Abstract
A number of enzymes have been shown to be involved in the process of activation and/or degradation of 5‐fluorouracil (5‐FU), and are potential candidates for predicting chemosensitivity to 5‐FU. Among these, orotate phosphoribosyltransferase (OPRT EC 2.4.2.10) is a key enzyme related to the first‐step activation process of 5‐FU and has been shown to be an important enzyme that helps to predict sensitivity to 5‐FU and its related derivatives. We developed a new enzyme‐linked immunosorbent assay (ELISA) to accurately assess intratumoral activity of OPRT. A new sandwich ELISA was established using anti‐OPRT polyclonal antibodies obtained from the rabbit immunized with the recombinant human peptides of the OPRT molecule. OPRT levels were measured in eight human cancer xenografts and in 75 gastric cancer tissues using both a newly established ELISA and a conventional enzyme assay, using radiolabeled 5‐FU as a substrate. There was a significant correlation between OPRT levels measured by this ELISA and OPRT enzyme activity the in eight human cancer xenografts (r 2 = 0.782) and gastric carcinoma tissue (r 2 = 0.617). The ELISA system for OPRT requires a minimal amount of carcinoma tissue, making it an easy‐to‐use assay system to predict sensitivity to 5‐FU and its derivatives in gastric carcinoma. There was a significant correlation between tumor growth inhibition rates against the oral administration of oral‐uracil/tegafur (UFT) and OPRT enzyme activity in the human cancer xenografts (r 2 = 0.574). These results suggest that this newly developed sandwich ELISA system for the quantification of OPRT levels is technically simple, feasible and a useful tool to predict sensitivity to fluoropyrimidine‐based anticancer chemotherapy in patients with gastric carcinoma and other cancers. (Cancer Sci 2006; 97)
Although 5‐fluorouracil (5‐FU) and its derivatives have been important anticancer agents and have been widely applied for patients with advanced gastrointestinal cancers, the overall response rate of 5‐FU alone is still at best 10–20% for gastric carcinoma.( 1 ) Mechanisms of the anticancer effect, metabolic pathway and the enzymes involved in the metabolic process of 5‐FU have been studied extensively (Fig. 1). A variety of attempts have been made to enhance anticancer effects and to reduce toxic effects by modulating enzymes involved in the metabolic pathway of 5‐FU.( 2 ) Some of the enzymes involved in the metabolic process of 5‐FU, including thymidylate synthase and dihydropyrimidine dehydrogenase, have been shown to predict sensitivity to 5‐FU and/or prognosis.( 3 , 4 ) Based on the fact that the inhibitor of dihydropyrimidine dehydrogenase (DPD) has been shown to enhance the anticancer effects of 5‐FU,( 2 ) DPD‐inhibitory fluoropyrimidines (DIF) have been developed to enhance anticancer effects.( 2 ) To date, DIFs have played a major role in neoadjuvant and/or adjuvant chemotherapy for patients with advanced gastric carcinoma.( 5 )
Figure 1.
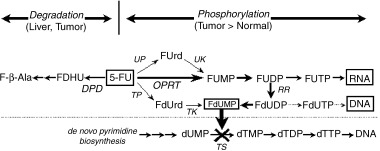
Phosphorylation and degradation pathway involved in 5‐fluorouracil (5‐FU) metabolism. DPD, dehydropyrimidine dehydrogenase (EC 1.3.1.2); OPRT, orotate phosphoribosyltransferase (EC 2.4.2.10); RR, ribonucleotide reductase (EC 1.17.4.1); TK, thymidine kinase (EC 2.7.1.21); TP, thymidine phosphorylase (EC 2.4.2.4); TS, thymidylate synthase (EC 2.1.1.45); UP, uridine phosphorylase (EC 2.4.2.3); UK, uridine/cytidine kinase (EC 2.7.1.48).
5‐FU administered in vivo is taken up by cancer cells and is phosphorylated to 5‐fluorouridine‐5′‐monophosphate (FUMP) with 5‐phosphoribosyl‐1‐phosphate (PRPP) as the cosubstrate,( 6 ) which is the first rate‐limiting step to elicit anticancer effects of 5‐FU. OPRT is the key enzyme involved in the main phosphorylation process. Along with TS and DPD, OPRT is another important enzyme in determining the anticancer effects of 5‐FU. Because the anticancer effect of DIF is unrelated to DPD activity, OPRT may be an important factor in predicting the anticancer effects of DIF. While enzyme assay of OPRT using radiolabeled 5‐FU as the substrate has previously been used, the assay procedure itself is quite complicated and tedious, which hampers processing of a large number of samples simultaneously. Furthermore, the accurate measurement of OPRT in patients in whom neoadjuvant anticancer chemotherapy with 5‐FU‐based anticancer agents is performed is hampered by the presence of 5‐FU in the resected cancer tissue specimens.
