Abstract
Members of the transforming growth factor‐β (TGF‐β) family, including TGF‐β, activin and bone morphogenetic proteins (BMPs), are multifunctional proteins that regulate a wide variety of cellular responses, such as proliferation, differentiation, migration and apoptosis. Alterations in their downstream signaling pathways are associated with a range of human diseases like cancer. TGF‐β family members transduce signals through membrane serine/threonine kinase receptors and intracellular Smad proteins. The ubiquitin–proteasome pathway, an evolutionarily conserved cascade, tightly regulates TGF‐β family signaling. In this pathway, E3 ubiquitin ligases play a crucial role in the recognition and degradation of target proteins by the 26S proteasomes. Smad degradation regulates TGF‐β family signaling; HECT (homologous to the E6‐accessory protein C‐terminus)‐type E3 ubiquitin ligases, Smad ubiquitin regulatory factor 1 (Smurf1), Smurf2, and a RING‐type E3 ubiquitin ligase, ROC1‐SCFFbw1a have been implicated in Smad degradation. Smurf1 and Smurf2 bind to TGF‐β family receptors via the inhibitory Smads, Smad6 and Smad7, to induce their ubiquitin‐dependent degradation. Arkadia, a RING‐type E3 ubiquitin ligase, induces the ubiquitination and degradation of Smad7 and corepressors, c‐Ski and SnoN, to enhance TGF‐β family signaling. Abnormalities in E3 ubiquitin ligases that control components of TGF‐β family signaling may lead to the development and progression of various cancers. (Cancer Sci 2008; 99: 2107–2112)
Abbreviations:
- TGF‐β
transforming growth factor‐β
- BMPs
bone morphogenetic proteins
- HECT
homologous to the E6‐accessory protein C‐terminus
- Smurfs
Smad ubiquitin regulatory factors
- Nedd4‐2
neural precursor cells‐expressed developmentally down‐regulated 4‐2
Transforming growth factor‐β (TGF‐β) family signaling
Members of the transforming growth factor‐β (TGF‐β) family including TGF‐β, activin and bone morphogenetic proteins (BMPs) are multifunctional proteins that regulate a diverse set of cellular responses, including proliferation, differentiation, migration and apoptosis.( 1 ) TGF‐β family members transduce signals as parts of heteromeric complexes containing type I and type II serine/threonine kinase receptors and intracellular Smad proteins (Fig. 1).( 2 , 3 , 4 , 5 ) Following ligand binding, the type II receptor phosphorylates the type I receptor, which in turn phosphorylates the receptor‐regulated Smads (R‐Smads), typically at a C‐terminal SXS motif (Fig. 2a). Smad1, Smad5 and Smad8 serve as substrates for the BMP receptors, whereas Smad2 and Smad3 are substrates for TGF‐β and activin. Activated R‐Smads associate with Smad4, a common‐partner Smad (Co‐Smad), and translocate into the nucleus. Once there, Smad complexes bind to transcriptional factor(s), such as FoxH1, Mixer, Runx‐related proteins and E2F, as well as transcriptional coactivators (e.g. p300 and CBP) and corepressors (e.g. TGIF, c‐Ski, and SnoN) to regulate transcriptional target genes.( 6 ) The third class of Smads, inhibitory Smads (I‐Smads) like Smad6 and Smad7, are induced by TGF‐β family ligands. I‐Smads compete with R‐Smads for binding to type I receptors, resulting in the inhibition of TGF‐β family signaling.( 7 , 8 )
Figure 1.
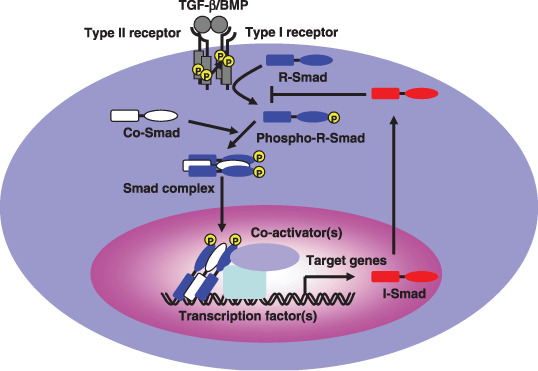
The transforming growth factor‐β (TGF‐β) family signaling pathway. Binding of TGF‐β family ligands induces the association of the type II and type I receptors into a heterodimeric complex. The type II receptor kinase phosphorylates the type I receptor, inducing its serine/threonine kinase activity. Receptor‐regulated Smads (R‐Smads) are then activated by phosphorylation by the type I receptor kinase. Activated R‐Smads form complexes with common‐partner Smads (Co‐Smad), and translocate into the nucleus. Once there, these proteins bind other transcriptional factors, including both transcriptional coactivators and corepressors.
Figure 2.
