Abstract
Dietary phytochemicals, including nobiletin and auraptene, have been shown to exert inhibiting effects in several chemically induced carcinogenesis models. We here investigated the influence of nobiletin and auraptene on prostate carcinogenesis using transgenic rats developing adenocarcinoma of the prostate (TRAP) bearing the SV40 T antigen transgene under control of the probasin promoter and human prostate cancer cells. Starting at 5 weeks of age, male TRAP rats received powder diet containing 500 p.p.m. nobiletin or auraptene, or the basal diet for 15 weeks and then were sacrificed for analysis of serum testosterone levels and histological changes. The body and relative prostate weights and serum testosterone levels did not differ among the groups. Since all animals developed prostate carcinomas, these were semiquantitatively measured and expressed as relative areas of prostate epithelial cells. Nobiletin caused significant reduction in the ventral (P < 0.01), lateral (P < 0.001) and dorsal (P < 0.05) prostate lobes, while decreasing high grade lesions (P < 0.05) in the ventral and lateral lobes. Feeding of auraptene also effectively reduced the epithelial component (P < 0.05) and high grade lesions (P < 0.05), in the lateral prostate. A further experiment demonstrated that growth of androgen sensitive LNCaP and androgen insensitive DU145 and PC3 human prostate cancer cells, was suppressed by both nobiletin and to a lesser extent auraptene in a dose‐dependent manner, with significant increase in apoptosis. In conclusion, these compounds, particularly nobiletin, may be valuable for prostate cancer prevention. (Cancer Sci 2007; 98: 471–477)
Prostate cancer is the most common malignant disease overall in the US and western countries and the second leading cause of cancer‐related deaths among men.( 1 ) It has been estimated that 234 460 new cases of prostate cancer will be diagnosed and that 27 350 deaths related to prostate cancer will occur in the US alone in 2006.( 1 ) There are potentially curative options such as radical prostatectomy or radiotherapy, but once the disease is metastatic, the outlook is poor. Therefore chemoprevention or chemical control of prostate cancer has become an important priority. Since the intake of citrus fruits has been found to be beneficial for cancer prevention by epidemiological survey,( 2 ) we here focused attention on nobiletin (5,6,7,8,3′,4′‐hexamethoxyflavone), a polymethoxy‐flavonoid extracted from citrus fruits such as oranges.( 3 ) It has been shown to exert antibacterial,( 4 ) anti‐inflammatory,( 5 , 6 ) antimutagenic,( 7 ) antioxidative( 6 ) and antitumor initiation( 8 ) effects, while also inhibiting proliferation of human squamous cancer cells,( 9 ) and hepatocarcinoma cells,( 10 ) and suppressing the production of matrix metalloproteinase‐1, ‐7 and ‐9.( 11 , 12 , 13 ) In vivo studies have demonstrated that nobiletin inhibits peritoneal dissemination of gastric cancer in severe combined immunodeficiency (SCID) mice,( 14 ) as well as azoxymethane (AOM)‐induced large bowel carcinogenesis.( 15 ) Thus this chemical appears to be a promising agent for suppression of cancer cell induction, invasion and metastasis.
Auraptene, a prenyloxycoumarin antioxidant agent also isolated from the citrus fruits such as natsumikans or grapefruit,( 16 , 17 ) has similarly been found to exert preventive potential, suppressing 12‐0‐tetradecanoylphorbol‐13‐acetate (TPA)‐induced tumor promotion in mouse skin,( 16 ) and chemically induced carcinogenesis in large bowel,( 18 ) oral cavity,( 19 ) esophagus,( 20 ) and liver( 21 , 22 ) in rats. Recently, it was reported that dietary administration of auraptene inhibits colitis‐related colon carcinogenesis,( 23 ) and lung metastasis of melanoma cells in mice.( 24 )
We hypothesized that citrus nobiletin or auraptene may afford chemopreventive effects against prostate cancer in in vivo and in vitro, and therefore investigated their influence on the transgenic rats developing adenocarcinoma of the prostate (TRAP) model which was generated in our laboratory and features well‐differentiated prostate adenocarcinomas in all prostatic lobes in a short period (15 weeks of age).( 25 , 26 , 27 , 28 , 29 ) The TRAP rat has a transgene which encodes SV40/Tag under probasin promoter, so that the developed carcinomas are androgen‐dependent. Furthermore, we examined chemotherapeutic effects and possible mechanisms using human prostate cancer cells in vitro.
Materials and Methods
Animals and chemicals. TRAP rats for the experiment were obtained by mating heterologous males, established in our laboratory with a Sprague‐Dawley (SD) genetic background,( 25 , 30 ) with wild‐type female SD rats (Clea, Tokyo, Japan). The hybrid litters were screened by PCR, as described previously,( 29 ) and male transgenic rats were selected for use in the experiment. All animals were housed 3/plastic cage on wood‐chip bedding in an air‐conditioned specific pathogen‐free (SPF) animal room at 22 ± 2°C and 55 ± 5% humidity with a 12 h light/dark cycle and fed powdered basal diet (Oriental MF, Oriental Yeast Co., Tokyo, Japan), with or without chemical supplements, and water ad libitum. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medical Sciences. Nobiletin (> 98% purity; lot no. 050621) and auraptene (>98% purity; lot no. 040220) were purchased from Nard Chemicals Co., Ltd. (Amagasaki, Hyogo, Japan). The doses of the test compounds for the animal experiments were selected based on previous chemopreventive studies.( 18 )
Animal experimental protocols, blood collection and tissue sampling. Starting at 5 weeks of age, 27 rats in three groups (9 rats per group) received powder diet containing 500 p.p.m. nobiletin or auraptene, or basal diet including 2% corn oil. Body weight and food consumption were recorded biweekly. At 20 weeks of age, animals in all groups were injected intraperitoneally 1 hour before sacrifice with 5‐bromo‐2′‐deoxyuridine (BrdU) solution (100 mg/kg body weight). Under deep ether anesthesia, blood was collected to measure testosterone hormone levels. The urogenital complex of each rat was harvested as a whole together with the seminal vesicles, then fixed in 10% phosphate‐buffered formalin. Livers, kidneys, spleens, lungs, testes and tongues were also removed, weighed and fixed. After fixation for 48 h, the ventral, dorsal, lateral, anterior prostate lobes and seminal vesicles were carefully dissected into individual lobes whenever possible, and each was weighed. The tissues were routinely processed to paraffin embedded sections and stained with hematoxylin and eosin (H&E).
