Abstract
(Cancer Sci 2010; 101: 609–615)
Valproic acid (VPA), a histone deacetylase inhibitor, upregulates NKG2D ligands (NKG2DLs) on some monocytic and lymphoid leukemic cells. However, its effect on myeloid leukemia cells and synergistic agents that can augment the effect of VPA remains unknown. Of the various myeloid cell lines examined, OUN‐1, a chronic myelogenous leukemia cell line, showed the most prominent upregulation of MICA/B and ULBP2 in response to VPA. The NKG2DL upregulation was observed only in leukemic cells without apoptosis and the effect was abrogated by pretreatment of cells with caffeine, an inhibitor of ATM/ATR. Several activators of ATM/ATR were screened for their effect on NKG2DL expression, but only hydroxyurea (HU) efficiently upregulated both MICA/B and ULPB2 expression on the cell line. VPA and HU synergistically upregulated the NKG2DLs on OUN‐1 cells as well as primary leukemic cells from some patients with acute myeloid leukemia. The upregulation of NKG2DLs by VPA and/or HU was associated with increased transcription of each NKG2DL gene. OUN‐1 cells treated with VPA + HU were more susceptible to killing by natural killer (NK) cells than untreated cells and the enhanced cytotoxicity of NK cells was blocked by the treatment of NK cells with anti‐NKG2D monoclonal antibodies. The same concentrations of VPA and HU did not affect the cytotoxicity of NK cells against OUN‐1 cells. These data suggest that VPA and HU might enhance the NK cell‐mediated antileukemia effect by increasing the susceptibility of myeloid leukemic cells to NK cells.
Natural killer (NK) cells play an essential role in the eradication of myeloid leukemia cells after allogeneic stem cell transplantation.( 1 , 2 , 3 ) Several lines of evidence indicate that the expression level of NKG2D ligand (NKG2DL) on leukemia cells affects the sensitivity of the leukemic cells to killing by NK cells.( 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ) Various agents have been evaluated for their inducibility of NKG2DLs on leukemic cells, to augment the NK cell‐mediated antileukemia effect.( 4 , 8 , 9 , 12 , 13 , 14 ) Valproic acid (VPA), a histone deacetylase inhibitor, is a potent inducer of NKG2DLs such as MICA/B and ULBPs on malignant cells.( 9 , 12 , 14 ) VPA augments the expression of MICA and ULBP2 on several monocytic and lymphoid leukemia cell lines and primary acute myeloid leukemia (AML) cells in vitro and in vivo.( 9 , 12 ) However, the mechanisms for the upregulation of NKG2DL on AML cells by VPA has not been studied extensively due to the lack of myeloid leukemia cell lines that display an upregulation of NKG2DLs in response to VPA. Clarifying the mechanisms associated with upregulation could identify other reagents that synergize with VPA to augment the expression of NKG2DL by myeloid leukemia cells and thereby enhance the susceptibility of leukemic cells to NK cells.
The expression of NKG2DL is mediated by the activation of ATR and ATM.( 10 , 15 , 16 ) Several reagents, including hydroxyurea (HU) and cisplastin, as well as physical stress, such as ionizing irradiation, activate ATM/ATR.( 17 , 18 ) Some of the ATR activators might not only induce apoptosis of myeloid leukemia cells( 19 ) but might also synergize with VPA in the induction of NKG2DL expression in leukemic cells, and thereby enhance the sensitivity of the leukemic cells to NK cell‐mediated cytotoxicity if the agents do not impair the NK cell function.
This study screened various myeloid leukemia cell lines for the upregulation of NKG2DL expression induced by VPA and investigated mechanisms for the upregulation. This report describes the synergistic effect of HU with VPA in the induction of NKG2DL on myeloid leukemia cells that were not susceptible to apoptosis.
Materials and Methods
Leukemia cells. Myeloid cell lines derived from chronic myeloid leukemia, including KH88, SAS413, and OUN‐1, and an AML cell line, NB4, were kindly provided by Dr. Masaki Yasukawa of Ehime University (Matsuyama, Japan). K562 was purchased from the Health Science Research Resources Bank (Osaka, Japan). A Burkitt’s lymphoma cell line Daudi, T‐cell leukemia cell lines Molt‐4 and Jurkat, an AML cell line HL60, a chronic myeloid leukemia cell line KU812, and monocytic leukemia cell lines U937 and THP‐1 were purchased from RIKEN BRC (Ibaraki, Japan). These cell lines were maintained in RPMI‐1640 supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FBS. Primary leukemic cells were isolated using the density gradient method from either the peripheral blood or bone marrow aspirates containing >90% leukemic cells of five patients with AML aged between 25 and 52 years. The leukemic cells were cryopreserved until use. All patients provided their informed consent for the use of their samples and this study protocol was approved by the institutional ethical committee.