To overcome these problems, we developed a novel enzyme‐linked immunosorbent assay (ELISA) system to accurately measure OPRT activity in tissue samples, requiring a minimal amount of tissue samples. This study describes the validity of OPRT ELISA and OPRT levels in gastric carcinoma tissue. This ELISA system enabled accurate measurement of OPRT activity and may be a useful tool for predicting sensitivity to fluoropyrimidine‐based anticancer chemotherapy for patients with gastric carcinoma.
Materials and Methods
Materials and human cancer xenografts in nude mice
PRPP was purchased from Sigma‐Aldrich (Japan) and [6‐14C] 5‐FU (45 Ci/mmol) was purchased from Moravek Biochemicals. All other chemicals and biochemicals used in this study were commercial products of the highest quality available. The 18 human cancer xenografts, gastric cancer xenograft SC‐2, SC‐4, ST‐40 4‐1ST colon cancer xenograft Co‐3, HCT‐15, KM12C, KM20C, lung cancer xenograft LC‐6, Lu‐99, Lu‐130, Lu‐134, LX‐1 prostate cancer xenograft PC‐3, uterine cancer xenograft ME‐180, bile duct carcinoma xenograft TGBC12TKB, pancreatic cancer xenograft PAN‐4, PAN‐12 were used. These cancer xenografts were maintained by the serial transplantation in the dorsum of nude mice. HCT‐15 and PC‐3 were obtained from ATCC (VA, USA). KM12C and KM20C were kindly provided by Dr K. Morikawa (Iwamizawa Worker's Compensation Hospital, Hokkaido, Japan). TGBC2TKB was obtained from Riken Bioresourses Center (Ibaraki, Japan) and ME‐180 was purchased from the Human Science Research Bank (Osaka, Japan). SC‐2, SC‐4, ST‐40, Co‐3 LC‐6, Lu‐99, Lu‐134, PAN‐4 and PAN‐12 were established at the Central Institute for Experimental Animals (Kawasaki, Japan). LX‐1 was purchased from JCRB (Osaka Japan).
Patients and specimens
A total of 75 patients with advanced gastric carcinoma gave written informed consent for the specimens to be used for measurement. In total, 75 gastric carcinoma tissue specimens were obtained from 75 patients with advanced gastric carcinoma who underwent surgical resections at the First Educational Hospital, Fujita Health University School of Medicine. Wet weight of the cancer tissues was in the range of 22–147 mg. All specimens were obtained immediately after surgical resection and were immersed in liquid nitrogen and stored in a freezer at −80°C until analysis.
Preparation of anti‐OPRT polyclonal antibodies
Two anti‐OPRT polyclonal antibodies, anti‐OPRT‐A and anti‐OPRT‐C, were obtained according to the method described by Sakamoto et al.( 7 ) Briefly, rabbits were immunized with two different portions of the peptides of the recombinant human orotate phosphoribosyl transferase (rhOPRT). The two amino acid sequences, peptide A (CSTNQIPMLIRRKETKDYGTKRL, C86‐108) and peptide C (ISAADRLEAAEMYRKAAWEAY, C454‐474), synthesized in our laboratory and conjugated with bovine cycloglobulin, were used as the immunogens. After confirming the sufficient rise of the antiserum titer in rabbits, whole blood was collected, and the antiserum was obtained by centrifugation at 1500 × g. It was then purified by affinity chromatography on the immunogen peptide solid‐phased column. The affinity‐purified antibodies, anti‐OPRT‐A antibody obtained against N‐terminal OPRT peptide and anti‐OPRT‐C antibody obtained against C‐terminal OPRT peptide, were used for the development of the OPRT ELISA.
Detection of OPRT by immunoblotting
Reactivity and specificity of anti‐OPRT antibodies were examined by immunoblot analysis. Human lung cancer xenograft LC‐11 and rhOPRT were used as the standard samples. Briefly, three volumes of protein extraction buffer (10 mM Tris‐HCl [pH 7.4], 1 mM EDTA, 0.5 mM DTT) was added to the frozen cancer tissue samples and homogenized by ultrasonic homogenizer. The homogenate was then centrifuged at 10 500 × g at 4°C for 60 min. After the protein contents were measured by the bicinchoninic acid (BCA) method, electrophoresis was performed at 200V for 45 min using Bis‐Tris gel (NuPAGE 10% Bis‐Tris Gels, MOPS running buffer, Invitrogen, Japan) in reduced conditions. The electrophorezed proteins were electrically transferred on polyvinglidene difluoride (PVDF) membrane with DC 1 A at 4°C for 180 min. After the blocking treatment, the PVDF membrane was incubated with anti‐OPRT‐A and anti‐OPRT‐C antibodies at room temperature for 60 min, and then reacted with antirabbit IgG goat polyclonal antibody conjugated with AP‐labeled dextran at room temperature for 60 min. Reactivity with OPRT antibodies was detected with a chemical substrate (CDP‐Star, PerkinElmer, Japan) using a chemical detecting system (ProXPRESS 2D Proteomic Imaging System, PerkinElmer, Japan).