Histopathology and immunohistochemistry. The prostatic lesions, including adenocarcinomas (ACs) and prostatic intraepithelial neoplasm (PINs), were histopathologically diagnosed as described previously.( 25 , 28 ) Since multiple proliferative epithelial lesions develop in this model, semiquantitative analysis was performed. First, additional slides stained with Azan, which showed clear contrast between epithelium and secreted material, were made. Then, relative areas of proliferating epithelium within acini were recorded with the help of an image processor for analytical pathology (IPAP: Sumika Technos Co., Osaka, Japan). Each acinus was then graded as 1 (predominantly consists of normal epithelium), 2 (predominantly consists of PIN) and 3 (predominantly consists of adenocarcinoma) for comparison among the groups. Immunohistochemistry for BrdU and SV/40 large T antigen was performed using the monoclonal mouse anti‐BrdU antibodies (Dako, Glostrup Denmark) and a mouse anti‐SV40 large T antigen monoclonal (Clone: PAb 101, BD PharMingen, San Diego, CA, USA), respectively. Binding was visualized with a Vectastain Elite ABC kit (Vector Laboratory, Burlingame, CA, USA) followed by light hematoxylin counterstaining to facilitate microscopic examination.
Cell culture. LNCaP, DU145, and PC3 human prostate carcinoma cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured as monolayers in RPMI medium 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% heat‐inactivated fetal bovine serum (FBS) and 0.5% penicillin/streptomycin in a 5% CO2 atmosphere at 37°C in a humidified incubator. Nobiletin and auraptene were dissolved in dimethyl sulfoxide (DMSO, 1% v/v final) with incubation at 37°C for 2–5 h and diluted with medium to prepare a 10 mL 1 × 10−3 M solution, then filtered through a 0.22 µm millipore filter (Millipore, Billerica, MA, USA) to avoid bacteria infection just before treatment.
Cell viability and dose responses. The effect of nobiletin or auraptene on cell viability was determined with a 2‐(4‐iodophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulfophenyl)‐2H‐tetrazolium salt monosodium salt (WST‐1) assay.( 31 ) LNCaP (0.4 × 104 cells/well), DU145 (0.3 × 104 cells/well) and PC3 (0.4 × 104 cells/well) cells were plated in 96‐well culture plates with varying concentrations of nobiletin (0, 1 × 10−6, 1 × 10−5, 5 × 10−5, 1 × 10−4 and 5 × 10−4 mol/L) or auraptene (0, 1 × 10−5, 5 × 10−5, 1 × 10−4, 5 × 10−4 and 1 × 10−3 mol/L) in 200 µL of culture medium in quadruplicate. After 48 h of treatment, 20 µL WST‐1 was added to each well and incubated for 90 min at 37°C, then each well was measured for absorbance at the wavelength of 430 nm. Percentage cell viability was determined relative to vehicle‐treated control cells, arbitrarily assigned 100% viability.
Dose response effect of these chemicals was also investigated at narrow range of concentrations in either a T25 flask or 6‐well plate, in which apoptosis and cell cycle analysis would be performed. LNCaP cells were preincubated in T25 flasks (1 × 105 cells/flask), and DU145 and PC3 cells in 6‐well plates (1.8 × 104 and 3 × 104, respectively). After 24 h incubation, the medium was exchanged by a fresh medium containing test chemicals at various concentrations, as shown in Fig. 1a–c, or the vehicle. After 72 h incubation, trypan blue resistant, live cell numbers were counted under a microscope. This was performed in triplicate
Figure 1.
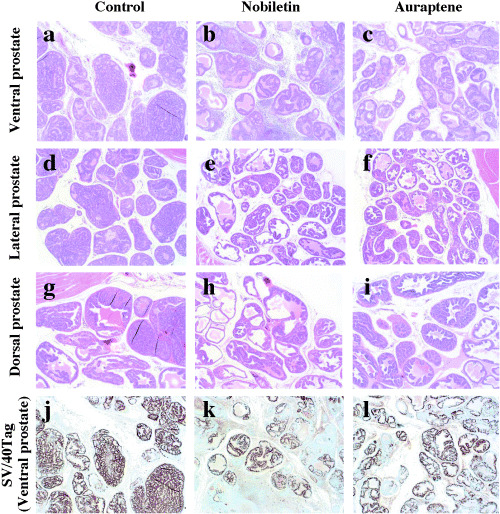
Representative histological obseravation of ventral (a–c), lateral (d–f) and dorsal (g–i) prostate (hematoxylin and eosin, ×4) of transgenic rats developing adenocarcinoma of the prostate treated with vehicle control, nobiletin or auraptene. a, d, g: vehicle control group with moderatly differentiated adenocarcinoma composed of atypical epithelial cells forming glandular and cribriform structures. b, e, h: nobiletin‐treated group with reduced epithelial area in ventral, lateral and dorsal prostate. c, f, i: auraptene‐treated group with reduced epithelial area in lateral prostate (f). j–l: representative imunohistochemical staining of SV/40 Tag in ventral prostate. The SV/40 Tag expression in nobiletin‐treated group (k) are found decreased in adenocarcinoma as compared with control group (j).