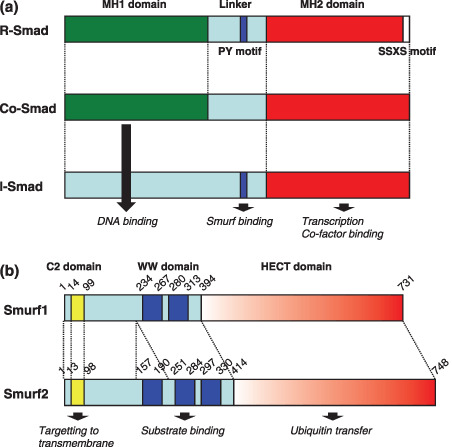
Schematic diagram displaying the structural organization of Smad proteins and Smad ubiquitin regulatory factor (Smurf) proteins. (a) Diagrammatic representation of the three subfamilies of Smads. Smad proteins consist of two conserved domains, the MH1 and MH2 domains, and the linker region. The PY motif of the linker that is recognized by the homologous to the E6‐accessory protein C‐terminus (HECT)‐type E3 ubiquitin ligases, and receptor‐mediated phosphorylation occurs at the carboxy‐terminal SSXS motif of R‐Smads. (b) Diagrammatic representation of Smurf1 and Smurf2. The C2 domain is responsible for localization of Smurfs to the plasma membrene in a Ca2+‐dependent manner. The WW domain binds substrate proteins containing PY motifs. The HECT domain catalyzes the transfer of ubiquitin to target substrates.
Ubiquitin‐proteasome system
The ubiquitin‐proteasome pathway is a major pathway for the targeted degradation of proteins. This process plays critical roles in a wide range of biological processes, including cell‐cycle progression, signal transduction, transcriptional regulation, receptor down‐regulation and endocytosis.( 9 , 10 ) In general, protein ubiquitination is catalyzed by a cascade of enzymes, including an ubiquitin‐activating enzyme E1, an ubiquitin‐conjugating enzyme E2 and an ubiquitin ligase E3. E3 ubiquitin ligases are crucial in the selective recognition of target proteins and also function in subsequent protein degradation by the 26S proteasomes.( 11 ) E3 ubiquitin ligases exist and act a single peptide (such as Mdm2 and XIAP) or as a multiple‐component complex (such as Skp1‐Cullin‐F‐box protein [SCF]). Frequently, genetic alterations and aberrations in the expression of E3 ubiquitin ligases result in cancer development.( 12 , 13 )
Smad ubiquitin regulatory factors (Smurfs)
Smad ubiquitin regulatory factor 1 (Smurf1) and Smurf2 are HECT (homologous to the E6‐accessory protein C‐terminus)‐type E3 ubiquitin ligases that regulate TGF‐β and BMP signaling.( 14 , 15 , 16 , 17 , 18 , 19 ) Smurfs contain an N‐terminal C2 domain for membrane binding, a central region containing two or three WW domains for protein‐protein interaction and a C‐terminal HECT domain for ubiquitin protein ligation (Fig. 2b). Smurf1 was originally identified as an E3 ubiquitin ligase that interacted with Smad1 and Smad5 through a specific interaction between the Smurf1 WW domain and the PY motif in linker region of Smad1, and induces degradation of Smads (Fig. 3).( 19 ) In addition to regulating the degradation of R‐Smads, Smurf1 and Smurf2 facilitate the inhibitory activities of I‐Smads (see below for details). Phosphorylation of the Smad1 linker primes Smad1 for proteasome‐dependent degradation by facilitating the Smurf1‐dependent polyubiquitination of Smad1.( 20 ) In addition to inducing Smad1 ubiquitination, Smurf1 binding inhibits Smad1 from interacting with the nuclear translocation factor Nup214.( 20 ) Thus, linker phosphorylation‐dependent Smurf1 binding results in Smad1 degradation or cytoplasmic retention. Smurf2 interacts with both Smad1 and Smad2 to induce their ubiquitin‐mediated degradation.( 16 , 18 )
Figure 3.
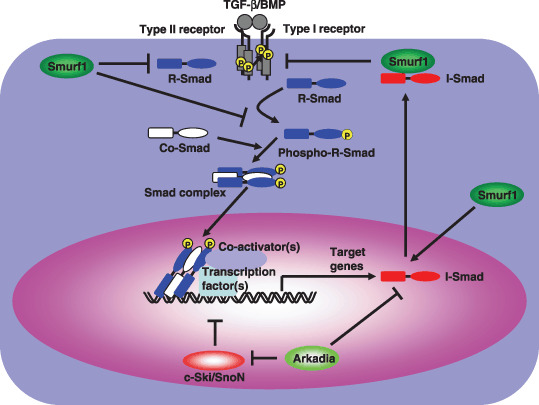
Smad ubiquitin regulatory factors (Smurfs) and Arkadia regulate transforming growth factor‐β (TGF‐β) family signaling. Smurfs induce the ubiquitination and degradation of R‐Smads. Smurfs also mediate the degradation of type I receptors by associating with inhibitory‐Smads (I‐Smads) in response to ligands, leading to the repression of TGF‐β family signaling. In contrast, Arkadia enhances TGF‐β signaling by down‐regulating the negative regulators Smad7 and SnoN/c‐Ski.