Monoclonal antibodies and reagents. Valproic acid, HU, cisplatin, CPT‐11, and doxorubicin were purchased from Sigma (Kyowa Hakko Kogyo, Tokyo, Japan). Caffeine, cyclosporine, FK506, SB202190, BAPTA‐AM, PD98059, and JNK‐1were purchased from Sigma. mAbs specific to MICA/B, ULBP1, ULBP2, ULBP3, and anti‐NKG2D were purchased from R&D Systems (Minneapolis, MN, USA). FITC‐conjugated goat antimouse IgG, annexin V–phycoerythrin (PE), anti‐CD3‐PE, anti‐CD56‐PE‐Cy5, and anti‐CD107a‐FITC were all purchased from BD PharMingen (San Diego, CA, USA).
Flow cytometry. The cells were stained with the appropriate Abs or the respective isotype control Abs, followed by incubation with FITC‐conjugated goat antimouse IgG. An annexin V–PE apoptosis detection kit I from BD PharMingen was used according to the instructions of the manufacturer. Data acquisition and a flow cytometric analysis were carried out on a BD FACSCalibur using the CellQuest software package (BD Biosciences).
RNA extraction and real‐time PCR. RNA was isolated using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. The reverse transcription of 1 μg RNA into cDNA was carried out using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and stored at −20°C until use. The quantification of NKG2DL gene expression was carried out using a LightCycler (Roche Diagnostics, Tokyo, Japan) with specific primers. The LightCycler with a GAPDH primer kit (Search‐LC, Heidelberg, Germany) was used for quantification of mRNA for GAPDH, a housekeeping gene, in the same samples. The relative amount of NKG2DL mRNA to GAPDH mRNA (NKG2DL/GAPDH) was used to represent expression levels of the NKG2D‐L gene.( 20 ) PCR was carried out using standard conditions. The PCR used the sense primer 5′‐GCCATGAACGTCAGGAATTT‐3′ and antisense primer 5′‐GACGCCAGCTCAGTGTGATA‐3′ for MICA, and the sense primer 5′‐TTACTTCTCAATGGGAGACTGT‐3′ and antisense primer 5′‐TGTGCCTGAGGACATGGCGA‐3′ for ULBP2. The housekeeping gene GAPDH was used as a loading control sense primer 5′‐CTATTCGATGCCGTGTATGC‐3′, and antisense primer 5′‐GCCTGGTCCAGACTTCTTTC‐3′.
Natural killer cell preparation. Peripheral blood mononuclear cells (PBMCs) were isolated from a healthy individual and two patients with AML using density gradient centrifugation. Then 106 PBMCs were cultured with 2 × 105 irradiated (45 Gy) K562 cells transfected with the membrane‐bound form of interleukin‐15 and human 4‐1BBL (K562‐mb15‐41BBL) in RPMI‐1640 containing 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 100 IU/mL interleukin‐2 for 14 d.( 21 ) The cultured PBMCs contained >90% CD3−CD56+CD16+ NK cells. In some experiments, the cultured NK cells were incubated in the presence of VPA or VPA + HU, washed once with PBS, then used as effect cells.
Drug treatment of leukemic cells. Leukemic cell lines were cultured for 24 h in 24‐well tissue culture plates at 37°C and 5% CO2 in the absence or presence of different drug concentrations. The following therapeutic drugs were tested: 200 μg/mL VPA; 20 μg/mL HU; 10 μM cisplatin; 100 μM CPT‐11; and 0.05 μM doxorubicin. Primary AML cells were cultured for 48 h in the complete medium containing growth factors including Flt3 ligand at 100 ng/mL, stem cell factor at 100 ng/mL, and granulocyte macrophage colony stimulating factor at 20 ng/mL (all from Amgen, Thousand Oaks, CA, USA)( 22 ) with or without VPA and/or HU. In some experiments, leukemic cells were incubated for 1 h at 37°C in the presence of 5 mM caffeine, 1 μM cyclosporine, 1 μM FK506, 10 μM SB202190, 5 μM BAPTA‐AM, 10 μM PD98059, or 5 μM JNK‐1 prior to culture with VPA.