Quantification of apoptotic cells. We optimized the concentrations of the chemicals so that growth inhibition rates were about 50% of the vehicle‐treated control value (IC50) after 3 days incubation for further study. Nobiletin and auraptene‐induced apoptosis in human prostate cancer cells was determined by annexin assays with a Guava Nexin kit (Guava Technologies, Inc. Hayward CA, USA) according to the manufacturer's protocol. Briefly, prostate cancer cells were treated with chemicals as described above for 72 h, trypsinized and washed three times with 1 mL of Nexin buffer. They were then centrifuged for collection and resuspended in 50 µL of Nexin buffer. Aliquots of 5 µL of Annexin V‐PE and 5 µL of 7‐amino‐actinomycin D (7‐AAD) were added to 40 µL of cells, and the mixtures were incubated on ice for 20 min under shielding from light, then analyzed with a cell analyzer, Guava PCA (Guava Technologies, Inc.)
Cell cycle analysis. After 72 h treatment of prostate cancer cells with nobiletin or auraptene, cells were trypsinized, washed twice with ice‐cold phosphate‐buffered saline containing 1% FBS, resuspended in 500 µL of staining solution containing 2% RNase soluion, 2.5% propidium iodide and 0.1% Triton X‐100 for each sample and the cell cycle was analyzed with Guava PCA and Multicycle AV software for Windows (Phoenix Flow Systems, San Diego, CA, US).
Statistical analysis. The statistical significance of differences among the control and chemical treatment groups was determined by Scheffe's tests. Dose‐dependent cell growth inhibition with regard to chemical concentration and cell viability was assessed with Spearman's correlation coefficients (ρ). P < 0.05 was considered statistically significant.
Results
General observations in the animal experiment. Dietary administration of test compounds of nobiletin or auraptene did not cause any adverse effects such as on the growth of rats during the study. There was no significant differences in food intake (data not shown) or the final body weights as well as absolute and relative prostate weights among the groups (Table 1). Histologically, there were no pathological lesions in the liver, kidneys, spleen, lung and testes suggesting toxicity of nobiletin or auraptene. The serum testosterone levels in rats given nobiletin or auraptene did not significantly differ from the control values (Table 1). All the TRAP rats developed prostate adenocarcinomas to some degree, so that no differences were present in the incidences of PIN and prostate cancer among the three groups. However, clear differences were noted in histopathological appearances of the prostate. The ventral and lateral prostate of untreated control TRAP rats were densely occupied by adenocarcinoma clusters (Fig. 2a.d.g) with reduced acinic lumina, while those of nobiletin or auraptene‐treated TRAP rats were less dense, and presence of luminal spaces was more prominent (Fig. 2b,c,e,f,h,i). Atrophic glands with degenerative alteration of tumor epithelial cells were observed with infiltration of inflammatory cells, this being particularly frequent in the ventral prostate of nobiletin‐treated TRAP rats.
Table 1.
Final body weights, relative prostate weights and serum testosterone concentrations
| Treatment | No. of rats | Body weights (g) | Relative prostate weights (%) | Serum testosterone(ng/mL) | |
|---|---|---|---|---|---|
| Whole | Ventral | ||||
| Control | 9 | 493.71 ± 40.35 | 0.96 ± 0.23 | 0.088 ± 0.021 | 2.02 ± 0.68 |
| Nobiletin | 9 | 473.15 ± 47.17 | 0.88 ± 0.23 | 0.087 ± 0.035 | 1.99 ± 1.08 |
| Auraptene | 9 | 492.56 ± 36.97 | 0.83 ± 0.20 | 0.075 ± 0.024 | 2.28 ± 0.79 |
Values are means ± SD.
Figure 2.
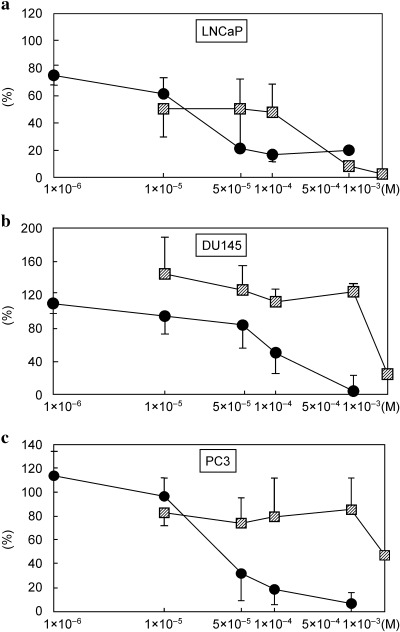
Effects of nobiletin and auraptene on cell viability of prostate cancer cells (Fig. 2). The cells were exposed to a wide range of nobiletin or auraptene concentrations for 48 h, and viability of the cells was determined by WST‐1 assay. Both nobiletin and auraptene caused significant dose‐dependent growth inhibition in LNCaP (a) P < 0.0001 (ρ = −0.87) and P < 0.0001 (ρ = −0.90), respectively) and nobiletin also was effective in DU145 cells (b) P = 0.0002 (ρ = −0.78) and PC3 cells (c) P < 0.0001 (ρ = −0.87). Vehicle‐treated cells were regarded as 100% viable, cell viabilities are depicted as percentages. The data represent the means of quadruplicate data. –•– Nobiletin, –▨– Auraptene.
