental factors such as fibroblast recruitment, angiogenesis, lymphogenesis, immunological reaction, and invasion to the surrounding tissue. Xenograft study help to clarify the effect of targeting certain tumors with specific molecules, however, may not be applicable when studying biological response to radiation.( 25 ) Syngenic systems are beneficial for clarifying the physiological effects of a treatment on the tumor and body.( 26 , 27 )
Anemia has been reported to reduce the response to radiation. The results of a clinical study showed that even though human tumors are heterogeneous in terms of oxygen status, the hemoglobin level was an important factor for the radiation response.( 28 ) A low hemoglobin level may reflect a reduced oxygen supply to the tumor, and thus be associated with tumor tissue hypoxia. In the present study, the animals were not anemic, but the artificial oxygen carrier may have delivered an extra supply of oxygen to the tumor.
The current study showed that rHSA‐FeP had no effect on tumor growth without irradiation. However, the combination of rHSA‐FeP with irradiation resulted in regression of tumor growth. Time to reach 30 g (TR30) became longer when irradiation was added. Moreover, it revealed that in the rHSA‐FeP + irradiation group, TR30 was much longer compared to the irradiation group. These phenomenon indicate that rHSA‐FeP enhanced the irradiation effect both spatially and temporally.
Even if the rise in oxygen tension in the tumor tissue is limited, it seems important to achieve the maximum radiation effect within the period when the tumor PtO2 is elevated from the hypoxic conditions. Although there seemed slightest change in the surrounding normal tissue in this experiment, conventional high‐dose radiation causes side effects such as skin and soft tissue fibrosis and ulcers. To prevent these side effects and achieve good tumor control, stereotaxic irradiation may be used when rHSA‐FeP is the irradiation enhancer.
Linberg and coworkers reported that the administration of polyethylene glycol (PEG)‐conjugated hemoglobin increased the surface tissue oxygen tension of UMR‐106 osteogenic sarcoma within 2 h of injection.( 6 ) Nevertheless, the delivery of oxygen was rather limited, with only a 78% increase from baseline. The differences observed in the degree of oxygenation of the hypoxia by the albumin‐heme and PEG‐conjugated hemoglobin could be due to the characteristics of each product (e.g. molecular size, viscosity, surface charge, colloid osmotic pressure, and oxygen‐binding affinity). The high viscosity and colloid osmotic pressure of the PEG‐conjugated hemoglobin should influence the plasma volume and shear stress on the capillary wall. Perfluorochemical emulsion and modified hemoglobin solution have been reported to improve tumor hypoxia.( 21 , 23 ) Teicher and colleagues reported that ultrapurified stabilized bovine hemoglobin solution with a high concentration of oxygen inhalation enhanced the radiation response and increased tumor growth delay.( 7 ) In previous studies, radiation‐enhancing effects were observed when large amounts of oxygen‐carrying material or high concentrations of oxygen were used. In the present study, albumin‐heme with air breathing showed an enhanced radiation effect. These materials could be candidates for enhancing radiotherapy and chemotherapy.
Conclusion
Albumin‐heme (rHSA‐FeP) appears to enhance the effect of radiation in an animal tumor model. Thus, the intra‐arterial administration of this artificial oxygen carrier followed by a bolus of radiation could be a potential candidate for clinical study.
Acknowledgments
The present study was partially supported by Grant in Aid for Scientific research (C‐16591410) from the Ministry of Education, Culture, Sports, Science and Technology, and Health Science Research Grants (Regulatory Science) from the Ministry of Health, Labor and Welfare.
References
- 1. Hockel M, Knoop C, Schlenger K et al . Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiather Oncol 1993; 26: 45–50. [DOI] [PubMed] [Google Scholar]
- 2. Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol 1996; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 3. Chapman JD, Dugle DL, Reuvers AP, Meeker BE, Borsa J. Studies on the radiosensitizing effect of oxygen in Chinese hamster cells. Int J Radiat Biol 1974; 26: 383–9. [DOI] [PubMed] [Google Scholar]
- 4. Henk JM. Late results of a trial of hyperbaric oxygen and radiotherapy in head and neck cancer: a rationale for hypoxic cell sensitizers? Int J Radiat Oncol Biol Phys 1986; 12: 1339–41. [DOI] [PubMed] [Google Scholar]
- 5. Guichard M. The use of fluorocarbon emulsions in cancer radiotherapy. Radiother Oncol 1991; 20 (Suppl.): 59–64. [DOI] [PubMed] [Google Scholar]
- 6. Linberg R, Conover CD, Shum KL, Shorr RGL. Increased tissue oxygenation and enhanced radiation sensitivity of solid tumors in rodents following polyethylene glycol conjugated bovine hemoglobin administration. In Vivo 1998; 12: 167–74. [PubMed] [Google Scholar]
