Abstract
TCDD (2,3,7,8‐tetrachlorodibenzo‐p‐dioxin) is a highly toxic environmental contaminant. When exposed to TCDD, mammalian cells undergo malignant transformation via abnormal intracellular signaling cascades, and the robust inductions of cytochrome P450 (CYP) enzymes are considered to mediate carcinogenesis by producing genotoxic metabolites. We here examined whether curcumin has preventive activity against TCDD‐induced CYP production and cell transformation. Initially, the cellular levels of cytochrome P450 (CYP) 1A1 and 1B1 were examined, because these are known to generate estrogen metabolites that mediate genotoxic stress. Curcumin inhibited CYP1A1 and 1B1 induction by TCDD at the mRNA and protein levels. Notably, the nuclear levels of arylhydrocarbon receptor (AhR) and AhR nuclear translocator (ARNT) were decreased by curcumin, but those in the cytoplasm were not. It was also found that oxidative stress mediated the curcumin‐induced degradations of AhR and ARNT. Furthermore, in vitro transformation assays showed that in normal human embryonic kidney cells and normal prostate cells curcumin prevents the anchorage‐independent growth induced by TCDD. In conclusion, curcumin attenuates AhR/ARNT‐mediated CYP induction by dioxin and presumably this mode‐of‐action may be responsible for the curcumin prevention of malignant transformation. The findings of this study should be found helpful in the design stage of pharmacodynamic studies for developing curcumin as a chemopreventive or anticancer agent. (Cancer Sci 2008; 99: 2518–2524)
The halogenated hydrocarbon 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD), which is viewed as the most harmful dioxin, detrimentally affects reproduction, the neural network, and immunity.( 1 ) TCDD is also classified as a human carcinogen because it activates xenobiotic metabolisms that are closely related to tumor initiation and promotion.( 2 ) During TCDD‐induced carcinogenesis, arylhydrocarbon receptor (AhR) and AhR nuclear translocator (ARNT, also known as HIF‐1α) play crucial roles in the activations of carcinogenic pathways. Briefly, TCDD binds and activates AhR in the cytoplasm, which then translocates into the nucleus and dimerizes with ARNT. The AhR/ARNT complex then targets XRE (xenobiotic response element) in the genes of xenobiotic enzymes and transactivates them. During this process, AhR functions as the prime transcription factor and ARNT as a partner for DNA binding.( 3 , 4 )
AhR/ARNT complex up‐regulates microsomal cytochrome P450 (CYP) enzymes that play diverse roles in the metabolisms of endogenous substances, environmental chemicals, and drugs.( 5 ) Of these CYPs, CYP1A1 and CYP1B1 have been reported to mediate carcinogenesis by inducing the productions of reactive estrogen metabolites.( 6 , 7 ) In particular, CYP1A1 and CYP1B1 hydroxylate 17‐β‐estradiol at the c‐2 or c‐4 positions to produce 2‐hydroxyestradiol or 4‐hydroxyestradiol, which contains the quinine moiety responsible for tumor initiation.( 8 , 9 ) Thus, AhR inhibition can reduce the expressions of CYP1A1 and CYP1B1 and in turn may prevent TCDD‐induced carcinogenesis.
Curcumin (diferuloylmethane), a major component of turmeric, has attracted public attention because of its cancer preventative effect. Several animal studies have demonstrated that curcumin inhibits carcinogenic initiation and postinitiation carcinogenic events in colon, stomach, and skin cancers.( 10 , 11 , 12 ) In addition, curcumin suppresses the carcinogenic activities of genotoxic chemicals.( 13 , 14 ) However, the underlying mechanism has not been elucidated. Recently, we investigated the mechanism of tumor growth inhibition by curcumin, and found that curcumin inhibits hypoxia‐inducible factor‐1 (HIF‐1), which plays a crucial role in tumor promotion. In detail, curcumin induces the ubiquitination and proteasomal degradation of ARNT, which prevents the alpha subunit of HIF‐1 (HIF‐1α) dimerizing with ARNT and binding to DNA.( 15 ) Here, we highlight that curcumin suppresses ARNT, which is also the partner of AhR. Therefore, we hypothesized that curcumin inhibits TCDD‐induced, AhR‐mediated carcinogenesis by targeting ARNT. Curcumin was found to degrade both AhR and ARNT via oxidative stress and in so doing to inhibit CYP1A1 and CYP1B1 inductions by TCDD. In particular, curcumin was observed to inhibit transformation of non‐cancerous cells. Accordingly, our findings indicate that the anticarcinogenic effect of curcumin is dependent upon the ROS‐mediated degradations of AhR and ARNT.