Quantitative analysis of carcinoma development. Data for relative areas of proliferating epithelial components in the ventral, lateral, dorsal and anterior prostate lobes are summarized in Table 2. Dietary administration of nobiletin significantly reduced the relative epithelial component of the ventral (P < 0.01), lateral (P < 0.001) and dorsal prostate (P < 0.05) lobes as compared with the control values. In addition, nobiletin‐treated TRAP rats significantly increased grade 2 acini, predominantly containing PIN, and decreased grade 3 acini with carcinomas in the ventral and lateral prostate as compared with control TRAP rats (Table 3). Feeding with auraptene also effectively reduced the relative epithelial component in the lateral prostate (P < 0.05) compared with the control rats (Table 2) and raised the proportion of grade 2 acini (P < 0.05), and lowered grade 3 acini (P < 0.05) in the lateral prostate as compared with the control values (Table 3).
Table 2.
Relative areas of epithelial components within acini
| Treatment | No. of rats | Epithelium/acinus (%) | ||||
|---|---|---|---|---|---|---|
| Ventral | Lateral | Dorsal | Anterior | Seminal vesicle | ||
| Control | 9 | 77.63 ± 3.28 | 88.09 ± 3.91 | 64.75 ± 7.08 | 25.22 ± 5.41 | 13.58 ± 4.38 |
| Nobiletin | 9 | 65.58 ± 7.64** | 75.34 ± 6.68*** | 54.11 ± 8.69* | 25.91 ± 4.25 | 13.92 ± 3.42 |
| Auraptene | 9 | 70.36 ± 6.42 | 78.58 ± 7.13* | 55.26 ± 8.76 | 22.99 ± 3.71 | 14.22 ± 4.03 |
P < 0.05,
P < 0.01,
P < 0.001 vs. untreated cotrol transgenic rats developing adenocarcinoma of the prostate, Scheffe's test. Values are means ± SD.
Table 3.
Quantitative evaluation of prostate lesions (%)
| Treatment | No. of rats | Ventral | Lateral | ||||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | ||
| Control | 9 | 0.00 ± 0.00 | 2.94 ± 1.95 | 97.06 ± 1.95 | 0.00 ± 0.00 | 4.93 ± 3.38 | 95.07 ± 3.38 |
| Nobiletin | 9 | 0.20 ± 0.44 | 8.52 ± 6.83* | 91.28 ± 7.22* | 0.00 ± 0.00 | 18.23 ± 9.35* | 81.77 ± 9.35* |
| Auraptene | 9 | 0.05 ± 0.14 | 4.57 ± 3.33 | 95.39 ± 3.46 | 0.00 ± 0.00 | 18.05 ± 12.48* | 81.95 ± 12.48* |
P < 0.05 vs. untreated control transgenic rats developing adenocarcinoma of the prostate, Scheffe's test; values are mean ± SD; Grade 1: Predominantly consists of normal epithelium; Grade 2: Predominantly consists of PIN; Grade 3: Predominantly consists of adenocarcinoma.
Immunohistochemical findings. The mean BrdU labeling indices (Table 4) of adenocarcinomas in the ventral prostate were significantly decreased by nobiletin treatment (P < 0.05), with a slight tendency for reduction with auraptene (P = 0.198) as compared with the control. Suppression of BrdU labeling indices was also observed in the lateral, dorsal and anterior prostate lobes of the nobiletin group and in the lateral prostate of the auraptene group, but without significance. In PIN, BrdU indices tend to be reduced by nobiletin or auraptene in the ventral and lateral prostate, but the differences were not significant. On the other hand, the PIN BrdU labeling indices were lower than those for adenocarcinomas in all the prostate lobes, while only the dorsal and anterior prostate lobes of control (both P < 0.01) and auraptene‐treated animals (P < 0.01 and 0.05, respectively) showed significant differences.
Table 4.
BrdU labeling indices in prostate cancers and PINs (%)
| Treatment | No. of rats | Ventral | Lateral | Dorsal | Anterior |
|---|---|---|---|---|---|
| In adenocarcinoma | |||||
| Control | 9 | 11.72 ± 3.49 | 13.49 ± 7.20 | 9.78 ± 3.68 | 8.41 ± 2.19 |
| Nobiletin | 9 | 8.03 ± 2.42* | 9.32 ± 2.09 | 6.97 ± 1.93 | 6.34 ± 1.86 |
| Auraptene | 9 | 9.42 ± 1.60 | 9.67 ± 3.75 | 9.57 ± 3.15 | 8.07 ± 2.37 |
| In PIN | |||||
| Control | 9 | 9.60 ± 1.35 | 8.16 ± 1.24 | 5.13 ± 1.42 ‡ | 4.91 ± 1.55 ‡ |
| Nobiletin | 9 | 8.22 ± 3.06 | 7.79 ± 1.89 | 5.63 ± 1.93 | 5.16 ± 2.46 |
| Auraptene | 9 | 8.17 ± 2.51 | 7.79 ± 1.82 | 5.66 ± 0.91 ‡ | 5.54 ± 1.68 † |
P < 0.05 vs. control of corresponding lobes and lesion;
P < 0.05,
P < 0.01 vs. adenocarcinoma of corresponding lobes and treatment; Scheffe's test, Values are mean ± SD; BrdU, 5‐bromo‐2′‐deoxyuridine; PIN, prostatic intraepithelial neoplasm.
SV40/T‐antigen oncoprotein was clearly detected immunohistochemically in areas of PIN and adenocarcinomas in the ventral, lateral, dorsal and anterior prostate (Table 5, Fig. 2j–l). The incidence of the SV40/T‐antigen positive cells of adenocarcinoma in ventral prostate was significantly decreased (P < 0.05) in the nobiletin‐treated animals. In the auraptene‐treated rats, values for adenocarcinomas in the ventral prostate were lower than in control TRAP rats, but the difference was not statistically significant (Table 5).