Cytotoxicity assay. NK cell cytotoxicity against leukemic cell lines was assessed using the standard chromium release assay, as described previously.( 23 ) In blocking experiments, anti‐NKG2D Abs were added to the NK cell suspension at 10 μg/mL and incubated at 37°C for 30 min before the addition of target cells. The percentage of specific lysis was calculated using the formula: 100 × (count per minute [cpm] released from test sample − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release).
CD107a mobilization assay. Two microliters of FITC‐CD107a mAb was added to the suspension of effector and target cell mixtures in 96‐well round microplates and incubated for 3 h at 37°C. One microliter of 2 mM monencin (Sigma) in 100% ethanol was included in the cell suspension to prevent the acidification of the endosomal compartment, which could alter the fluorescence of internalized CD107a:FITC‐CD107a mAb complexes. After the incubation, the plate was centrifuged to the pellet cells and the supernatant was removed. Cell–cell conjugate was disrupted by washing the cell with PBS supplemented with 0.02% azide and 0.5 mM EDTA. Samples were then mixed vigorously then stained with mAbs specific for CD3 and CD56, followed by flow cytometric analysis.( 24 )
Statistical method. Differences in the expression levels of NKG2DL in leukemia cell lines were assessed using Student’s t‐test.
Results
Upregulation of NKG2DL on leukemic cell lines induced by VPA. Several myeloid and lymphoid leukemic cells were examined for the expression of MICA/B and ULBP1–3 before and after incubation in the presence of VPA in order to identify a cell line suitable for the screening of the agents that can augment VPA‐induced NKG2DL expression. Three myeloid cell lines, as well as Molt‐4 and Jurkat, showed apparent upregulation of both MICA/B and ULBP2 after incubation with VPA (Fig. 1a,b). Time‐course experiments revealed 24 h of incubation in the presence of VPA to be optimal for the maximum induction of MICA/B and ULBP2 in OUN‐1 cells. The upregulation shown in the histogram was most evident in OUN‐1 cells (Fig. 1a), thus, this cell line was chosen for the further analyses.
Figure 1.
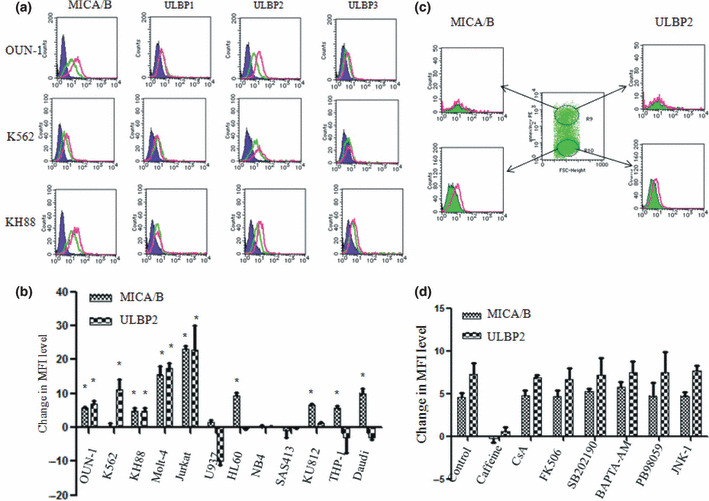
Upregulation of NKG2D ligand (NKG2DL) expression on leukemic cell lines by treatment with valproic acid (VPA). (a) Histograms of NKG2DL expression on three myeloid leukemia cell lines are shown. Green lines, untreated cells; purple lines, isotype control; red lines, VPA treated cells. (b) Changes in the mean fluorescence intensity (MFI) level of MICA/B and ULBP2 in various leukemic cell lines. Each column represents the difference in the MFI level calculated by subtracting the MFI level of untreated cells from that of VPA treated cells. The differences in the MFI levels are indicated as the mean + SD from three independent experiments. *P < 0.05. (c) Selective upregulation of NKG2DLs by VPA treatment on non‐apoptotic OUN‐1 cells. Apoptotic and non‐apoptotic cells defined by the expression of annexin V were separately assessed for the expression of NKG2DLs. Green lines, untreated cells; red lines, VPA treated cells. (d) Effect of various reagents on VPA‐induced MICA/B and ULBP2 expression by OUN‐1 cells. OUN‐1 cells were pretreated with the indicated reagent for 1 h and were incubated in the presence of VPA. Each column represents the difference in the MFI level calculated by subtracting the MFI level of untreated cells from that of VPA treated cells. BAPTA‐AM, calcium chelator; CsA, cyclosporine; FK506, tacrolimus; JNK‐1, an inhibitor of JNK; PD98059, an inhibitor of ERK1/2; SB202190, an inhibitor of p38MAPK.