Measurement of OPRT enzymatic activity
OPRT enzyme activity was determined by a conventional enzyme assay using [6‐14C] 5‐FU as the substrate, according to the method originally described by Shirasaka et al.( 8 ) Briefly, protein extract was prepared from the cancer tissue specimens according to the method described for immunoblotting. Protein extracts were mixed with enzyme reaction buffer (50 mM Tris‐s‐HCl, pH 8.0, containing 100 mM NaF, 50 mM MgCl2, 40 mM PRPP, 5 mM DTT, 20 µM [6‐14C] 5‐FU) and incubated at 37°C for 10 min. After deproteinization caused by adding an equal volume of methanol, the amount of the substrate and the product was measured using radio‐high‐performance liquid chromatography (HPLC) (HPLC System; LC‐10AD, Shimadzu, Japan) and a detector (Flow‐One A500, Perkins Elmer, Japan). OPRT activity was determined from the quantities of the substrate and the product.
Establishment of sandwich ELISA system for the measurement of OPRT level
Sandwich type ELISA was established using anti‐OPRT‐A and anti‐OPRT‐C antibodies as the catcher and the tracer, respectively. Fifty microliters of 5.0 µg/mL anti‐OPRT‐C antibody dissolved in 0.1 M carbonate buffer (pH 9.6) was coated onto an EIA plate (Nunc 469949) at 4°C for 16 h. After washing with distilled water without phosphate‐buffered saline (PBS), blocking was performed with SabilCoat (SurModics) at room temperature for 60 min. Protein extracts of cancer tissue samples were diluted and were adjusted so the final concentration was 1 mg/mL. One‐hundred µL of protein extracts of tissue samples per well were added and incubated at room temperature for 60 min and were then washed five times with PBS. One‐hundred µL of peroxidase‐labeled anti‐OPRT‐A antibody per well was added and incubated at room temperature for 30 min. After seven washes with PBS, 100 µL of orthophenylene diamine (OPD) dissolved in substrate buffer (0.05 M citrate buffer, 0.01% H2O2, 1 mM EDTA) solution was added and incubated at room temperature for 30 min. The reaction was stopped by adding 0.5 N sulfate solution and the absorbance at 492 nm was measured with a plate reader (Spectramax 3400PC, Molecular Devices, Japan). Protein extract derived from human breast cancer cell line MDA‐MB‐435S, in which the OPRT protein was previously quantified by immunoblotting, was used as the standard.
Antitumor activities of UFT on cancer xenografts
Human tumor xenografts were prepared and maintained by serial transplantation into the right dorsum of nude mice. When the tumor volume reached approximately 200 mm3, the mice were randomly assigned into the treatment group and the control groups (six mice/group). Nude mice in the treatment group received oral‐uracil/tegafur (UFT) orally at the maximum tolerated dose (MTD) of 20 mg/kg/day, once daily for 14 consecutive days. Mice in the control group received 0.5% (w/v) HPMC solution orally for 14 consecutive days. Tumor volume was measured once a week after the start of drug administration. Tumor volume and relative tumor volume were calculated according to the following formula: tumor volume = (width)2 × (length)/2, and relative tumor volume = (mean tumor volume during treatment)/(mean tumor at the start of treatment). The following formula was used to calculate the antitumor effect: tumor growth inhibition rate (%) = (1‐the mean relative tumor volume of the treatment groups/the mean relative tumor volume of the control group). The tumor growth inhibition rate (%GIR) on day 15 was used as the indicator of the antitumor effect. Tumors were obtained on day 15 and immersed in liquid nitrogen and stored at −80°C for the measurement of OPRT enzyme activity and DPD levels.
DPD activity in cancer xenografts
DPD activity of the 18 human cancer xenografts was measured according to a conventional enzyme assay using a radiolabeled substrate. Briefly, enzyme solution obtained from a tumor was incubated with a reaction mixture containing 2 mM dithithreitol, 5 mM MgCl2, 20 mM [6‐14C]‐FU and 100 mM NADPH at 37°C for 10 or 30 min. The reaction was suspended by boiling and the mixture was centrifuged at 1500 × g for 10 min. An aliquot of supernatant was applied to a thin‐layer chromatography plate (silica gel 60 F254, Merk Darmstadt, Germany), which was first developed for approximately 5 cm with a mixture of 99% ethanol and 1 M ammonium acetate (5:1, v/v) and was then developed for approximately 15 cm in ethylether : acetone : chloroform : water (50:50:40:1, v/v/v/v). Each sample product was visualized and quantified using an imaging analyzer (BAS‐2000; Fuji, Tokyo, Japan).
Measurement of thymidine phosphorylase (TP) and DPD in human gastric carcinoma tissues
Tissue levels of TP and DPD in 46 gastric carcinoma tissues were measured using the sandwich ELISA established previously.( 9 ) TP and DPD values were measured to examine the correlations with OPRT levels.