Table 5.
SV40/Tag expression ratios in prostate cancers (%)
| Treatment | No. of rats | Ventral | Lateral | Dorsal | Anterior |
|---|---|---|---|---|---|
| Control | 9 | 79.02 ± 10.53 | 88.36 ± 3.72 | 67.33 ± 8.28 | 58.66 ± 6.87 |
| Nobiletin | 9 | 62.09 ± 11.06* | 82.69 ± 6.14 | 68.09 ± 10.56 | 54.80 ± 7.96 |
| Auraptene | 9 | 68.25 ± 10.66 | 82.94 ± 7.50 | 64.74 ± 8.28 | 62.37 ± 6.58 |
P < 0.05 vs. untreated control transgenic rats developing adenocarcinoma of the prostate, Scheffe's test. Values are mean ± SD.
Dose‐dependent growth inhibition in human prostate cancer cells. WST‐1 assays performed in 96‐well plates with a wide range of concentrations of nobiletin or auraptene demonstrated significant dose‐dependent growth inhibition in LNCaP, DU145 and PC3 cells, except in the auraptene‐case with DU145 and PC3 cells (Fig. 3a–c). Cell counting for a narrow range of concentrations in T25 flasks or 6‐well plates also revealed both nobiletin and auraptene to cause significant dose‐dependent growth inhibition in these three cell lines (Fig. 1a–c). Significantly greater growth inhibition was consistently observed with lower doses of nobiletin than with auraptene.
Figure 3.
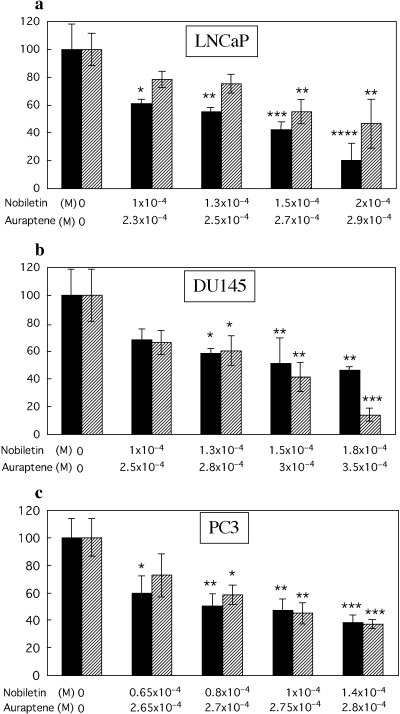
Effects of nobiletin and auraptene in narrow range of concentration on cell viability of LNCaP (a), DU145 (b) and PC3 (c), evaluated with alive cell numbers after 72 h incubation. Significantly dose‐dependent growth inhibition in all the three cells is seen LNCaP (a): nobiletin, P = 0.0004 (ρ = −0.95), auraptene, P = 0.0007 (ρ = −0.90); DU145 (b): nobiletin, P = 0.001 (ρ = −0.87), auraptene, P = 0.0004 (ρ = −0.95); PC3 (c): nobiletin, P = 0.001 (ρ = −0.87), auraptene, P = 0.0006 (ρ = −0.92). Columns, mean of three experiments; bars, SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, versus control. ▪ Nobiletin, ▨ Auraptene.
Annexin assays. At the optimal IC50 concentrations (LNCaP, nobiletin 1.3 × 10−4, auraptene 2.8 × 10−4 mol/L; DU145, nobiletin 1 × 10−4, auraptene 2.7 × 10−4 mol/L; PC3, nobiletin 0.65 × 10−4, auraptene 2.7 × 10−4 mol/L), nobiletin and auraptene induced more apoptosis of LNCaP, DU145 and PC3 cells as compared with the vehicle (Fig. 4). Values were significantly increased for early, late and/or total apoptosis in all these cell lines treated with nobiletin or auraptene.
Figure 4.
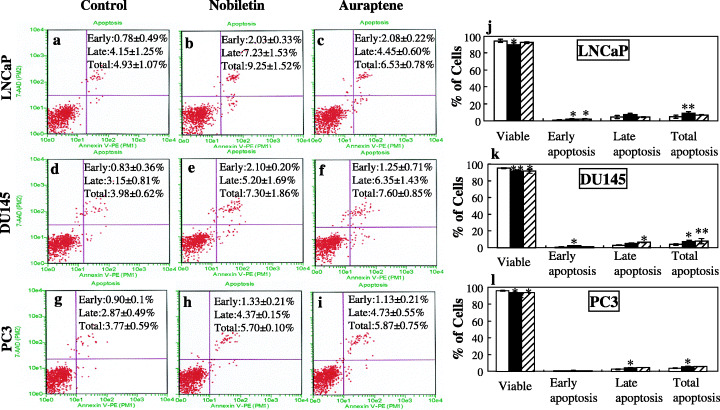
Effects of nobiletin and auraptene on apoptosis induction in human prostate carcinoma cells. LNCaP cells (a–c); DU145 cells (d–f) and PC3 cells (g–i) were treated with vehicle or nobiletin or auraptene for 72 h, then harvested for analysis of apoptosis with the Guava Nexin Kit. Lower right quadrant, indicates early apoptotic cells (Annexin V‐PE stained cells); upper right quadrant, indicates late apoptotic cells (Annexin V‐PE and 7‐AAD stained cells); lower left quadrant shows viable cells. The percentage of viable cells and early, late and total apoptosis data are summarized in j–l. Columns, mean of three experiments; bars, SD. *P < 0.05; **P < 0.01, versus control. □ Control, ▪ Nobiletin, ▨ Auraptene.