Agents that upregulate NKG2DLs on leukemic cells induce apoptosis( 19 , 25 , 26 , 27 ) and examination of the total cell population after VPA treatment might underestimate the inducibility of NKG2DL by the agents due to the presence of apoptotic cells. Indeed, when apoptotic and non‐apoptotic cells were examined separately after VPA treatment, the apparent upregulation of MICA/B and ULBP2 was only observed in annexin V‐negative non‐apoptotic cells (Fig. 1c). The mean fluorescence intensity levels of MICA/B on VPA treated or untreated OUN‐1 cells were 10.4 ± 2.3 and 4.8 ± 1.5 (P < 0.05), respectively; the mean fluorescence intensity levels of ULBP2 on VPA treated or untreated OUN‐1 cells were 12.9 ± 2.9 and 6.1 ± 1.7 (P < 0.05), respectively. Therefore, only the annexin V‐negative cell population was observed in the subsequent analyses.
Although little is known about the mechanisms for the upregulation of NKG2DL by VPA, the expression of NKG2DL is controlled by ATM/ATR kinase and various signaling pathways affect the expression of NKG2DL by T cells.( 16 , 28 ) Various inhibitors were tested for their ability to block NKG2DL expression to determine which pathway mediates the upregulation of NKG2DL in OUN‐1 cells (Fig. 1d). Only caffeine, an ATM/ATR inhibitor, abrogated the upregulation of NKG2DL induced by VPA, suggesting ATM/ATR kinases are involved in NKG2DL induction.
Effect of ATM/ATR activators on OUN‐1. Several reagents capable of stimulating ATM/ATR were examined for the inducibility of MICA/B and ULBP2 on OUN‐1 cells. HU induced expression of both MICA and ULBP2 on OUN‐1 cells, whereas cisplatin and CPT‐11 failed to show such stimulatory effects (Fig. 2a). Doxorubicin, an agent capable of upregulating NKG2DLs on multiple myeloma cells,( 10 ) upregulated MICA/B alone. In addition, the upregulation of the NKG2DL by HU was only evident in non‐apoptotic cells (Fig. 2b).
Figure 2.
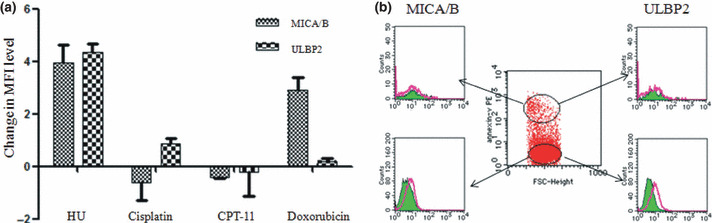
Effect of ATM/ATR activators on the expression of NKG2D ligand (NKG2DL) by chronic myelogenous leukemia cell line OUN‐1. (a) OUN‐1 cells were incubated in the presence of 20 μg/mL hydroxyurea (HU), 10 μM cisplatin, 100 μM CPT‐11, or 0.05 μM doxorubicin for 24 h. Each column represents the difference in the mean fluorescence intensity (MFI) level calculated by subtracting the MFI level of untreated cells from that of the cells treated with the indicated reagent. (b) Selective upregulation of NKG2DLs by HU treatment on non‐apoptotic OUN‐1 cells. Apoptotic and non‐apoptotic cells defined by the expression of annexin V were separately assessed for the expression of NKG2DLs. Green lines, untreated cells; red lines, HU treated cells.
Synergism of HU and VPA in the upregulation of NKG2DL expression. The treatment of OUN‐1 cells with HU and VPA upregulated NKG2DL expression to a greater degree than HU or VPA alone (Fig. 3a,b). This synergistic effect was also observed in leukemic cells from two of three patients with AML from whom a sufficient number of leukemic cells could be obtained. The synergistic effect on OUN‐1 cells was abolished by pretreatment of the cells with caffeine (Fig. 3c). When the mRNA levels of the NKG2DLs were compared between untreated and treated cells using real‐time PCR, both VPA and HU treated cells showed 2.5‐fold higher MICA/B RNA and ULBP2 mRNA levels than untreated cells. The combined use of VPA and HU further increased the mRNA levels to 3.5‐fold of the control (Fig. 3d). These findings indicate the transcriptional upregulation of each gene to underlie the augmentation of the NKG2DL expression induced by these reagents.
Figure 3.