Statistical analysis
The significance of correlation was evaluated with Fischer's Z transformation. P < 0.05 was considered statistically significant.
Results
Reactivity of anti‐OPRT antibodies in immunoblot analysis
Reactivity and specificity of OPRT‐A and OPRT‐C antibodies were examined using immunoblot analysis. A single band was clearly detected in the immunoblot analysis, indicating both OPRT‐A and OPRT‐C antibodies reacted with the 52.2 kDa OPRT molecule of the protein extract obtained from human lung cancer xenograft, LC‐11 (Fig. 2).
Figure 2.
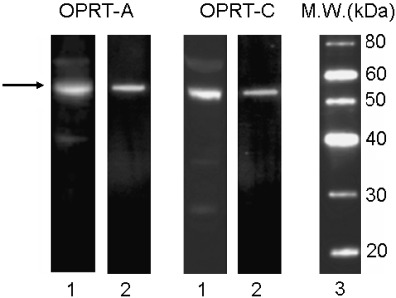
Reactivity of anti‐orotate phosphoribosyltransferase (OPRT) antibodies, OPRT‐A and OPRT‐C with human cancer xenograft and rhOPRT protein. Lane 1, protein extract of human lung cancer xenograft LC‐11; lane 2, rhOPRT protein; lane 3, molecular markers.
Standard curve of OPRT ELISA
Absorbance at 492 nm of the serially diluted rhOPRT protein, eight through 0.125 ng/well, was examined to evaluate the quantitative potential of the OPRT ELISA system (Fig. 3). A significant and linear correlation was observed between the absorbance and the OPRT concentration, for the range of OPRT concentrations tested (y = 0.0133x, r 2 = 0.983, P < 0.0001).
Figure 3.
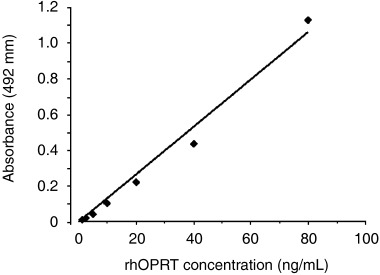
Standard curve of orotate phosphoribosyltransferase (OPRT) enzyme‐linked immunosorbent assay (ELISA). Absorbance at 492 nm and the serially diluted rhOPRT protein was closely correlated. P < 0.0001 by Fischer's Z‐transformation, y = 0.0133x, r 2 = 0.983.
Correlation between OPRT ELISA and enzyme assay in cancer xenografts
Correlation between OPRT values measured by OPRT ELISA and OPRT enzymatic activities obtained by the conventional enzyme assay were examined in eight human cancer xenografts in nude mice (Fig. 4). OPRT levels measured by OPRT ELISA were in the range between 50.8 and 232.8 ng/mg protein/min and the OPRT level in the tumor of the highest expression was 4.6 times the OPRT level in the tumor of the lowest expression. A significant correlation was observed between OPRT levels obtained by the OPRT ELISA system in human cancer xenografts and OPRT enzymatic activities measured by enzyme assay (y = 1.223x, r 2 = 0.782, P < 0.0001).
Figure 4.
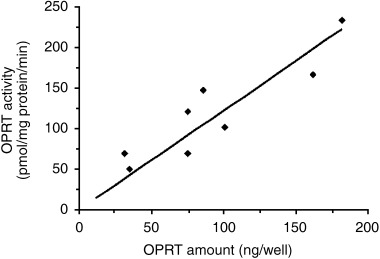
Correlation between orotate phosphoribosyltransferase (OPRT) level measured by enzyme‐linked immunosorbent assay (ELISA) and OPRT enzymatic activity measured by enzyme assay in human cancer xenografts. P < 0.0001 by Fischer's Z‐transformation, y = 1.223x, r 2 = 0.782.
OPRT levels measured by OPRT ELISA in gastric carcinoma tissues
The distribution of OPRT levels measured by OPRT ELISA in 75 gastric carcinoma tissues is shown in Figure 5. OPRT levels in gastric carcinoma tissue were in the range between 0.2 and 15.7 ng/mg protein and the mean value was 5.4 ± 3.6 ng/mg protein. OPRT levels varied widely and the highest OPRT level was 12.9 times the lowest OPRT level in gastric carcinoma. A significant correlation was observed between OPRT levels obtained by the OPRT ELISA system and OPRT enzymatic activities in gastric carcinoma tissue (y = 0.545x − 0.017, r 2 = 0.617, P < 0.0001) (Fig. 6).
Figure 5.
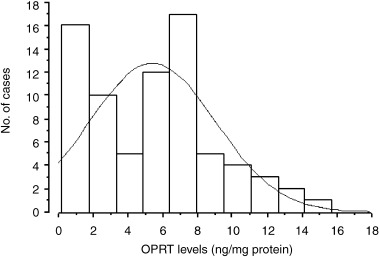
Distribution of orotate phosphoribosyltransferase (OPRT) levels in gastric carcinoma tissues (n = 75).