Cell cycle analysis. Based on the effects of nobiletin or auraptene on the inhibition of cell proliferation and viability, we also examined possible inhibitory effects of the chemicals on progression through the cell cycle. Compared with the vehicle (G0/G1, 59.6 ± 2.3%; S, 5.5 ± 0.4%; G2/M, 24.5 ± 1.4%), treatment of LNCaP cells with nobiletin or auraptene at about IC50 concentration for 72 h resulted in a significantly higher ratio of cells in the G0/G1 phase (75.2 ± 2.2%, P < 0.0001; 74.0 ± 1.7%, P < 0.0001, respectively), with reduction in S phase (3.2 ± 0.4%, P < 0.0001; 3.6 ± 0.3%, P < 0.001, respectively) and G2/M phase (13.3 ± 5.1%, P < 0.01; 16.8 ± 0.6%, P < 0.05, respectively) cells (Fig. 5a–c,j). Treatment of DU145 cells with nobiletin or auraptene also resulted in significantly higher levels of G0/G1 phase arrest (62.8 ± 3.1%, P < 0.05 and 66.3 ± 2.2%, P < 0.01, respectively), compared with the vehicle (G0/G1, 56.9 ± 2.1%) (Fig. 5d–f,k). With PC3 cells, nobiletin treatment resulted in a significantly higher level of G2/M accumulation (31.9 ± 2.2%, P < 0.05) compared with the control (G2/M, 26.3 ± 0.9%). Such increase was not found with the auraptene treatment (26.5 ± 1.8%, P = 0.98) (Fig. 5g–i,l).
Figure 5.
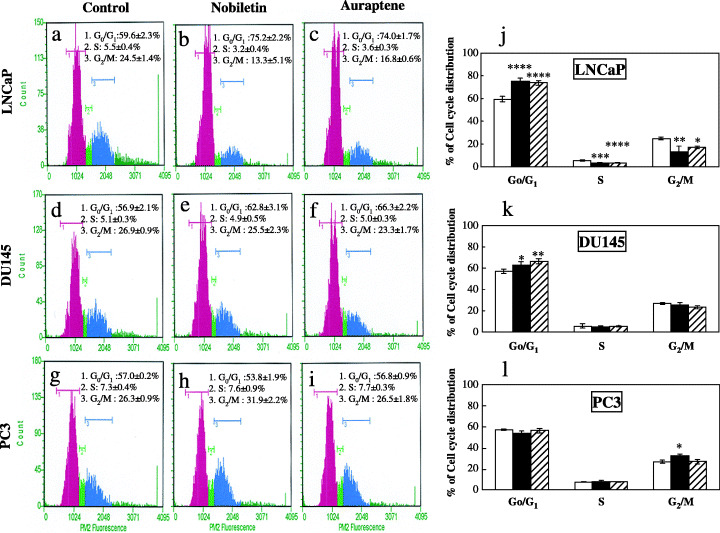
Effects of nobiletin and auraptene on cell cycle progression of human prostate carcinoma cells. Cell cycle distribution in LNCaP cells (a–c) after treatment with vehicle and nobiletin or auraptene. Cell cycle distribution of DU145 and PC3 are shown in d–f and g–i, respectively. Summaries of cell cycle distribution data for LNCaP, DU145 and PC3 cells are shown in j–l. Columns, mean of three independent experiments; bars, SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, versus non‐treated control group. □ Control, ▪ Nobiletin, ▨ Auraptene.
Discussion
The present study provided clear evidence that the two antioxidants nobiletin and auraptene can protect against prostate cancer development in a transgenic model and also suppress proliferation by cell lines. This is in line with the earlier finding that antioxidants such as green tea polyphenols can significantly inhibit prostate cancer development and increase survival rate in transgenic adenocarcinoma mouse prostate (TRAMP) mice,( 32 ) also bearing the SV/40 T antigen transgene under control of the probasin promoter.
Compared with chemical carcinogen induced models, including examples with 3,2′‐dimethyl‐4‐aminobiphenyl (DMAB) and 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP),( 33 ) which take 40 weeks (20 weeks after DMAB treatment for 20 weeks) and over a year, respectively, induction of prostate cancer in TRAP rats is extremely strong and rapid, even compared to the TRAMP mouse model. In TRAP rats, PIN and adenocarcinoma are found at 4 weeks and 15 weeks, respectively,( 25 , 26 ) but in the TRAMP mice they are formed at 10 weeks and 18 weeks of age, respectively.( 34 ) Cancer development in the TRAP rat largely depends on androgens, because SV40 T antigen production and cancer as well as prostate glands themselves are under the control of androgen action.( 25 , 29 ) Therefore it might be expected that no effects on the incidence would be exerted without reducing the testosterone level and/or expression of SV40 T antigen in the prostate. In consequence, it is biologically significant that nobiletin or auraptene treatment here clearly suppressed tumor expansion without change in testosterone level. The fact that nobiletin and auraptene caused a shift from adenocarcinoma to PIN indicates delayed or suppressed prostate tumor progression. This might be related to inhibition of cell proliferation as revealed by reduced BrdU‐labeling indices in nobiletin‐treated rats and morphometric analysis of the relative areas of epithelium in both nobiletin and auraptene‐treated rats.