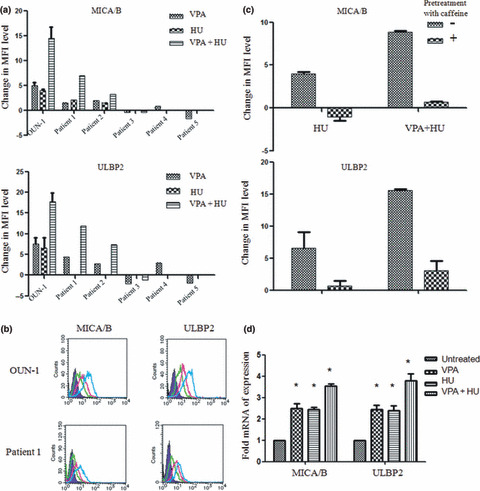
Synergistic effect of valproic acid (VPA) and hydroxyurea (HU) on NKG2D ligand (NKG2DL) expression by OUN‐1 chronic myelogenous leukemia cells. (a) OUN‐1 cells and primary leukemic cells from five patients with acute myeloid leukemia were treated with VPA, HU, or VPA + HU for either 24 h (OUN‐1) or 48 h (primary leukemic cells). Each column represents the difference in the mean fluorescence intensity (MFI) level calculated by subtracting the MFI level of untreated cells from that of the cells treated with the indicated condition. Leukemic cells from Patients 3–5 were only examined for the inducibility of MICA/B and ULBP2 by VPA or HU alone. (b) Representative histograms of OUN‐1 and Patient 1’s leukemic cells treated with VPA and HU. Green lines, untreated cells; green lines, cells treated with VPA + HU; purple lines, isotype control; red lines, VPA treated cells. (c) Effect of caffeine on the expression of NKG2DLs induced by VPA + HU. OUN‐1 cells were pretreated with 5 mM caffeine then incubated with either HU or VPA + HU for 24 h. (d) Effect of VPA and HU on the expression of MICA/B and ULBP2 genes. The gene expression levels relative to GAPDH were determined using real‐time PCR. Each column and error bar represents the mean ± SD of the ratios of the NKG2DL mRNA level to the GAPDH mRNA level from three independent experiments. *P < 0.01.
Effect of leukemic cell pretreatment with VPA and HU on NK cell‐mediated cytotoxicity. The cytotoxicity of NK cells isolated from healthy individuals was examined against OUN‐1 cells treated with or without NKG2DL inducers. Treatment of OUN‐1 cells with VPA or VPA + HU significantly increased the cytotoxicity, although the enhancing effect by VPA + HU was comparable to that by VPA alone (Fig. 4A). The enhancing effect of VPA was blocked by the pretreatment of OUN‐1 cells with anti‐NKG2D mAbs (Fig. 4B). VPA treatment of leukemic cells obtained from two AML patients also increased the cytotoxicity by autologous NK cells and the enhancing effect of VPA was blocked by the pretreatment of leukemic cells with anti‐NKG2D mAbs (Fig. 4C). A 51Cr‐release assay and degranulation assay indicated that the treatment of NK cells with VPA or VPA + HU did not affect their cytotoxicity against OUN‐1 cells (Fig. 4D,E), thus suggesting that NK cells can exert cytotoxicity against leukemic cells in the presence of these agents.
Figure 4.
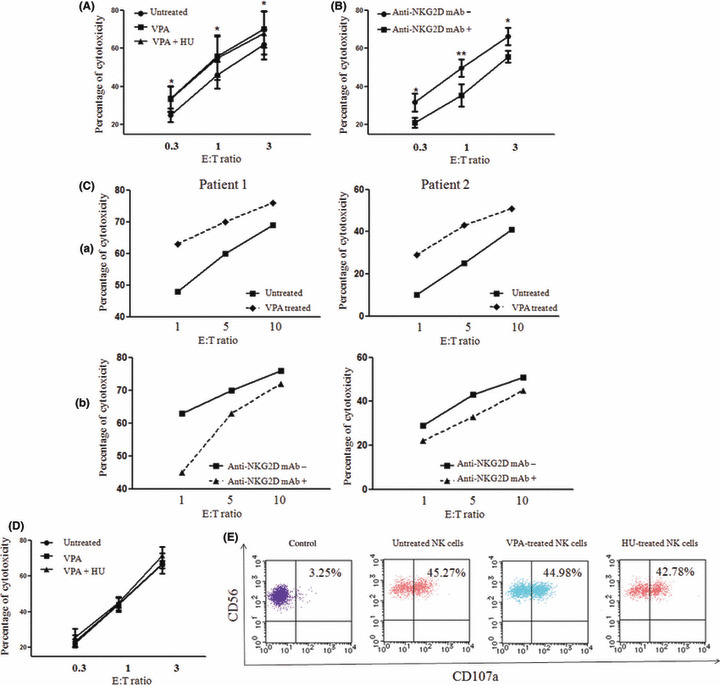
Enhanced natural killer (NK) cell‐mediated lysis of OUN‐1 chronic myelogenous leukemia cells following treatment with valproic acid (VPA) and hydroxyurea (HU). (A) OUN‐1 cells were incubated with or without 200 μg/mL VPA or in combination with 20 μg/mL HU for 24 h then examined for their sensitivity to killing by cultured NK cells from a healthy individual. *P < 0.05. (B) OUN‐1 cells treated with VPA + HU were incubated in the presence or absence of anti‐NKG2D mAb, then tested for their sensitivity to NK cell‐mediated lysis. *P < 0.05; **P < 0.01. (C) (a) Acute myeloid leukemia cells from two patients were incubated with or without 200 μg/mL VPA for 48 h, and the cytotoxicity of cultured NK cells from the patients against autologous leukemic cells was assessed by chromium release assay. (b) Cultured NK