Materials and Methods
Materials. Curcumin, 17‐β‐estradiol, N‐acetyl cysteine (NAC), and hydrogen peroxide were purchased from Sigma‐Aldrich (St. Louis, MO, USA). TCDD and MG‐132 were purchased from Supelco (Bellefonte, PA, USA) and Alexis Biochemicals (Lausen, Switzerland), respectively. Culture media and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA). Antisera against AhR, ARNT, CYP1A1, CYP1B1, and β‐tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antihistone H3 antiserum from Abcam (Cambridge, MA, USA).
Cell culture. Hep3B hepatoma, MCF‐7 mammary carcinoma, and HEK 293 human embryonic kidney cell line were obtained from ATCC (Manassas, VA, USA). PNT2, a non‐tumorigenic, SV‐40‐immotalized prostate epithelial cell line was obtained from ECACC (London, UK). Cells were cultured with 10% of heat‐inactivated fetal bovine serum, 100 U/mL of penicillin, and 100 µg/mL of streptomycin in a humidified condition with 5% CO2/95% O2 at 37°C. Hep3B was cultured in α‐MEM, MCF‐7, and HEK293 in Dulbecco's modified Eagle's medium, and PNT2 in RPMI‐1640 medium.
Western blot analysis. Total proteins were separated on 6.5% or 10% sodium dodecylsulfate–polyacrylamide gel, and transferred to Immobilon‐P membranes (Millipore, Bedford, MA, USA). Membranes were incubated overnight at 4°C with primary antibodies diluted 1:5000 in 5% non‐fat milk, and sequentially with horseradish peroxidase–conjugated secondary antibodies (1:5000 dilution). The immune complexes were visualized using an Enhanced Chemiluminescence Plus kit (Amersham Biosciences, Piscataway, NJ, USA).
Semiquantitative reverse transcription–polymerase chain reaction (RT‐PCR). Total RNA was isolated using TRIZOL (Invitrogen) and was reverse‐transcribed at 48°C for 20 min and the cDNAs were amplified over 18 PCR cycles in a reaction mixture containing 5 µCi [α‐32P]dCTP. PCR products were electrophoresed on a 4% polyacrylamide gel, and dried gels were autoradiographed. Primer sequences are 5′‐TCTACACCTTCACCCTCATC‐3′ and 5′‐TAGTGCTCCTTGACCATCTT‐3′ for CYP1A1; 5′‐AGGACACTGTGGTTTTTGTC‐3′ and 5′‐GGCTTGTAAATTTTGGACAG‐3′ for CYP1B1; and 5′‐ACACCTTCTACAATGAGCTG‐3′ and 5′‐CATGATGGAGTTGAAGGTAG‐3′ for β‐actin.
Chromatin immunoprecipitation (ChIP). Cells were treated with 10% formaldehyde for 10 min to cross‐link DNAs and proteins, and 150 mM glycine was added to stop the cross‐linking. After cells were lyzed and sonicated, soluble chromatin samples were immunoprecipitated with anti‐AhR at 4°C overnight. DNAs were isolated from immunoprecipitated material and amplified by PCR with 5 µCi [α‐32P]dCTP. Primer sequences are 5′‐ACCCGCCACCCTTCGACAGTTC‐3′ and 5′‐TGCCCAGGCGTTGCGTGAGAAG‐3′ for CYP1A1 promoter; and 5′‐GTGCGCACGGAGGTGGCGATA‐3′ and 5′‐GCTCCTCCCGCGCTTCTCAC‐3′ for CYP1B1 promoter. PCR products were electrophoresed in a 4% polyacrylamide gel, and dried gels were autoradiographed.