Figure 6.
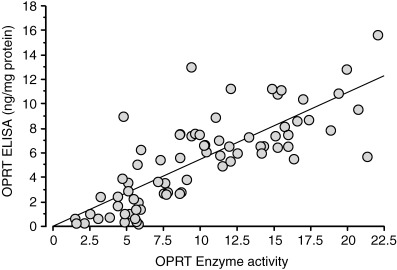
Correlation between orotate phosphoribosyltransferase (OPRT) level measured by enzyme‐linked immunosorbent assay (ELISA) and OPRT enzymatic activity measured by enzyme assay in 75 patients with advanced gastric carcinoma tissues. P < 0.001 by Fischer's Z‐transformation, y = 0.545x − 0.017, r 2 = 0.617.
Correlation between sensitivity to fluoropyrimidine and OPRT enzyme activity
Figure 7 (A,B) shows the correlation between the tumor growth inhibition rate (%GIR) against the administration of UFT and the OPRT enzyme activity, and between percentage GIR and the DPD levels in 18 cancer xenografts transplanted in nude mice. There was a significant correlation between percentage GIR and OPRT enzyme activity (P < 0.0001) by Fischer's Z‐transformation, y = 0.152x ± 27.6, r 2 = 0.574.
Figure 7.
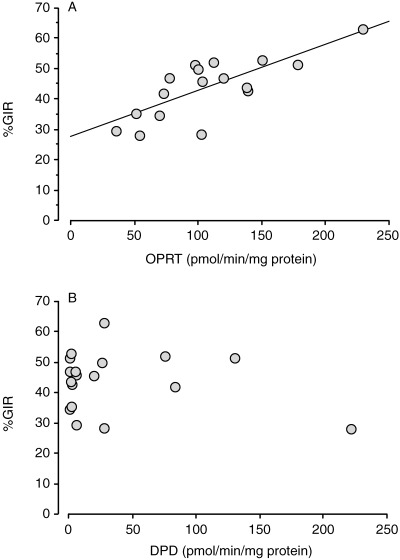
Correlation between the tumor growth inhibition rate (%GIR) against the administration of oral‐uracil/tegafur (UFT) and orotate phosphoribosyltransferase (OPRT) enzyme activity (A), and between percentage GIR and dihydropyrimidine dehydrogenase (DPD) levels (B) in 18 cancer xenografts transplanted in nude mice. There was a significant correlation between percentage GIR and OPRT enzyme activity. P < 0.0001 by Fischer's Z‐transformation, y = 0.152x ± 27.6, r 2 = 0.574.
Correlation of OPRT level with TP and DPD in human gastric carcinoma tissues
Correlation coefficients of OPRT levels measured by ELISA with TP and DPD were 0.042 (P = 0.80) and −0.292 (P = 0.071), respectively (Fig. 8A,B).
Figure 8.
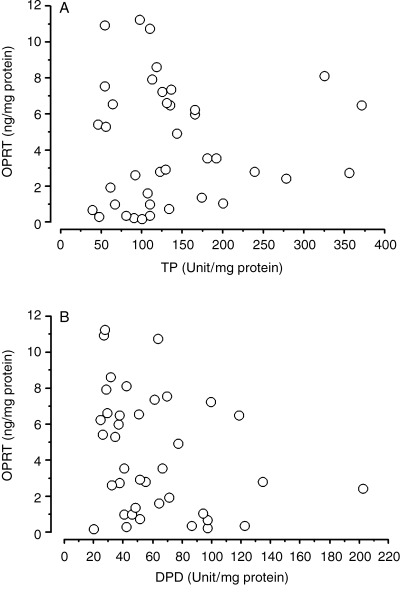
Correlation of orotate phosphoribosyltransferase (OPRT) level measured by enzyme‐linked immunosorbent assay (ELISA) and serum thymidine phosphorylase (TP) level (A), and dihydropyrimidine dehydrogenase (DPD) level (B).
Discussion
The primary aim of the present study was to establish a quick, simple and efficient assay system to accurately measure OPRT levels in gastric carcinoma tissue requiring a minimal amount of tissue. We have successfully developed a sandwich ELISA system to measure OPRT levels using two distinct polyclonal antibodies specifically raised against rhOPRT. OPRT levels were measured in eight human cancer xenografts and in 75 gastric carcinoma tissues. We found that there was a significant correlation between OPRT levels measured by the newly developed ELISA and OPRT enzyme activity, which validated the quantification of OPRT levels with the newly established OPRT ELISA. There was a significant correlation between the tumor growth inhibition rates against the oral administration of UFT and OPRT enzyme activity in the human cancer xenografts. OPRT levels were neither correlated with TP or DPD, suggesting that the OPRT level in gastric carcinoma tissues was independent of TP or DPD levels. The present study clearly demonstrated that a newly developed ELISA was technically simple and feasible and required a minimal amount of cancerous tissue. It also enabled the processing of a large number of samples simultaneously and to accurately measure OPRT levels, regardless of the amount of 5‐FU contained in cancer tissue after neoadjuvant chemotherapy.