Smurf1 and Smurf2 interact with Smad7 with higher affinities than those for the R‐Smads, inducing the ubiquitin‐dependent degradation of Smad7 and the associated receptors for members of the TGF‐β family.( 14 , 15 , 17 ) Although Smurf1 and Smurf2 are localized to the nucleus, their binding to Smad7 induces their export and recruitment to activated receptors, resulting in the degradation of the receptors and Smad7. Smurf1 also binds to CRM1 (chromosomal region maintenance 1) through a C‐terminal nuclear export signal (NES).( 21 ) Smurf1 induces the export of Smad7 from the nucleus to the cytoplasm, allowing Smad7 to associate with activated type I receptors at the plasma membrane. This localization of Smad7 to the plasma membrane requires the C2 domain of Smurf1.( 22 )
Although the C‐terminal MH2 domains of Smad6 and Smad7 are essential for the inhibition of TGF‐β and BMP signaling, the N domain of Smad7 (Smad7N) is required for efficient inhibition of TGF‐β signaling by Smad7 (Fig. 2a).( 23 ) Smad7N physically interacts with the MH2 domain of Smad7, which may induce a conformational change in the MH2 domain that enhances its affinity for the TGF‐β receptor complex. Smad7N also positively regulates the catalytic activity of the Smurf2 HECT domain by facilitating E2 recognition.( 24 ) The interaction of the Smurf2 HECT domain with the UbcH7 E2 is weak; Smad7N strongly enhanced the interaction of Smurf2 and UbcH7, stimulating Smurf2 ligase activity. Thus, Smad7 both regulates Smurf2 activity by promoting E2 binding to its HECT domain and aids in recruiting Smurf2 to membrane receptors.
E3 ubiquitin ligases catalyze the ubiquitination of both their substrates and themselves. In many cases, E3 autoubiquitination induces their proteasome‐dependent degradation, suggesting that autoubiquitination controls E3 ubiquitin ligase abundance. Wiesner et al. demonstrated that intramolecular interactions between the C2 and HECT domains of Smurf2 inhibited its catalytic activity and stabilized Smurf2 levels.( 25 ) This interaction inhibited Smurf2 ligase activity by interfering with ubiquitin thioester formation, which served to stabilize steady‐state levels of both Smurf2 and the substrate. The Smad7N domain disrupted the interaction between the C2 and HECT domains. These data suggest that autoinhibition of the Smurf2 HECT domain by the C2 domain maintains the steady‐state levels of this E3 ubiquitin ligase and can be relieved by adaptor‐mediated substrate targeting.( 25 )
In addition to Smad7, Smad2 plays an important role in regulating the activity of Smurf2.( 26 ) In response to TGF‐β ligation, Smad2 forms a complex with Smurf2, which mediates the recruitment of Smurf2 to the transcriptional corepressor SnoN. Smurf2 subsequently promotes the ubiquitination and proteasomal degradation of SnoN.( 26 ) The anaphase‐promoting complex (APC) similarly uses Smad3 as an adaptor for SnoN recruitment, resulting in the ubiquitin‐dependent degradation of SnoN.( 27 , 28 ) SnoN degradation is an essential initial step in TGF‐β signaling.( 29 ) Therefore, the TGF‐β‐dependent degradation of SnoN, either through the Smurf2 pathway or the APC pathway, is thought to be required for activation of TGF‐β signaling in a context‐dependent manner.
WWP1, Nedd4‐2 and Itch
In addition to Smurf1 and Smurf2, WWP1/TGIF‐interacting ubiquitin ligase 1 (Tiul1), neural precursor cells‐expressed developmentally down‐regulated 4 (Nedd4)‐2, and Itch/atrophin‐1 interacting protein 4 (AIP4) also regulate TGF‐β family signaling. These molecules share a characteristic domain organization with Nedd4 and the Smurf proteins. We identified WWP1 and Nedd4‐2 as Smad7‐binding proteins by yeast two‐hybrid screening.( 30 , 31 ) Both proteins interact with the TGF‐β type I receptor (TβR‐I) via Smad7 to induce ubiquitin‐mediated degradation of TβR‐I. Although Nedd4‐2 is capable of enhancing the ubiquitination and degradation of Smad2 in the presence of activated TβR‐I, a similar function for WWP1 remains controversial. Smad2 ubiquitination by WWP1 may require TGIF and other factors involved in TGF‐β signaling.( 32 ) Smad7 also functions as an adaptor for WWP1 and Nedd4‐2 in the ubiquitination of Smad4.( 33 )
Itch/AIP4, which is involved in immune responses,( 34 , 35 ) also regulates TGF‐β signaling. Loss of Itch from MEF results in reduced susceptibility to TGF‐β‐induced cell growth arrest and decreased Smad2 phosphorylation, without any alterations in the protein levels of either Smad2 or TβR‐I.( 36 ) Itch mediates the TGF‐β‐induced ubiquitination of Smad2, which enhances the interaction of Smad2 with activated TβR‐I in a manner dependent on E3 ubiquitin ligase activity. In contrast, Lallemand et al. demonstrated that Itch interacts with Smad7 to inhibit TGF‐β signaling.( 37 ) Itch enhances the association of Smad7 with the activated TβR‐I, independent of ubiquitin ligase activity. This difference may be due to tissue‐ or cell‐type‐specific effects; further studies will need to delineate the molecular mechanism by which Itch participates in the regulation of TGF‐β signaling.