Nuclear extract. After centrifugation at 1500 g for 5 min at 4°C, the cell pellets were re‐suspended in a buffer A (20 mM Tris, pH 7.8, 1.5 mM MgCl2, 10 mM KCl, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, protease inhibitor cocktail, and 1 mM Na3VO4) and cooled on ice. After being resuspended in 0.6% NP‐40, the lyzed cells were centrifuged and the supernatants were collected as the cytosolic fraction. Nuclear pellets were resuspended in a buffer B which was prepared by adding NaCl (finally 400 mM) and glycerol (finally 5%) to the buffer A. After launching on shaking incubator at 4°C for 30 min, the pellets were centrifuged and kept the supernatant as the nuclear fraction at –70°C.
ROS assay. Culture media were replaced with a Hank's Balanced Salt Solution that had been preincubated at 37°C in a 5% CO2 atmosphere. Cells were then treated with 50 µM 2′,7′‐dichlorofluorescein diacetate (DCFDA) for 30 min in the dark, and detached with trypsin‐EDTA solution. After a brief washing with phosphate‐buffered saline (PBS), the oxidized form of DCFDA, fluorescent dichlorofluorescein was excited at 488 nm and detected at 530 nm, using a FACStar flow cytometer.
Immunofluorescence staining. Hep3B cells, which were cultured onto cover glasses, were fixed with 3.7% paraformaldehyde, washed with PBS, and blocked with 3% bovine serum albumin. Anti‐ARNT (1:300) and anti‐AhR (1:300) antisera were incubated overnight at 4°C. Each cover glass was incubated with biotinylated secondary antibodies (1:1.000) for 1 h and reacted with ALEXA fluor‐488 (green)‐conjugated streptavidine (1:1000) or ALEXA fluor‐594(red)‐conjugated streptavidine (1:1000) for 1 h. All nuclei were counterstained with DAPI (0.1 µg/mL). Fluorescence images were observed using an IX71 microscope (Olympus, Hamburg, Germany). Images were captured using an UIS 2 camera running Image proPlus 5.1 software (Olympus).
Focus and colony formation. Cells were treated with 1 nM TCDD and 10 nM 17‐β‐estradiol twice a week for 2 weeks. Foci (colonies formed by over‐grown cells) were stained with 0.05% crystal violet. Foci were counted under a microscope at 40× magnification. For colony formation analysis, 0.6% agar in fetal bovine serum–containing medium was poured into 35‐mm culture dishes. After solidification, 2 × 104 cells were mixed with 0.4% agar and laid on top of the agar. When solidified, 1.5 mL of RPMI‐1640 medium was added. After 24 h, the medium was changed with the fresh medium and drugs were administered twice a week. The culture dishes were further incubated for 4–6 weeks until the colonies appeared countable. Crystal violet–stained colonies were counted and the images were captured.
Preparation of siRNA and transfection. siRNA duplexes were synthesized by Samchully Pharm. (Seoul, Korea). Sequences of the ARNT siRNA are 5′‐GCAGAGAAUUUCAGGAAUATT‐3′ and 5′‐UAUUCCUGAAAUUCUCUGCTT‐3′. The siRNAs were transfected into cells using Lipofectamine.
Statistical analysis. All data were analyzed using Microsoft Excel 2003 software, and results are expressed as mean and SD. The Mann–Whitney U‐test was used to compare numbers of foci or colony, and differences were considered statistically significant at the P < 0.05 level.