OPRT is a 5‐FU‐anabolyzing enzyme and is a primary enzyme in the process of initial phosphorylation in human cancers.( 6 , 10 ) Orotate phosphoribosyltransferase (EC 2.4.2.10) and orotidine‐5′‐monophosphate decarboxylase (EC 4.1.1.23) were involved in the reactions of adding ribose‐5′‐phosphate to orotic acid decarboxylation of orotidine‐5′‐monophosphate. In vivo, OPRT is the enzyme that catalyzes the final two steps in the process of de novo pyrimidine synthesis.( 11 ) Three distinct pathways by which 5‐FU is activated into a nucleotide have been identified (Fig. 1):( 10 ) (1) directly converted to FUMP by OPRT in the presence of 5‐phosphoribosyl‐1‐pyrophosphate (PRPP) as a cosubstrate; (2) indirectly converted to FUMP in a sequence of reactions with conversion of 5‐FU to 5‐fluorouridine (FUR) catalyzed by a pyrimidine nucleoside phosphorylase (UP) with ribose‐1‐phosphate (Rib‐1‐P) as the cosubstrate; (3) indirectly to FdUMP by 2′‐deoxy‐5‐fluorouridine (FUdR) catalyzed by TP with deoxy‐Rib‐1‐P (dRib‐1‐P). Among these pathways, the direct pathway converting 5‐FU to FUMP has been shown to be predominant in tumor tissues,( 10 ) indicating that OPRT activity is mainly the rate limiting step in the phosphorylation process of 5‐FU. OPRT activity is therefore likely to be associated with the anticancer effects of 5‐FU.
Numerous basic and clinical evidence suggests that OPRT levels are associated with sensitivity to 5‐FU. Increased sensitivity to 5‐FU has been reported in vitro by the introduction of the E. coli UPRT gene that corresponds to the OPRT gene in humans in gastric cancer cell line.( 12 ) Recent clinical studies also demonstrated that increased expression of the OPRT gene is associated with an increased sensitivity to 5‐FU,( 13 , 14 ) suggesting that the expression of the OPRT gene is a useful predictor of sensitivity to 5‐FU. It is therefore reasonable to conclude that upregulation of OPRT activity increases phosphorylation of 5‐FU to FUMP, which is an active form of 5‐FU causing inhibition of f‐RNA synthesis. This was apparent by the fact that a significant positive correlation of OPRT with f‐RNA that integrated into RNA and thymidylate synthase inhibition rates after the intravenous administration of 5‐FU in patients with colorectal cancer,( 15 ) which suggested that OPRT may be closely related to sensitivity to 5‐FU. No data has been available in gastric carcinoma tissues. Because our preliminary results in gastric carcinoma in whom S‐1‐based adjuvant chemotherapy was performed indicated that survival of patients with high OPRT levels is better than those with low OPRT levels (data not published), it is likely that OPRT levels are related to the response to anticancer chemotherapy and prognosis in patients with gastric carcinoma. The results of the present study demonstrated that there was a significant correlation between the tumor growth inhibition rate (%GIR) against the administration of UFT and the OPRT enzyme activity, whereas there was no correlation between %GIR and DPD level, suggesting that OPRT levels could be used as a significant predictor of sensitivity against a fluoropyrimidine derivative. These results are in line with previous reports.
In the present study, there was a significant correlation between the OPRT levels measured by ELISA and OPRT enzyme activity, indicating that OPRT levels by the ELISA reflect OPRT enzyme activity. There are certain advantages of the ELISA system for OPRT over the conventional enzyme assay. OPRT enzyme activity measured by enzyme assay in tissues containing 5‐FU after neoadjuvant chemotherapy may be inaccurate, because unlabeled 5‐FU contained in cancer tissue causes underestimation of OPRT values by the competitive reaction with unlabeled 5‐FU. In contrast, the OPRT value measured by this sandwich ELISA is theoretically not influenced by the presence of 5‐FU in targeted cancerous tissues. Therefore, OPRT levels are accurately measured by this ELISA even in cases after neoadjuvant chemotherapy with fluoropyrimidines. The other advantage of OPRT ELISA was that this ELISA allows processing of a large number of samples simultaneously with minimal quantity of cancer tissue specimens required. In fact, whereas larger than 20 mg of cancerous tissues is required to measure OPRT enzyme activities with the enzyme assay, only 5 mg of tissue is required to measure OPRT levels with the OPRT ELISA. Furthermore, for the measurement of 60 samples, results are obtained within approximately 6 h with ELISA, whereas 2 days are at least required to obtain OPRT enzyme activities with a conventional enzyme assay. No radioisotope is needed for OPRT ELISA. As long as the antibodies, the standard substrate used in the ELISA system and the protein extracts of tissue samples are prepared immediately before the OPRT ELISA, OPRT levels obtained by the ELISA are quite reproducible and the interassay variability was confirmed to be within the minimal range (data not shown).