cells were incubated in the presence of anti‐NKG2D Abs or medium alone for 30 min then used for chromium release assay against VPA treated leukemic cells. (D) NK cells were incubated in the presence or absence of VPA or VPA + HU for 24 h, and their cytotoxicity against VPA‐treated OUN‐1 cells was compared. (E) Degranulation of NK cells that were treated with VPA or HU for 24 h in response to OUN‐1 cells. The scattergrams represent the CD107a expression by CD3−CD56+ NK cells after 3 h of incubation with OUN‐1 cells. E:T, Effector: Target.
Discussion
NKG2DL expression on leukemic cells plays a key role in the antileukemia effect by NK cells because NKG2D can mediates cytotoxicity depending on the NKG2DL expression levels on leukemic cells, even in the presence of inhibitory signals through a KIR‐KIR‐L interaction.( 5 ) A previous AML patient relapsed after allogeneic stem cell transplantation in association with the loss of ULBP2 expression by AML cells.( 21 ) If the agents non‐toxic to NK cells are capable of upregulating NKG2DLs on leukemic cells, then those agents might potentiate NK cell‐mediated leukemic cell killing.
Of the various agents that have been tested for their inducibility of NKG2DL expression, this study focused on the effect of VPA because it has an antileukemia effect by itself, and its low toxicity allows the use of other agents in combination.( 9 , 19 , 29 ) The screening of various myeloid leukemia cell lines identified OUN‐1 as a suitable cell line for the analysis of mechanisms underlying the NKG2DL upregulation by VPA. The successful blocking of the NKG2DL‐inducing effect by caffeine led to screening ATM/ATR activators, and HU was found, for the first time, to be a potent NKG2DL inducer on myeloid leukemia cells. This antileukemic agent, with low toxicity, upregulated NKGD2L expression on OUN‐1 cells as well as on primary leukemic cells from some patients with AML, synergistically with VPA.
The effect of VPA on various cancer cells has been extensively studied both in vitro and in vivo.( 9 , 12 , 14 , 16 ) VPA increases the transcription of MICA/B without affecting ULBPs in hepatoma cells.( 14 ) Diermayr et al. showed the upregulation of ULBP1 and MICA/B on primary leukemic cells, thus resulting in their increased sensitivity to NK cell‐mediated killing.( 5 ) A recent clinical trial showed VPA increases the expression level of MICA, ULBP2, and ULBP3 by AML cells in vivo.( 9 ) However, despite the large body of evidence for the upregulation of NKG2DLs on leukemic cells, the mechanisms for NKG2DL induction remained unknown, possibly due to the lack of suitable cell lines for in vitro studies. The identification of OUN‐1 as an NKG2DL inducible myeloid leukemia cell line allowed the determination that the ATR/ATM pathway is involved in NKG2DL induction. Genotoxic stress and stalled DNA replication induce NKG2DL expression by activating a DNA damage checkpoint pathway initiated by ATM or ATR protein kinase( 15 ) and caffeine, an inhibitor of ATM/ATR catalytic activity, interferes with the induction of MICA expression on activated T cells.( 16 ) In addition to HU identified in the present study, the identification of other ATM/ATR activators could further help to upregulate NKG2DL expression by myeloid leukemia cells.