Results
Curcumin inhibited TCDD‐induced CYP1A1 and CYP1B1 expression. Given that AhR also requires ARNT for DNA binding, we examined whether curcumin inhibits the transcriptional activity of AhR, as it does for HIF‐1α.( 15 ) We treated Hep3B and MCF7 cells with TCDD to activate AhR, and determined the protein and mRNA levels of CYP1A1 and CYP1B1. TCDD induced CYP1A1 and CYP1B1 protein expressions and the effects of TCDD were noticeably reduced by curcumin (Fig. 1a). Likewise, both CYP1A1 and CYP1B1 mRNA inductions by TCDD were also suppressed by curcumin (Fig. 1b). In the absence of TCDD, however, curcumin did not substantially suppress the basal levels of CYP1A1 and CYP1B1 proteins and mRNAs (Fig. 1a,b). This suggests that curcumin blocks the cellular process for the CYP induction, but not the CYP transcription or translation per se. To examine whether curcumin inhibits AhR binding to XRE in CYP1A1 or CYP1B1 promoter, ChIP assays were performed. TCDD enhanced AhR recruitment to XRE, and this was inhibited by curcumin (Fig. 1c). These data suggest that curcumin inhibits xenobiotic gene induction in response to TCDD by preventing AhR binding to XRE.
Figure 1.
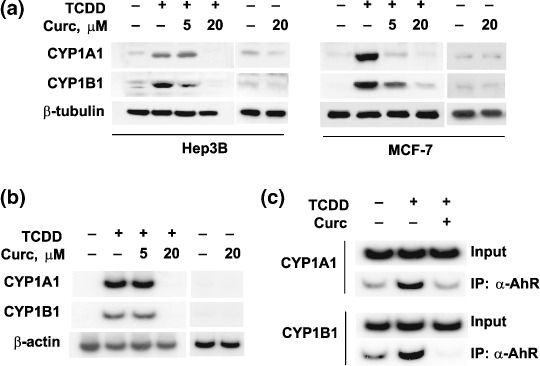
Inhibitory effects of curcumin (Curc) on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced cytochrome P450 (CYP) 1A1 and CYP1B1 expression. (a) CYP1A1 and CYP1B1 protein levels. Hep3B and MCF‐7 cells were treated with 250‐pM TCDD in the absence or presence of 5 or 20 µM of curcumin for 24 h. CYP and β‐tubulin proteins were analyzed by western blotting. (b) CYP 1A1 and 1B1 mRNA levels. After incubating cells with TCDD or/and curcumin for 24 h, CYP and β‐actin mRNA levels were analyzed by semiquantitative reverse transcription–polymerase chain reaction using [α‐32P]dCTP. (c) Arylhydrocarbon receptor (AhR) binding to the CYP1A1 and CYP1B1 genes. AhR recruitments to the XRE of CYP genes were analyzed using chromatin immunoprecipitation assays. After Hep3B cells had been treated with 250‐pM TCDD or 20‐µM curcumin for 8 h, cells were lyzed and fixed with formaldehyde. Soluble chromatin samples were then immunoprecipitated with anti‐CYP1A1 or anti‐CYP1B1 antibody, and immunoprecipitated DNA was amplified by semiquantitative PCR, electrophoresed, and autoradiographed.
Curcumin suppressed AhR and ARNT in the nucleus, but not in the cytoplasm. To investigate the mechanism by which curcumin inhibits the XRE binding of AhR, we first analyzed AhR and ARNT levels in total cell lysates. As previously reported,( 15 ) ARNT levels were reduced during curcumin treatment, but AhR was also down‐regulated by curcumin (Fig. 2a). In nuclear fractions, AhR and ARNT levels were found to be enhanced by TCDD (Fig. 2b, left panel), whereas their total levels were not significantly altered (Fig. 1a). This suggests that AhR is activated by TCDD and that it is then translocated to the nucleus with ARNT. Furthermore, curcumin noticeably reduced nuclear AhR and ARNT levels in TCDD‐treated cells, but not their cytosolic levels (Fig. 2b). Presumably, curcumin targets AhR and ARNT in the nucleus rather than in the cytoplasm. Fig. 2(c) shows that TCDD induced nuclear accumulations of AhR and ARNT and that the nuclear proteins were down‐regulated by curcumin without altering their protein levels in cytoplasm. These results further support that AhR and ARNT are mainly targeted in the nucleus by curcumin.