It has been reported that TS and/or DPD are associated with sensitivity to 5‐FU and the prognosis of patients with colorectal cancer.( 3 , 4 ) Despite a number of experimental results using colorectal, breast, or head and neck cancer cells suggested that overexpression of TS protein or high TS activity were associated with resistance to 5‐FU, Ishikawa et al.( 16 ) demonstrated that while there was no correlation between tumoral TS and sensitivity to 5‐FU, high DPD activity and DPD mRNA levels were associated with tumors with low sensitivity to 5‐FU in patients with gastric carcinoma. DPD is likely to be the most important factor that can predict sensitivity of 5‐FU, and thereby an inhibitor of DPD is an important research target to enhance the anticancer effects of 5‐FU based on the present results. Recent development of DIF has dramatically changed fluoropyrimidine‐based anticancer chemotherapeutic regimens.( 5 , 17 ) In fact, because the majority of recent first‐line chemotherapeutic regimens include DIF, S‐1, sensitivity to DIF, S‐1, rather than to 5‐FU, has now become more important to predict the response rate of new generation chemotherapeutic regimens for patients with gastric carcinoma. Prediction of sensitivity to S‐1 is therefore essential to determine the effective chemotherapeutic regimen for patients with gastric carcinoma as well as to determine whether or not neoadjuvant chemotherapy should be performed. Because response to S‐1 is unrelated to the level of DPD, the 5‐FU metabolism‐related enzymes, except for DPD, appear to be more important in predicting the effects of S‐1. Therefore, OPRT activity may be one of the most important factors predicting the sensitivity to S‐1‐based chemotherapy. Given the fact that DIF has been widely used as the primary fluoropyrimidine for gastric carcinoma in neoadjuvant or adjuvant settings, the significance of the measurement of OPRT activity in patients with advanced gastric carcinoma may further be increased in the future.
OPRT levels measured by the newly developed ELISA reflect OPRT enzyme activity. Our immunohistochemical analysis demonstrated that the OPRT protein has been shown to be expressed preferentially in cancerous tissue compared with its normal counterpart,( 18 , 19 ) suggesting that 5‐FU is phosphorylated preferentially in cancer cells, and thereby expression of the OPRT protein may determine anticancer effects to 5‐FU. We are now examining if there is a correlation between OPRT levels OPRT gene expression in gastric carcinoma. Because gene expression of OPRT has been reported to be related to the prognosis of colorectal carcinoma,( 20 ) OPRT gene expression seems to be associated with sensitivity to fluoropyrimidine‐based anticancer chemotherapy.
The present study clearly demonstrated no correlation of OPRT levels with the levels of TP and DPD, suggesting that OPRT level is a novel factor independent of the levels of other previously known enzymes related to 5‐FU metabolism. Because previous studies demonstrated that TP and/or DPD are related to sensitivity against 5‐FU,( 20 , 21 , 22 ) further studies are required to evaluate the clinical significance of OPRT levels in gastric carcinoma.
In summary, the ELISA system established in the present study for the measurement of OPRT appears to be clinically simple and feasible. Compared with enzyme assay, this method enables the processing of a large number of samples simultaneously with a minimal quantity of cancer tissue specimen required, and to accurately measure OPRT levels in patients in whom neoadjuvant chemotherapy with fluoropyrimidine is performed. Thus, the measurement of OPRT in cancer tissue may be useful for the prediction and monitoring of the anticancer effects of S‐1‐based anticancer chemotherapy for patients with advanced gastric carcinoma and other types of cancers. Further accumulation of evidence is required to determine the clinical relevance and significance of the measurement of OPRT levels in patients with gastric carcinoma who receive neoadjuvant and/or adjuvant chemotherapy.
Acknowledgments
This study was supported by a grant from Fujita Health University.