The expression of NKG2D‐L induced by various agents has been assessed by flow cytometry, but all the studies analysed the total cell populations rather than a specific cell population.( 4 , 12 , 14 , 22 ) The present study revealed that this method underestimates the upregulation of NKG2DL expression by test agents because many of the agents that upregulate NKG2DLs induce apoptosis of the target cells and the apoptotic cells might fail to express NKG2DLs. The understanding of this phenomenon should be particularly important when primary leukemic cells are screened for their response to NKG2DL inducers, because sensitive screening methods are required to identify the optimal combination of such inducers in AML cells of individual patients.
Hydroxyurea is often used to control the blood cell count in patients with myeloproliferative disorders and AML as a palliative treatment.( 30 , 31 , 32 ) A recent study showed that VPA and HU can modulate the cell cycle and cooperatively induce apoptosis of cancer cells.( 19 ) Several clinical trials of VPA and all‐trans retinoic acid for patients with myeloid malignancies used HU to control leukocytosis.( 9 , 29 ) HU might synergize with VPA in patients responsive to the combination therapy, not only by augmenting apoptosis of leukemic cells but also by increasing the susceptibility of leukemic cells to NK cell‐mediated cytotoxicity. It is plausible that giving VPA and HU simultaneously might further augment the NK cell‐mediated killing of myeloid leukemia cells by upregulating NKG2DLs. Clinical trials using VPA in combination with HU are therefore warranted for the palliative treatments of elderly patients with either AML or myeloproliferative disorders.
Acknowledgments
We thank Dr. Dario Campana at University of Tennessee College of Medicine (Memphis, TN, USA) for providing us with K562‐mb15‐41BBL cells and Dr Ken‐ichi Yamamoto at the Cancer Research Institute of Kanazawa University (Kanazawa, Japan) for helpful discussions. This investigation was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Technology, Sports, and Culture of Japan.
References
- 1. Ruggeri L, Capanni M, Urbani E et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295 (5562): 2097–2100. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen S, Kuentz M, Vernant JP et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem‐cell transplantation. Leukemia 2008; 22 (2): 344–352. [DOI] [PubMed] [Google Scholar]
- 3. Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49‐mismatched NK cells from MHC‐matched donors. Blood 2007; 109 (8): 3603–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang C, Niu J, Zhang J, Wang Y, Zhou Z, Tian Z. Opposing effects of interferon‐alpha and interferon‐gamma on the expression of major histocompatibility complex class I chain‐related A in tumors. Cancer Sci 2008; 99 (6): 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diermayr S, Himmelreich H, Durovic B et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK‐cell lines with single KIR‐HLA class I specificities. Blood 2008; 111 (3): 1428–1436. [DOI] [PubMed] [Google Scholar]
- 6. Andresen L, Jensen H, Pedersen MT, Hansen KA, Skov S. Molecular regulation of MHC class I chain‐related protein A expression after HDAC‐inhibitor treatment of Jurkat T cells. J Immunol 2007; 179 (12): 8235–8242. [DOI] [PubMed] [Google Scholar]
- 7. Fuertes MB, Girart MV, Molinero LL et al. Intracellular retention of the NKG2D ligand MHC class I chain‐related gene A in human melanomas confers immune privilege and prevents NK cell‐mediated cytotoxicity. J Immunol 2008; 180 (7): 4606–4614. [DOI] [PubMed] [Google Scholar]
- 8. Kato N, Tanaka J, Sugita J et al. Regulation of the expression of MHC class I‐related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D‐expressing cells. Leukemia 2007; 21 (10): 2103–2108. [DOI] [PubMed] [Google Scholar]
- 9. Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all‐trans‐retinoic acid or sodium valproate. Leukemia 2009; 23 (4): 641–648. [DOI] [PubMed] [Google Scholar]
- 10. Soriani A, Zingoni A, Cerboni C et al. ATM‐ATR‐dependent up‐regulation of DNAM‐1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK‐cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113 (15): 3503–3511. [DOI] [PubMed] [Google Scholar]
- 11. Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase‐3‐dependent expression of MHC class I‐related chain A and B. Cancer Res 2005; 65 (23): 11136–11145. [DOI] [PubMed] [Google Scholar]