Figure 2.
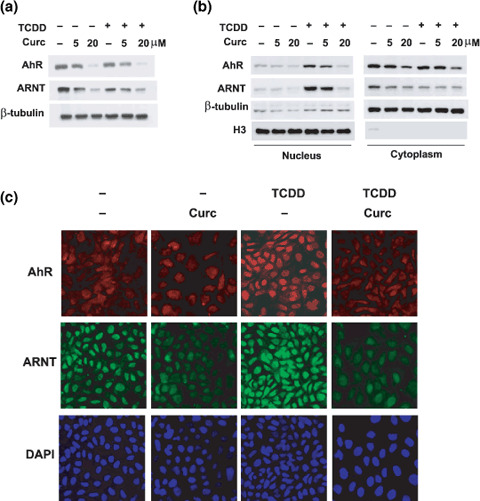
The effects of curcumin (Curc) on the expressions of arylhydrocarbon receptor (AhR) and AhR nuclear translocator (ARNT). (a) AhR and ARNT levels in total cell lysates. Hep3B cells were treated with 250‐pM 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD) or/and curcumin for 8 h, and protein levels were analyzed by western blotting. (b) AhR and ARNT levels in nuclear and cytoplasmic fractions. After Hep3B cells had been treated for 8 h, samples were fractionated as nuclear and cytoplasmic proteins, and proteins were analyzed by western blotting. β‐tubulin and α‐H3 (histone‐H3) were analyzed to qualify cell fractionation and to verify equal protein loadings. (c) Loss of nuclear proteins induced by curcumin. Hep3B cells were treated with 250‐pM TCDD or 20‐µM curcumin for 8 h, and then AhR and ARNT levels in the nucleus and cytoplasm were analyzed using immunofluorescence assays. All nuclei were counterstained with DAPI (0.1 µg/mL).
Curcumin inhibited cellular responses to TCDD via a ROS‐dependant pathway. In terms of curcumin‐induced ARNT degradation, we previously demonstrated that oxidative stress facilitates the proteasomal degradation of ARNT.( 15 ) Therefore, we tested the possibility that oxidative stress and proteasomes are also involved in AhR degradation. A proteasome inhibitor MG132 partially recovered AhR and ARNT suppressed by curcumin, but failed to increase CYP1A1 and CYP1B1 levels (Fig. 3a). Although AhR can be stabilized by MG132, forcibly stabilized AhR may lose its transcriptional ability. Likewise, despite substantial HIF‐1α induction by MG132, HIF‐1 downstream genes are known to be down‐regulated due to loss of HIF‐1 activity.( 16 ) We next examined whether ROS mediate AhR and ARNT degradation by curcumin. Intracellular ROS levels noticeably increased in curcumin‐treated MCF‐7 or Hep3B cells versus untreated cells (Fig. 3b). This suggests that curcumin acted as a pro‐oxidant in our experimental setting. We next used an antioxidant NAC to examine the involvement of ROS, and found that NAC recovered AhR, ARNT, CYP1A1, and CYP1B1 expressions (Fig. 3c). Moreover, a pro‐oxidant H2O2 inhibited AhR/ARNT expressions and TCDD‐mediated CYP inductions (Fig. 3d). These results suggest that curcumin degrades AhR and ARNT redox‐dependently through proteasomes, and that this impairs xenobiotic responses to TCDD.
Figure 3.