In our in vitro experiment, nobiletin or auraptene also significantly inhibited the proliferation of human prostate cancer cells, LNCaP, DU145 and PC3, in a dose‐dependent fashion, and reduced their viabilities accompanied by cell cycle arrest and apoptosis induction, good markers of preventive activity.( 35 ) It was earlier reported that the flavonoid baicalin also caused G0/G1 arrest in LNCaP with enhanced expression of p27kip1( 36 ) and in DU145( 37 ) and the isoflavone genistein caused G2/M arrest both in LNCaP and PC3 cells,( 38 , 39 ) with down‐regulation of cyclin B and up‐regulation of p21WAF1( 39 ) and in DU145.( 40 ) Whether the same mechanisms contribute to nobiletin and auraptene induction of G0/G1 arrest in LNCaP and DU145 cells, and G2/M arrest in PC3 remains to be elucidated. Apoptosis induction is arguably the most potent defense against cancer progression,( 41 ) and our findings for early, late or total apoptosis in LNCaP, PC3 and DU145 cells are consistent with other in vivo studies regarding nobiletin and auraptene( 15 , 21 ) as well as in vitro,( 36 , 37 , 42 ) studies of other candidates in prostate cancer cell lines.
It has been reported that lycopene, another antioxidant, exerted beneficial effects on prostate cancer prevention in an observation study and neoadjuvant intervention trial.( 43 )β‐carotene and green tea were found to be chemopreventive for prostate cancer development in a case‐control study, but had no influence in phase III and phase II studies, respectively.( 43 ) From the present experiments, both nobiletin and auraptene should have good potential as prostate cancer preventors, but further careful investigations are required before human application.
In conclusion, the present study provided the first evidence of inhibitory effects of nobiletin and auraptene on prostate cancer in both in vivo and in vitro systems. The mechanisms appear to involve a nonandrogen‐mediated pathway with induction of apoptosis and cell cycle arrest and the data suggest that nobiletin and auraptene may possess strong potential for development as chemopreventive agents against human prostate cancer.
Acknowledgments
This research was supported partly by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan, a Grant‐in‐Aid for the 2nd Term Comprehensive 10‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan, and a grant from the Society for Promotion of Pathology of Nagoya, Japan.
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–30. [DOI] [PubMed] [Google Scholar]
- 2. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991; 2: 325–57. [DOI] [PubMed] [Google Scholar]
- 3. Li S, Yu H, Ho CT. Nobiletin: efficient and large quantity isolation from orange peel extract. Biomed Chromatogr 2006; 20: 133–8. [DOI] [PubMed] [Google Scholar]
- 4. Jayaprakasha GK, Negi PS, Sikder S, Rao LJ, Sakariah KK. Antibacterial activity of Citrus reticulata peel extracts. Z Naturforsch [C] 2000; 55: 1030–4. [DOI] [PubMed] [Google Scholar]
- 5. Murakami A, Nakamura Y, Ohto Y et al. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti‐inflammatory polymethoxyflavonoid. Biofactors 2000; 12: 187–92. [DOI] [PubMed] [Google Scholar]
- 6. Murakami A, Nakamura Y, Torikai K et al. Inhibitory effect of citrus nobiletin on phorbol ester‐induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 2000; 60: 5059–66. [PubMed] [Google Scholar]
- 7. Wall ME, Wani MC, Manikumar G et al. Plant antimutagenic agents, 2. Flavonoids. J Nat Prod 1988; 51: 1084–91. [DOI] [PubMed] [Google Scholar]
- 8. Nishino H, Tokuda H, Satomi Y et al. Cancer prevention by antioxidants. Biofactors 2004; 22: 57–61. [DOI] [PubMed] [Google Scholar]
- 9. Kandaswami C, Perkins E, Soloniuk DS, Drzewiecki G, Middleton E Jr. Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Lett 1991; 56: 147–52. [DOI] [PubMed] [Google Scholar]
- 10. Ohnishi H, Asamoto M, Tujimura K et al. Inhibition of cell proliferation by nobiletin, a dietary phytochemical, associated with apoptosis and characteristic gene expression, but lack of effect on early rat hepatocarcinogenesis in vivo. Cancer Sci 2004; 95: 936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishiwa J, Sato T, Mimaki Y, Sashida Y, Yano M, Ito A. A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J Rheumatol 2000; 27: 20–5. [PubMed] [Google Scholar]
- 12. Sato T, Koike L, Miyata Y et al. Inhibition of activator protein‐1 binding activity and phosphatidylinositol 3‐kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases‐1 production and suppression of production of matrix metalloproteinases‐1 and ‐9 in human fibrosarcoma HT‐1080 cells. Cancer Res 2002; 62: 1025–9. [PubMed] [Google Scholar]
- 13. Kawabata K, Murakami A, Ohigashi H. Nobiletin, a citrus flavonoid, down‐regulates matrix metalloproteinase‐7 (matrilysin) expression in HT‐29 human colorectal cancer cells. Biosci Biotechnol Biochem 2005; 69: 307–14. [DOI] [PubMed] [Google Scholar]
- 14. Minagawa A, Otani Y, Kubota T et al. The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn J Cancer Res 2001; 92: 1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki R, Kohno H, Murakami A et al. Citrus nobiletin inhibits azoxymethane‐induced large bowel carcinogenesis in rats. Biofactors 2004; 22: 111–4. [DOI] [PubMed] [Google Scholar]