References
- 1. Macdonald JS, Gohmann JJ. Chemotherapy of advanced gastric cancer: present status, future prospects. Semin Oncol 1988; 15: 42–9. [PubMed] [Google Scholar]
- 2. Shirasaka T, Nakano K, Takechi T et al. Antitumor activity of 1 M tegafur‐0.4 M 5‐chloro‐2,4‐dihydroxypyridine‐1 M potassium oxonate (S‐1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 1996; 56: 2602–266. [PubMed] [Google Scholar]
- 3. Etienne MC, Cheradame S, Fischel JL et al. Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol 1995; 13: 1663–70. [DOI] [PubMed] [Google Scholar]
- 4. Johnston PG, Lenz HJ, Leichman CG et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5‐fluorouracil in human colorectal and gastric tumors. Cancer Res 1995; 55: 1407–12. [PubMed] [Google Scholar]
- 5. Schoffski P. The modulated oral fluoropyrimidine prodrug S‐1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 2004; 15: 85–106. [DOI] [PubMed] [Google Scholar]
- 6. Fukushima M, Murakami Y, Suzuki K. The analysis of the innate pathways of 5‐fluorouracil phosphorylation in human gastrointestinal cancer cell lines in vitro and in vivo. Oncol Rep 1997; 4: 1189–94. [DOI] [PubMed] [Google Scholar]
- 7. Sakamoto K, Sugimoto Y, Miyadera K, Oka T, Fukushima M. Preparation of anti‐OPRT antibody for immunochemical detection. Jpn J Cancer Chemother 2005; 32: 653–8. [PubMed] [Google Scholar]
- 8. Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5‐fluorouracil without loss of its antitumor activity in rats. Cancer Res 1993; 53: 4004–9. [PubMed] [Google Scholar]
- 9. Kurebayashi J, Yamamoto Y, Udagawa K, Okubo S, Fukushima M, Sonoo H. Establishment of enzyme‐linked immunosorbent assays for thymidylate synthase and dihydropyriminide dehydrogenase in cancer tissues. Oncol Rep 2004; 11: 973–9. [PubMed] [Google Scholar]
- 10. Peters GJ, Van Groeningen CJ, Laurensse EJ, Pinedo HM. A comparison of 5‐fluorouracil metabolism in human colorectal cancer and colon mucosa. Cancer 1991; 68: 1903–199. [DOI] [PubMed] [Google Scholar]
- 11. Suttle DP, Bugg BY, Winkler JK, Kanalas JJ. Molecular cloning and nucleotide sequence for the complete coding region of human UMP synthase. Proc Natl Acad Sci USA 1988; 85: 1754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inaba M, Sawada H, Sadata A, Hamada H. Circumvention of 5‐fluorouracil resistance in human stomach cancer cells by uracil phosphoribosyltransferase gene transduction. Jpn J Cancer Res 1999; 90: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujii R, Seshimo A, Kameoka S. Relationships between the expression of thymidylate synthase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase and cell proliferative activity and 5‐fluorouracil sensitivity in colorectal carcinoma. Int J Clin Oncol 2003; 8: 72–8. [DOI] [PubMed] [Google Scholar]
- 14. Isshi K, Sakuyama T, General T et al. Predicting 5‐FU sensitivity using human colorectal cancer specimens: comparison of tumor dihydropyrimidine dehydrogenase and orotate phosphoribosyl transferase activities with in vitro chemosensitivity to 5‐FU. Int J Clin Oncol 2002; 7: 335–42. [DOI] [PubMed] [Google Scholar]
- 15. Katsumata K, Tomioka H, Sumi T et al. Correlation between clinicopathologic factors and kinetics of metabolic enzymes for 5‐fluorouracil given to patients with colon carcinoma by two different dosage regimens. Cancer Chemother Pharmacol 2003; 51: 155–60. [DOI] [PubMed] [Google Scholar]
- 16. Ishikawa Y, Kubota T, Otani Y et al. Dihydropyrimidine dehydrogenase and messenger RNA levels in gastric cancer: possible predictor for sensitivity to 5‐fluorouracil. Jpn J Cancer Res 2000; 91: 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshida I, Sakurai Y, Komori Y et al. Successful downstaging by S‐1‐based chemotherapy followed by surgical resections for gastric carcinoma with extensive distant lymph node metastasis – report of two cases and a review of cases with surgical resection after downstaging by S‐1‐based chemotherapy. Hepatogastroenterology 2005; 52: 978–84. [PubMed] [Google Scholar]
- 18. Kamoshida S, Shiogama K, Shimomura R et al. Immunohistochemical demonstration of fluoropyrimidine‐metabolizing enzymes in various types of cancer. Oncol Rep 2005; 14: 1223–30. [PubMed] [Google Scholar]
- 19. Kamoshida S, Sakamoto N, Matsuoka H et al. Heat‐assisted stretching of paraffin sections on hot plate weakens immunoreactivity of orotate phosphoribosyltransferase. Acta Histochem Cytochem 2005; 38: 69–74. [Google Scholar]
- 20. Ichikawa W, Uetake H, Shirota Y et al. Both gene expression for orotate phosphoribosyltransferase and its ratio to dihydropyrimidine dehydrogenase influence outcome following fluoropyrimidine‐based chemotherapy for metastatic colorectal cancer. Br J Cancer 2003; 89: 1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evrard A, Cuq P, Robert B, Vian L, Pelegrin A, Cano JP. Enhancement of 5‐fluorouracil cytotoxicity by human thymidine‐phosphorylase expression in cancer cells: in vitro and in vivo study. Int J Cancer 1999; 80: 465–70. [DOI] [PubMed] [Google Scholar]
- 22. Salonga D, Danenberg KD, Johnson M et al. Colorectal tumors responding to 5‐fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 2000; 6: 1322–7. [PubMed] [Google Scholar]