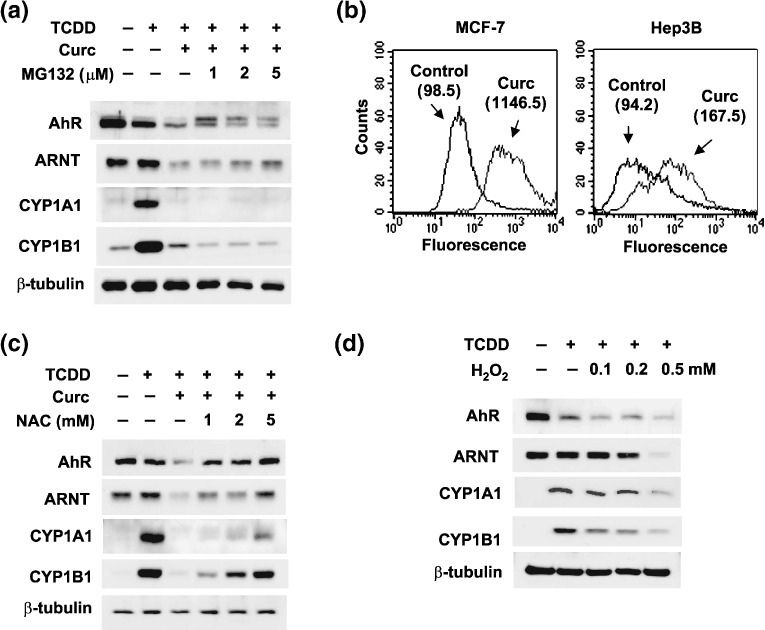
ROS‐mediated, proteasomal degradations of arylhydrocarbon receptor (AhR) and AhR nuclear translocator (ARNT) by curcumin (Curc). (a) Proteasome‐dependent degradations of AhR and ARNT. MCF‐7 cells were treated with 250‐pM 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD), 10‐µM curcumin, and/or MG‐132 at the indicated concentrations for 16 h. Proteins were analyzed by western blotting. (b) ROS production by curcumin. MCF‐7 cells were treated with phosphate‐buffered saline (Control) or 20‐µM curcumin (Curc) for 4 h, and stained for intracellular ROS using DCFDA, and the fluorescence was measured using a FACStar flow cytometer. The numbers given in parentheses are the mean fluorescence values from two separate experiments. (c) AhR and ARNT rescue by N‐acetyl cysteine (NAC). MCF‐7 cells were treated with NAC at the indicated dose for 16 h, and harvested for western blotting. (d) AhR and ARNT degradation by H2O2. MCF‐7 cells were incubated in serum‐free medium in the presence/absence of hydrogen peroxide (H2O2) for 8 h, and protein levels were analyzed by western blotting.
TCDD‐induced transformation depended on the transcription factor AhR/ARNT. Since the role of AhR in dioxin‐induced carcinogenesis has been well established,( 17 , 18 ) we here examined the involvement of ARNT in cellular transformation. For this purpose, we used a normal human prostate cell‐line (PNT2) as a target for TCDD‐induced transformation. Fig. 4a shows that ARNT was effectively knocked‐down by siRNA. After TCDD treatment, many foci of transformed cells were observed in both mock and control RNA‐treated cells. However, TCDD‐induced focus formation was significantly reduced in ARNT‐knocked‐down cells (Fig. 4b), which suggests that ARNT, as well as AhR, is also a potential target in terms of preventing TCDD‐induced transformation.
Figure 4.
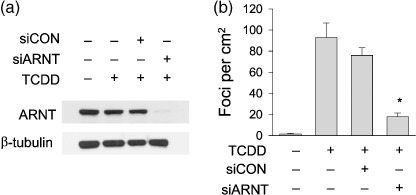
2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced focus formation was reduced by arylhydrocarbon receptor nuclear translocator (ARNT) knock‐down. (a) The effect of ARNT silencing. PNT2 cells were transfected with 40‐nM ARNT siRNA (siARNT) or control siRNA (siCON), and ARNT levels were analyzed by western blotting. (b) Effect of ARNT knock‐down on focus formation. PNT2 cells were transfected with 40 nM of siRNA and the next day were incubated with 1‐nM TCDD and 10‐nM 17‐β‐estradiol. Media were changed daily and the drugs were administered twice a week for 2 weeks. Foci (cell clusters) on culture plates were stained with crystal violet (0.05%) and counted. Bars represent the mean ± SD of three separate experiments. *P < 0.05 versus siCON.