- 16. Murakami A, Kuki W, Takahashi Y et al. Auraptene, a citrus coumarin, inhibits 12‐O‐tetradecanoylphorbol‐13‐acetate‐induced tumor promotion in ICR mouse skin, possibly through suppression of superoxide generation in leukocytes. Jpn J Cancer Res 1997; 88: 443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogawa K, Kawasaki A, Yoshida T et al. Evaluation of auraptene content in citrus fruits and their products. J Agric Food Chem 2000; 48: 1763–9. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka T, Kawabata K, Kakumoto M et al. Citrus auraptene exerts dose‐dependent chemopreventive activity in rat large bowel tumorigenesis: the inhibition correlates with suppression of cell proliferation and lipid peroxidation and with induction of phase II drug‐metabolizing enzymes. Cancer Res 1998; 58: 2550–6. [PubMed] [Google Scholar]
- 19. Tanaka T, Kawabata K, Kakumoto M et al. Chemoprevention of 4‐nitroquinoline 1‐oxide‐induced oral carcinogenesis by citrus auraptene in rats. Carcinogenesis 1998; 19: 425–31. [DOI] [PubMed] [Google Scholar]
- 20. Kawabata K, Tanaka T, Yamamoto T et al. Suppression of N‐nitrosomethylbenzylamine‐induced rat esophageal tumorigenesis by dietary feeding of auraptene. J Exp Clin Cancer Res 2000; 19: 45–52. [PubMed] [Google Scholar]
- 21. Sakata K, Hara A, Hirose Y et al. Dietary supplementation of the citrus antioxidant auraptene inhibits N,N‐diethylnitrosamine‐induced rat hepatocarcinogenesis. Oncology 2004; 66: 244–52. [DOI] [PubMed] [Google Scholar]
- 22. Hara A, Sakata K, Yamada Y et al. Suppression of beta‐catenin mutation by dietary exposure of auraptene, a citrus antioxidant, in N,N‐diethylnitrosamine‐induced hepatocellular carcinomas in rats. Oncol Rep 2005; 14: 345–51. [PubMed] [Google Scholar]
- 23. Kohno H, Suzuki R, Curini M et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis‐related colon carcinogenesis in mice. Int J Cancer 2006; 118: 2936–42. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka T, Kohno H, Murakami M, Kagami S, El‐Bayoumy K. Suppressing effects of dietary supplementation of the organoselenium 1,4‐phenylenebis (methylene) selenocyanate and the Citrus antioxidant auraptene on lung metastasis of melanoma cells in mice. Cancer Res 2000; 60: 3713–6. [PubMed] [Google Scholar]
- 25. Asamoto M, Hokaiwado N, Cho YM et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 2001; 61: 4693–700. [PubMed] [Google Scholar]
- 26. Cho YM, Takahashi S, Asamoto M et al. Age‐dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: lack of influence of carcinogen or testosterone treatment. Cancer Sci 2003; 94: 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hokaiwado N, Asamoto M, Cho YM, Tsuda H, Shirai T. Lack of effect of human c‐Ha‐ras proto‐oncogene overexpression on prostate carcinogenesis in probasin/SV40 T antigen transgenic rats. Cancer Sci 2003; 94: 1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kandori H, Suzuki S, Asamoto M et al. Influence of atrazine administration and reduction of calorie intake on prostate carcinogenesis in probasin/SV40 T antigen transgenic rats. Cancer Sci 2005; 96: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Said MM, Hokaiwado N, Tang M et al. Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by leuprorelin, a luteinizing hormone‐releasing hormone agonist. Cancer Sci 2006; 97: 459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asamoto M, Hokaiwado N, Cho YM, Shirai T. Effects of genetic background on prostate and taste bud carcinogenesis due to SV40 T antigen expression under probasin gene promoter control. Carcinogenesis 2002; 23: 463–7. [DOI] [PubMed] [Google Scholar]
- 31. Ishiyama M, Tominaga H, Shiga M et al. A combined assay of cell viability and in vitro cytotoxicity with a highly water‐soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 1996; 19: 1518–20. [DOI] [PubMed] [Google Scholar]
- 32. Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA 2001; 98: 10350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucia MS, Bostwick DG, Bosland M et al. Workgroup I. rodent models of prostate cancer. Prostate 1998; 36: 49–55. [DOI] [PubMed] [Google Scholar]
- 34. Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen‐independent prostate cancer progression in the TRAMP model. Cancer Res 1997; 57: 4687–91. [PubMed] [Google Scholar]
- 35. Chen C, Kong AN. Dietary cancer‐chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci 2005; 26: 318–26. [DOI] [PubMed] [Google Scholar]
- 36. Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC‐SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate 2001; 49: 285–92. [DOI] [PubMed] [Google Scholar]
- 37. Gu ZQ, Sun YH, Xu CL, YL. Study of baicalin in inducing prostate cancer cell line DU145 apoptosis in vitro. Zhongguo Zhong Yao Za Zhi 2005; 30: 63–6. [PubMed] [Google Scholar]
- 38. Cao F, Jin TY, Zhou YF. Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene. Biomed Environ Sci 2006; 19: 35–41. [PubMed] [Google Scholar]
- 39. Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein‐induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer 1998; 32: 123–31. [DOI] [PubMed] [Google Scholar]
- 40. Oki T, Sowa Y, Hirose T et al. Genistein induces Gadd45 gene and G2/M cell cycle arrest in the DU145 human prostate cancer cell line. FEBS Lett 2004; 577: 55–9. [DOI] [PubMed] [Google Scholar]
- 41. Tolomeo M, Simoni D. Drug resistance and apoptosis in cancer treatment: development of new apoptosis‐inducing agents active in drug resistant malignancies. Curr Med Chem Anticancer Agents 2002; 2: 387–401. [DOI] [PubMed] [Google Scholar]
- 42. Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett 2000; 160: 219–28. [DOI] [PubMed] [Google Scholar]
- 43. Nelson PS, Montgomery B. Unconventional therapy for prostate cancer: good, bad or questionable. Nat Rev Cancer 2003; 3: 845–58. [DOI] [PubMed] [Google Scholar]