Arkadia
Arkadia was originally identified as a protein that enhances nodal signaling, inducing mammalian nodes during embryonic development.( 38 , 39 ) Arkadia possesses multiple nuclear localization signals in its N‐terminus and a RING‐finger domain at its C‐terminus. Arkadia, which is widely expressed throughout mammalian tissues, enhances the signaling activities of both BMP and TGF‐β. Arkadia interacts with Smad7 and induces its ubiquitination and degradation (Fig. 3).( 40 ) In contrast to the Smurf proteins, Arkadia does not interact with TβR‐I and fails to induce receptor degradation. Liu et al. demonstrated that Axin activates TGF‐β signaling upon forming a multimeric complex containing Smad7 and Arkadia.( 41 ) Axin, a scaffold protein in the Wnt pathway, is required for constitutive degradation of β‐catenin.( 42 ) Axin also enhances TGF‐β signaling in an Arkadia‐dependent manner. By promoting Smad7 polyubiquitination, Axin cooperates with Arkadia to reduce Smad7 stability. Wnt‐1 attenuates Axin‐induced Smad7 ubiquitination, which is consistent with the observation that Wnt‐1 down‐regulates Axin protein levels.( 41 ) Thus, Axin acts as an intrinsic regulator in both Wnt and TGF‐β signaling, which may play an important role in regulating the cross‐talk between these two signaling pathways.
Recently, several groups have reported that Arkadia targets SnoN and c‐Ski as well as Smad7 for degradation.( 43 , 44 ) SnoN and c‐Ski are potent negative regulators that inhibit activated Smad complex.( 45 ) Arkadia therefore enhances TGF‐β signaling by inducing the ubiqitination and degradation of Smad7, SnoN, and c‐Ski, all of which are independently acting negative regulators of TGF‐β signaling.
Others
The SCF complexes are multisubunit RING‐type E3 ligases that participate in the degradation of a wide variety of proteins. The SCF complex contains three invariable components, ROC1/Rbx1 (RING‐finger protein), Cul1 (scaffold protein), and Skp1 (adaptor protein), as well as the variable component that confers specific substrate recognition, known as an F‐box protein, that binds to Skp1 via its F‐box motif. ROC1‐SCFFbw1a interacts with Smad3 to trigger the degradation of Smad3 in a ligand‐dependent manner.( 46 ) Transcriptional coactivator p300 potentiates the transcriptional activity of Smad3, but also induces the interaction of Smad3 with the ROC1‐SCFFbw1a complex. ROC1‐SCFFbw1a‐induced proteasomal degradation may be necessary to terminate Smad3‐mediated transcriptional activity. Wan et al. reported that SCFFbw1a also regulates Smad4 protein stability.( 47 )
Smad4 mutants isolated from cancer cells exhibit accelerated induction of ubiquitin‐dependent proteasomal degradation in comparison to wild‐type Smad4.( 48 ) Liang et al. demonstrated that the SCFSkp2 complex physically interacts with Smad4; several cancer‐associated Smad4 mutants exhibit a significantly increased affinity for Skp2. Skp2 promotes the ubiquitination‐dependent degradation of these Smad4 cancer mutants, but not the wild‐type protein.( 49 ) Skp2, which targets tumor suppressor proteins such as p27 for degradation, is up‐regulated in a multitude of human cancers.( 12 ) Thus, the ubiquitin‐dependent degradation of cancer‐associated Smad4 mutants by the SCFSkp2 complex may be the molecular mechanism mediating both the oncogenic role of Skp2 and the tumor suppressor function of Smad4.
CHIP (carboxyl terminus of Hsc70‐interacting protein), a U‐Box‐dependent E3 ligase, interacts with Smad1/4 to regulate the BMP signaling pathway.( 50 ) CHIP also associates with Smad3 to function as a negative regulator of TGF‐β signal transduction.( 51 ) Unlike ROC1–SCFFbw1a however, CHIP decreases total Smad3 levels independent of TGF‐β activation. Overexpression of CHIP attenuates the cytostatic effects of TGF‐β. Reductions in endogenous CHIP protein levels by knock‐down enhance cellular sensitivity to TGF‐β signaling. CHIP may desensitize the cell to TGF‐β by decreasing basal Smad3 levels.