- 7. Teicher BA, Holden SA, Ara G, Herman TS, Hopkins RE, Menon K. Effect of a bovine hemoglobin preparation (SBHS) on the response of two murine solid tumors to radiation therapy or chemotherapeutic alkylating agents. Biomat Art Cells Immob Biotech 1992; 20: 657–60. [DOI] [PubMed] [Google Scholar]
- 8. Thews O, Kelleher DK, Vaupel P. Dynamics of tumor oxygenation and red blood cell flux in response to inspiratory hyperoxia combined with different levels of inspiratory hypercapnia. Radiother Oncol 2002; 62: 77–85. [DOI] [PubMed] [Google Scholar]
- 9. Kaanders JHAM, Pop LAM, Marres HAM et al . ARCON: experience in 215 patients with advanced head and neck cancer. Int J Radiation Oncol Biol Phys 2002; 52: 769–78. [DOI] [PubMed] [Google Scholar]
- 10. Kaanders JHAM, Bussink J, Van Der Kogel AJ. ARCON: a novel biology based approach in radiotherapy. Lancet Oncol 2002; 3: 728–37. [DOI] [PubMed] [Google Scholar]
- 11. Tsuchida E, Komatsu T, Hamamatsu K et al . Exchange transfusion with albumin heme as an artificial O2‐infusion into anesthetized rat: physiological responses, O2‐delivery, and reduction of the oxidized hemin sites by red blood cells. Bioconjug Chem 2000; 11: 46–50. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi K, Komatsu T, Iwamaru A et al . Oxygenation of hypoxic region in solid tumor by administration of human serum albumin incorporating synthetic hemes. J Biomed Mater Res A 2003; 64: 48–51. [DOI] [PubMed] [Google Scholar]
- 13. Tanda S, Hori K, Saito S, Zhang QH, Li HC, Suzuki M. Effects of intravenous infusion of dopamine on tumor blood flow in rat subcutis. Jpn J Cancer Res, 1994; 85: 556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komatsu T, Hamamatsu K, Wu J, Tsuchida E. Physicochemical properties and O2‐coordination structure of human serum albumin incorporating tetrakis (o‐pivalamido) phenylporphyrinatoiron (II) derivatives. Bioconjug Chem 1999; 10: 82–6. [DOI] [PubMed] [Google Scholar]
- 15. Komatsu T, Yamamoto H, Huang Y, Horinouchi H, Kobayashi K, Tsuchida E. Exchange transfusion with synthetic oxygen‐carrying plasma protein ‘albumin‐heme’ into an acute anemia rat model after seventy‐percent hemodilution. J Biomed Mater Res 2004; 71A: 644–51. [DOI] [PubMed] [Google Scholar]
- 16. Kubota T, Matsuzaki SW, Hoshiya S et al . Antitumor activity of paclitaxel against human breast carcinoma xenografts serially transplanted into nude mice. J Surg Oncol 1997; 64: 115–21. [DOI] [PubMed] [Google Scholar]
- 17. Rijpkema M, Kaanders JHAM, Joosten FBM et al . Effect of carbogen breathing on oxygenation and vascularity of head and neck tumors as measured by MRI. Int L Radiat Oncol Biol Phys 2002; 53: 1185–91. [DOI] [PubMed] [Google Scholar]
- 18. Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and locoregional control in advanced breast cancer. Br J Radiol 1995; 68: 215–18. 7537598 [Google Scholar]
- 19. Bussink J, Kaanders JHAM, Stirk AM, Van Der Kogel AJ. Effects of nicotinamide and carbogen on oxygenation in human tumor xenografts measured with luminescence based fiberoptic probes. Radiother Oncol 2000; 57: 21–30. [DOI] [PubMed] [Google Scholar]
- 20. Sieman DW, Hill RP, Bush RS. The importance of the preradiation breathing time of O2 and carbogen (5% CO2: 95% O2) on the in vivo radiation response of a murine sarcoma. Int J Radiother Oncol 2000; 62: 77–85. [Google Scholar]
- 21. Nozue M, Lee I, Manning JM, Manning LR, Jain RK. Oxygenation in tumors by modified hemoglobins. J Surg Oncol 1996; 62: 109–14. [DOI] [PubMed] [Google Scholar]
- 22. Robinson MF, Dupuis NP, Kusumoto T, Lie F, Menon K, Teicher BA. Increased tumor oxygenation and radiation sensitivity in two rat tumors by hemoglobin‐based, oxygen carrying preparation. Artif Cells Blood Substit Immobil Biothechnol 1995; 23: 431–8. [DOI] [PubMed] [Google Scholar]
- 23. Koch CJ, Oprysko PR, Shuman AL, Jenkins WT, Brandt G, Evans SM. Radiosensitization of hypoxic tumor cells by dodecafluoropentane: a gas phase perfluorochemical emulsion. Cancer Res 2002; 62: 3626–9. [PubMed] [Google Scholar]
- 24. Huang Y, Komatsu T, Yamamoto H, Horinouchi H, Kobayashi K, Tsuchida E. Safety evaluation of an artificial O2 carrier as a red cell substitute by blood biochemical tests and histopathology observations. ASAIO J 2004; 50: 525–9. [DOI] [PubMed] [Google Scholar]
- 25. Jouanneau E, Alberti L, Nejjari M et al . Lack of antitumor activity of recombinant endostatin in a human neuroblastoma xenograft model. J Neuro-Oncol 2001; 51: 11–18. [DOI] [PubMed] [Google Scholar]
- 26. Krishnan K, Khanna C, Helman LJ. The molecular biology of pulmonary metastasis. Thorac Surg Clin 2006; 16: 115–24. [DOI] [PubMed] [Google Scholar]
- 27. Walser TC, Xinrong Ma Kundy N, Dorsey R, Goloubeva O, Fulton AM. Immune‐mediated modulation of breast cancer growth and metastasis by chemokine Mig (CXCL9) in a murine model. J Immunother 2007; 30: 490–8. [DOI] [PubMed] [Google Scholar]
- 28. Harrison LB, Shasha D, Homel P. Prevalence of anemia in cancer patients undergoing radiotherapy: prognostic significance and treatment. Oncology 2002; 63: 11–18. [DOI] [PubMed] [Google Scholar]