- 12. Vales‐Gomez M, Chisholm SE, Cassady‐Cain RL, Roda‐Navarro P, Reyburn HT. Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res 2008; 68 (5): 1546–1554. [DOI] [PubMed] [Google Scholar]
- 13. Pende D, Rivera P, Marcenaro S et al. Major histocompatibility complex class I‐related chain A and UL16‐binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D‐dependent natural killer cell cytotoxicity. Cancer Res 2002; 62 (21): 6178–6186. [PubMed] [Google Scholar]
- 14. Armeanu S, Bitzer M, Lauer UM et al. Natural killer cell‐mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 2005; 65 (14): 6321–6329. [DOI] [PubMed] [Google Scholar]
- 15. Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436 (7054): 1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen‐activated human T lymphocytes express cell‐surface NKG2D ligands via an ATM/ATR‐dependent mechanism and become susceptible to autologous NK‐ cell lysis. Blood 2007; 110 (2): 606–615. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi M, Hirano A, Kumano T et al. Critical role for chicken Rad17 and Rad9 in the cellular response to DNA damage and stalled DNA replication. Genes Cells 2004; 9 (4): 291–303. [DOI] [PubMed] [Google Scholar]
- 18. Iwahori S, Yasui Y, Kudoh A et al. Identification of phosphorylation sites on transcription factor Sp1 in response to DNA damage and its accumulation at damaged sites. Cell Signal 2008; 20 (10): 1795–1803. [DOI] [PubMed] [Google Scholar]
- 19. Kramer OH, Knauer SK, Zimmermann D, Stauber RH, Heinzel T. Histone deacetylase inhibitors and hydroxyurea modulate the cell cycle and cooperatively induce apoptosis. Oncogene 2008; 27 (6): 732–740. [DOI] [PubMed] [Google Scholar]
- 20. Feng X, Chuhjo T, Sugimori C et al. Diazepam‐binding inhibitor‐related protein 1: a candidate autoantigen in acquired aplastic anemia patients harboring a minor population of paroxysmal nocturnal hemoglobinuria‐type cells. Blood 2004; 104 (8): 2425–2431. [DOI] [PubMed] [Google Scholar]
- 21. Lu X, Kondo Y, Takamatsu H et al. CD16+ CD56‐ NK cells in the peripheral blood of cord blood transplant recipients: a unique subset of NK cells possibly associated with graft‐versus‐leukemia effect. Eur J Haematol 2008; 81 (1): 18–25. [DOI] [PubMed] [Google Scholar]
- 22. Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar‐Filipowicz A. Differentiation‐promoting drugs up‐regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell‐mediated lysis. Leuk Res 2007; 31 (10): 1393–1402. [DOI] [PubMed] [Google Scholar]
- 23. Nakao S, Takami A, Takamatsu H et al. Isolation of a T‐cell clone showing HLA‐DRB1*0405‐restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood 1997; 89 (10): 3691–3699. [PubMed] [Google Scholar]
- 24. Rubio V, Stuge TB, Singh N et al. Ex vivo identification, isolation and analysis of tumor‐cytolytic T cells. Nat Med 2003; 9 (11): 1377–1382. [DOI] [PubMed] [Google Scholar]
- 25. Insinga A, Monestiroli S, Ronzoni S et al. Inhibitors of histone deacetylases induce tumor‐selective apoptosis through activation of the death receptor pathway. Nat Med 2005; 11 (1): 71–76. [DOI] [PubMed] [Google Scholar]
- 26. Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase‐dependent and ‐independent apoptotic signaling pathways. Leuk Res 2002; 26 (5): 495–502. [DOI] [PubMed] [Google Scholar]
- 27. Kaiser M, Zavrski I, Sterz J et al. The effects of the histone deacetylase inhibitor valproic acid on cell cycle, growth suppression and apoptosis in multiple myeloma. Haematologica 2006; 91 (2): 248–251. [PubMed] [Google Scholar]
- 28. Molinero LL, Fuertes MB, Fainboim L, Rabinovich GA, Zwirner NW. Up‐regulated expression of MICA on activated T lymphocytes involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase, and calcineurin. J Leukoc Biol 2003; 73 (6): 815–822. [DOI] [PubMed] [Google Scholar]
- 29. Kuendgen A, Gattermann N. Valproic acid for the treatment of myeloid malignancies. Cancer 2007; 110 (5): 943–954. [DOI] [PubMed] [Google Scholar]
- 30. Fruchtman SM. Treatment paradigms in the management of myeloproliferative disorders. Semin Hematol 2004; 41 (2 Suppl 3): 18–22. [DOI] [PubMed] [Google Scholar]
- 31. Thiele J, Kvasnicka HM. Comparative effects of interferon and hydroxyurea on bone marrow fibrosis in chronic myelogenous leukemia. Leuk Lymphoma 2001; 42 (5): 855–862. [DOI] [PubMed] [Google Scholar]
- 32. Motomura S, Sakai R, Tomita N et al. Chronic myelogenous leukemia with long‐term hypoplasia induced by alpha‐interferon and hydroxyurea. Rinsho Ketsueki 1998; 39 (4): 302–307. [PubMed] [Google Scholar]