Ectodermin/TIF1γ, a RING‐type E3 ligase, is essential for the specification of the ectoderm. This protein acts by restricting the mesoderm‐inducing activity of TGF‐β signals to the mesoderm, which favors neural induction.( 52 ) Ectodermin/TIF1γ binds to Smad4 and induces Smad4 ubiquitination and degradation. Depletion of Ectodermin/TIF1γ from human cancer cell lines enhances the cytostatic effects of TGF‐β. Ectodermin/TIF1γ mediates these biological responses by down‐regulating Smad4 expression, which results in the repression of both TGF‐β and BMP signaling. He et al. identified Ectodermin/TIF1γ as a protein that selectively bound only the receptor‐activated subset of Smad2 and Smad3 proteins.( 53 ) In these experiments, overexpression of Ectodermin/TIF1γ inhibited the binding of Smad2/3 to Smad4, whereas its depletion augmented the binding of Smad2/3 to Smad4. The converse was also true when Smad4 levels were manipulated. Although Ectodermin/TIF1γ inhibited Smad4‐dependent gene responses, He et al. were not able to demonstrate that Ectodermin/TIF1γ targets Smad4 for ubiquitination and degradation. The two models are not mutually exclusive, each scenario may dominate in different cellular contexts.
Genetic aberration and alterations in expression of E3 ubiquitin ligases in human cancer
As TGF‐β signaling is tightly regulated by numerous E3 ubiquitin ligases, dysregulated expression or functionality of such E3 ubiquitin ligases may affect the proper transmission of TGF‐β signaling, contributing to cancer development. In support of this theory, misregulated expression or aberrant function of E3 ligases, such as Smurfs, Arkadia, WWP1, Ectodermin/TIF1γ, Skp2, and Fbw1a, is observed in several human cancers.
High expression levels of Smurf2 correlates with increased depth of invasion and lymph node metastases and poor survival.( 54 ) An inverse correlation between Smurf2 expression and phospho‐Smad2 levels is also observed in cancers. In patients with esophageal squamous cell carcinoma, elevated expression levels of Smurf2 correlated with tumor development and a poor prognosis, suggesting that the repression of TGF‐β signaling by Smurf2 occurs during tumor development in humans. The Smurf1 gene, mapping to 7q21.1‐31.1, was amplified and overexpressed in pancreatic cancer.( 55 )
SnoN is overexpressed in a variety of tumors. Several esophageal cancer cell lines have lost the ability to degrade SnoN following TGF‐β ligation. Although all the components of the TGF‐β pathway are present and functional in SEG‐1 cells, a Barrett's‐associated esophageal adenocarcinoma cell line, this cell line is resistant to TGF‐β‐mediated growth inhibition.( 56 ) Levy et al. demonstrated that SEG‐1 cells have lost Arkadia expression and exhibit deficient SnoN degradation in response to TGF‐β.( 43 ) Reintroduction of Arkadia restored TGF‐β‐induced Smad3/4‐dependent transcription and SnoN degradation. These results suggest that the loss of Arkadia may contribute to tumorigenesis by increasing SnoN expression.
The amplification of 8q21 occurs in a large percentage of prostate and breast cancers; WWP1 is located at this region. WWP1 is frequently overexpressed in prostate and breast cancers; the degree of overexpression correlates significantly with copy number.( 57 , 58 ) Forced overexpression of WWP1 enhanced cell proliferation, whereas gene silencing of WWP1 mRNA suppressed it. Therefore, WWP1 likely functions as an oncogene in prostate and breast cancers.
Conclusion and perspectives
Recent progress studying TGF‐β signaling mechanisms has revealed the important role for ubiquitin‐dependent proteasomal degradation in regulating TGF‐β signaling (Table 1). Disruption of ubiquitin‐dependent degradation of the components of TGF‐β signaling can lead to cancer development. These E3 ubiquitin ligases regulating the TGF‐β signaling pathway may be candidates for pharmacological cancer therapies. Small molecule inhibitors of E3 ubiquitin ligases (e.g. Mdm2 and TRAF6) have recently been developed.( 59 ) Further research examining the role of the ubiquitin–proteasome system in regulating TGF‐β signaling will provide additional insight into the development of small‐molecule or peptide‐based inhibitors for future therapeutic treatments.
Table 1.
E3 ubiquitin ligases implicated in family signaling pathway
| E3 ubiquitin ligase | Target proteins | Adaptor | Regulatory mechanisms | Reference |
|---|---|---|---|---|
| Smurf1 | Smad1/5 | Smurf1 ubiquitinates Smad1/5 in a ligand‐independent manner | (19) | |
| TβR‐I | Smad6/7 | Smurf1 induces the degradation of Smad7 and the associated receptors | (14) | |
| Smad7 | (14) | |||
| Smurf2 | TβR‐I | Smad7 | Smurf2 induces the degradation of Smad7 and the associated receptors | (15) |
| Smad1 | Smad1 ubiquitination by Smurf2 is a ligand‐independent manner | (18) | ||
| Smad2 | The interaction between Smad2 and Smurf2 is enhanced after TGF‐β ligation | (16) | ||
| SnoN | Smad2 | Smurf2 interacts with SnoN via Smad2 to induce ubiquitin‐dependentdegradation of SnoN in response to TGF‐β | (26) | |
| Nedd4‐2 | TβR‐I | Smad7 | Nedd4‐2 interacts with TβR‐I via Smad7 to induce ubiquitin‐dependent degradation of TβR‐I | (31) |
| Smad2 | Smad2 ubiquitination by Nedd4‐2 is enhanced after TGF‐β ligation | (31) | ||
| Smad4 | Smad7 | Smad7 functions as an adaptor for Nedd4‐2 in the ubiquitination of Smad4 | (33) | |
| WWP1/Tiul1 | TβR‐I | Smad7 | WWP1 interacts with TβR‐I via Smad7 to induce ubiquitin‐dependent degradation of TβR‐I | (30, 32) |
| Smad2 | Smad2 ubiquitination by WWP1 may require TGIF | (32) | ||
| Smad4 | Smad7 | Smad7 functions as an adaptor for WWP1 in the ubiquitination of Smad4 | (33) | |
| Itch/AIP4 | Smad2 | The interaction between Smad2 and Itch is enhanced after TGF‐β ligation | (36) | |
| Arkadia | Smad7 | Axin | Axin cooperates with Arkadia to reduce Smad7 stability | (40, 41) |
| SnoN/c‐Ski | Arkadis enhances TGF‐β signaling by inducing ubiquitin‐dependent degradation of SnoN and c‐Ski | (43, 44) | ||
| ROC1‐SCFFbw1a | Smad3 | ROC1‐SCFFbw1a interacts with Smad3 to induce the degradation of Smad3 in a ligand‐dependent manner | (46) | |
| Smad4 | SCFFbw1a decreases Smad4 protein stability | (47) | ||
| SCFSkp2 | Smad4 | Several cancer‐associated Smad4 mutants exhibit a significantly increased affinity for Skp2 | (49) | |
| CHIP | Smad1/4 | CHIP decreases the protein levels of Smad1 and Smad4 in a ligand‐independent manner | (50) | |
| Smad3 | CHIP decreases total Smad3 levels independent of TGF‐β activation | (51) | ||
| Ectodermin/TIF1γ | Smad4 | Ectodermin binds to Smad4 and may induce Smad4 ubiquitination and degradation | (52) | |
| APC | SnoN | Smad3 | APC uses Smad3 as an adaptor for SnoN recruitment to induce the ubiquitin‐dependent degradation of SnoN | (27, 28) |
Acknowledgments
There are many important papers in this field, and for reasons of space, we have not been able to mention all of them. We apologize to those investigators whose papers could not be cited. This research was supported by KAKENHI (Grants‐in‐Aid for Scientific Research) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF‐β in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807–21. [DOI] [PubMed] [Google Scholar]
- 2. ten Dijke P, Hill CS. New insights into TGF‐β‐Smad signaling. Trends Biochem Sci 2004; 29: 265–73. [DOI] [PubMed] [Google Scholar]
- 3. Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev 2005; 19: 2783–810. [DOI] [PubMed] [Google Scholar]
- 4. Heldin CH, Miyazono K, ten Dijke P. TGF‐β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–71. [DOI] [PubMed] [Google Scholar]
- 5. Derynck R, Zhang YE. Smad‐dependent and Smad‐independent pathways in TGF‐β family signalling. Nature 2003; 425: 577–84. [DOI] [PubMed] [Google Scholar]
- 6. Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF‐β superfamily signalling. Genes Cells 2002; 7: 1191–204. [DOI] [PubMed] [Google Scholar]
- 7. Hayashi H, Abdollah S, Qiu Y et al . The MAD‐related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 1997; 89: 1165–73. [DOI] [PubMed] [Google Scholar]
- 8. Imamura T, Takase M, Nishihara A et al . Smad6 inhibits signalling by the TGF‐β superfamily. Nature 1997; 389: 622–6. [DOI] [PubMed] [Google Scholar]
- 9. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67: 425–79. [DOI] [PubMed] [Google Scholar]
- 10. Jesenberger V, Jentsch S. Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol 2002; 3: 112–21. [DOI] [PubMed] [Google Scholar]
- 11. Laney JD, Hochstrasser M. Substrate targeting in the ubiquitin system. Cell 1999; 97: 427–30. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama KI, Nakayama K. Ubiquitin ligases. cell‐cycle control and cancer. Nat Rev Cancer 2006; 6: 369–81. [DOI] [PubMed] [Google Scholar]
- 13. Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin‐like proteins in cancer pathogenesis. Nat Rev Cancer 2006; 6: 776–88. [DOI] [PubMed] [Google Scholar]
- 14. Ebisawa T, Fukuchi M, Murakami G et al . Smurf1 interacts with transforming growth factor‐β type I receptor through Smad7 and induces receptor degradation. J Biol Chem 2001; 276: 12 477–80. [DOI] [PubMed] [Google Scholar]
- 15. Kavsak P, Rasmussen RK, Causing CG et al . Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell 2000; 6: 1365–75. [DOI] [PubMed] [Google Scholar]
- 16. Lin X, Liang M, Feng XH. Smurf2 is a ubiquitin E3 ligase mediating proteasome‐dependent degradation of Smad2 in transforming growth factor‐β signaling. J Biol Chem 2000; 275: 36 818–22. [DOI] [PubMed] [Google Scholar]
- 17. Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell 2003; 14: 2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Chang C, Gehling DJ, Hemmati‐Brivanlou A, Derynck R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci USA 2001; 98: 974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999; 400: 687–93. [DOI] [PubMed] [Google Scholar]
- 20. Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 2007; 25: 441–54. [DOI] [PubMed] [Google Scholar]
- 21. Tajima Y, Goto K, Yoshida M et al . Chromosomal region maintenance 1 (CRM1)‐dependent nuclear export of Smad ubiquitin regulatory factor 1 (Smurf1) is essential for negative regulation of transforming growth factor‐β signaling by Smad7. J Biol Chem 2003; 278: 10 716–21. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki C, Murakami G, Fukuchi M et al . Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J Biol Chem 2002; 277: 39 919–25. [DOI] [PubMed] [Google Scholar]
- 23. Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor‐β signaling. J Cell Biol 2001; 155: 1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogunjimi AA, Briant DJ, Pece‐Barbara N et al . Regulation of Smurf2 ubiquitin ligase activity by anchoring the E2 to the HECT domain. Mol Cell 2005; 19: 297–308. [DOI] [PubMed] [Google Scholar]
- 25. Wiesner S, Ogunjimi AA, Wang HR et al . Autoinhibition of the HECT‐type ubiquitin ligase Smurf2 through its C2 domain. Cell 2007; 130: 651–62. [DOI] [PubMed] [Google Scholar]
- 26. Bonni S, Wang HR, Causing CG et al . Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol 2001; 3: 587–95. [DOI] [PubMed] [Google Scholar]
- 27. Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase‐promoting complex for ubiquitination and degradation of SnoN. Genes Dev 2001; 15: 2822–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan Y, Liu X, Kirschner MW. The anaphase‐promoting complex mediates TGF‐β signaling by targeting SnoN for destruction. Mol Cell 2001; 8: 1027–39. [DOI] [PubMed] [Google Scholar]
- 29. Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF‐β signaling by the SnoN oncoprotein. Science 1999; 286: 771–4. [DOI] [PubMed] [Google Scholar]
- 30. Komuro A, Imamura T, Saitoh M et al . Negative regulation of transforming growth factor‐β (TGF‐β) signaling by WW domain‐containing protein 1 (WWP1). Oncogene 2004; 23: 6914–23. [DOI] [PubMed] [Google Scholar]
- 31. Kuratomi G, Komuro A, Goto K et al . NEDD4‐2 (neural precursor cell expressed, developmentally down‐regulated 4‐2) negatively regulates TGF‐β (transforming growth factor‐β) signaling by inducing ubiquitin‐mediated degradation of Smad2 and TGF‐β type I receptor. Biochem J 2005; 386: 461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo SR, Lallemand F, Ferrand N et al . The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 2004; 23: 3780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morén A, Imamura T, Miyazono K, Heldin CH, Moustakas A. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 2005; 280: 22 115–23. [DOI] [PubMed] [Google Scholar]
- 34. Fang D, Elly C, Gao B et al . Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol 2002; 3: 281–7. [DOI] [PubMed] [Google Scholar]
- 35. Perry WL, Hustad CM, Swing DA, O'Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet 1998; 18: 143–6. [DOI] [PubMed] [Google Scholar]
- 36. Bai Y, Yang C, Hu K, Elly C, Liu YC. Itch E3 ligase‐mediated regulation of TGF‐β signaling by modulating Smad2 phosphorylation. Mol Cell 2004; 15: 825–31. [DOI] [PubMed] [Google Scholar]
- 37. Lallemand F, Seo SR, Ferrand N et al . AIP4 restricts transforming growth factor‐β signaling through a ubiquitination‐independent mechanism. J Biol Chem 2005; 280: 27 645–53. [DOI] [PubMed] [Google Scholar]
- 38. Episkopou V, Arkell R, Timmons PM, Walsh JJ, Andrew RL, Swan D. Induction of the mammalian node requires Arkadia function in the extraembryonic lineages. Nature 2001; 410: 825–30. [DOI] [PubMed] [Google Scholar]
- 39. Niederlander C, Walsh JJ, Episkopou V, Jones CM. Arkadia enhances nodal‐related signaling to induce mesendoderm. Nature 2001; 410: 830–4. [DOI] [PubMed] [Google Scholar]
- 40. Koinuma D, Shinozaki M, Komuro A et al . Arkadia amplifies TGF‐β superfamily signaling through degradation of Smad7. EMBO J 2003; 22: 6458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W, Rui H, Wang J et al . Axin is a scaffold protein in TGF‐β signaling that promotes degradation of Smad7 by Arkadia. EMBO J 2006; 25: 1646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 2003; 94: 225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levy L, Howell M, Das D, Harkin S, Episkopou V, Hill CS. Arkadia activates Smad3/Smad4‐dependent transcription by triggering signal‐induced SnoN degradation. Mol Cell Biol 2007; 27: 6068–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagano Y, Mavrakis KJ, Lee KL et al . Arkadia induces degradation of SnoN and c‐Ski to enhance transforming growth factor‐β signaling. J Biol Chem 2007; 282: 20 492–501. [DOI] [PubMed] [Google Scholar]
- 45. Miyazono K, Suzuki H, Imamura T. Regulation of TGF‐β signaling and its roles in progression of tumors. Cancer Sci 2003; 94: 230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukuchi M, Imamura T, Chiba T et al . Ligand‐dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol Biol Cell 2001; 12: 1431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wan M, Tang Y, Tytler EM et al . Smad4 protein stability is regulated by ubiquitin ligase SCFβ‐TrCP1 . J Biol Chem 2004; 279: 14 484–7. [DOI] [PubMed] [Google Scholar]
- 48. Xu J, Attisano L. Mutations in the tumor suppressors Smad2 and Smad4 inactivate transforming growth factor β signaling by targeting Smads to the ubiquitin‐proteasome pathway. Proc Natl Acad Sci USA 2000; 97: 4820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liang M, Liang YY, Wrighton K et al . Ubiquitination and proteolysis of cancer‐derived Smad4 mutants by SCFSkp2 . Mol Cell Biol 2004; 24: 7524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li L, Xin H, Xu X et al . CHIP mediates degradation of Smad proteins and potentially regulates Smad‐induced transcription. Mol Cell Biol 2004; 24: 856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xin H, Xu X, Li L et al . CHIP controls the sensitivity of transforming growth factor‐β signaling by modulating the basal level of Smad3 through ubiquitin‐mediated degradation. J Biol Chem 2005; 280: 20 842–50. [DOI] [PubMed] [Google Scholar]
- 52. Dupont S, Zacchigna L, Cordenonsi M et al . Germ‐layer specification and control of cell growth by ectodermin, a Smad4 ubiquitin ligase. Cell 2005; 121: 87–99. [DOI] [PubMed] [Google Scholar]
- 53. He W, Dorn DC, Erdjument‐Bromage H, Tempst P, Moore MA, Massagué J. Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGFβ pathway. Cell 2006; 125: 929–41. [DOI] [PubMed] [Google Scholar]
- 54. Fukuchi M, Fukai Y, Masuda N et al . High‐level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Res 2002; 62: 7162–5. [PubMed] [Google Scholar]
- 55. Loukopoulos P, Shibata T, Katoh H et al . Genome‐wide array‐based comparative genomic hybridization analysis of pancreatic adenocarcinoma: Identification of genetic indicators that predict patient outcome. Cancer Sci 2007; 98: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Edmiston JS, Yeudall WA, Chung TD, Lebman DA. Inability of transforming growth factor‐β to cause SnoN degradation leads to resistant to transforming growth factor‐β‐induced growth arrest in esophageal cancer cells. Cancer Res 2005; 65: 4782–8. [DOI] [PubMed] [Google Scholar]
- 57. Chen C, Zhou Z, Ross JS, Zhou W, Dong JT. The amplified WWP1 gene is a potential molecular target in breast cancer. Int J Cancer 2007a; 121: 80–7. [DOI] [PubMed] [Google Scholar]
- 58. Chen C, Sun X, Guo P et al . Ubiquitin E3 ligase WWP1 as an oncogenic factor in human prostate cancer. Oncogene 2007b; 26: 2386–94. [DOI] [PubMed] [Google Scholar]
- 59. Guédat P, Colland F. Patented small molecule inhibitors in the ubiquitin proteasome system. BMC Biochem 2007; 8 (Suppl 1): S14. [DOI] [PMC free article] [PubMed] [Google Scholar]