Curcumin prevented the cancerous transformation induced by TCDD. To evaluate the effect of curcumin on TCDD‐induced transformation, we used two non‐cancerous cell lines, PNT2 and HEK293. We first confirmed that curcumin also effectively suppressed AhR and ARNT (Fig. 5a) and prevented the TCDD‐induced expressions of CYP1A1 and CYP1B1 mRNAs (Fig. 5b) in both cell lines. In the absence of TCDD, few foci were formed on the plates and also curcumin failed to affect the focus formation. After TCDD treatment, focus formation was markedly induced in both cell‐lines and curcumin suppressed the TCDD‐induced focus formation (Supporting Fig. S1). We counted the numbers of foci and plotted the data (Fig. 5c). Statistically, focus formation by TCDD was significantly reduced in curcumin‐treated cells. In the presence of NAC, the curcumin effect was significantly attenuated (Fig. 5c), which also supports the involvement of ROS in the curcumin action. To further investigate the anticarcinogenic effect of curcumin, we cultured cells in agar plates and evaluated anchorage‐independent cell growth. Several colonies were settled even in the absence of TCDD, but the colony formation was markedly induced by TCDD in both cell lines. Also, curcumin significantly inhibited the colony formation both in untreated and TCDD‐treated cells (Fig. 6a,b). These results suggest that curcumin has a preventive effect on non‐cancer cell transformation induced by TCDD.
Figure 5.
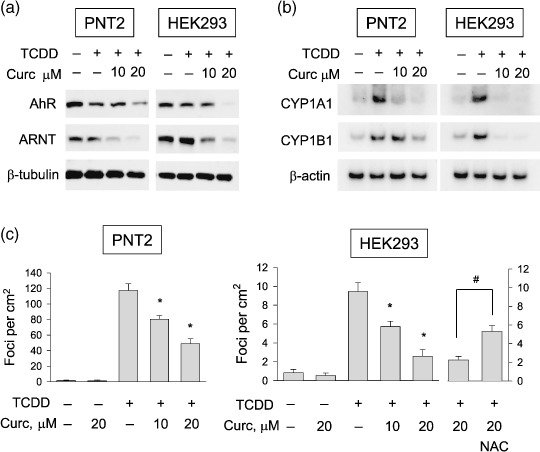
Effects of curcumin (Curc) on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced focus formation in two non‐cancerous cell‐lines. (a) Effects of curcumin on the expressions of arylhydrocarbon receptor (AhR) and AhR nuclear translocator (ARNT). PNT2 and HEK293 cells were treated with 1‐nM TCDD, 10‐nM 17‐β‐estradiol, and curcumin for 16 h, and proteins were analyzed by western blotting. (b) Effects of curcumin on the expressions of cytochrome P450 (CYP) 1A1 and CYP1B1. After cells had been treated with drugs for 16 h, CYP1A1 and CYP1B1 mRNA levels were analyzed by semiquantitative reverse transcription–polymerase chain reaction. (c) Effects of curcumin on TCDD‐induced focus formation. TCDD (1 nM), curcumin (10, 20 µM), or/and N‐acetyl cysteine (NAC) (5 mM) were administered twice a week for 2 weeks. Foci were stained with crystal violet and counted under a microscope at 40× (focus numbers are presented as numbers per cm2). Bars represent the mean ± SD of three separate experiments. *P < 0.05 versus the TCDD control, #: P < 0.05 versus the 20‐µM curcumin‐treated group.
Figure 6.
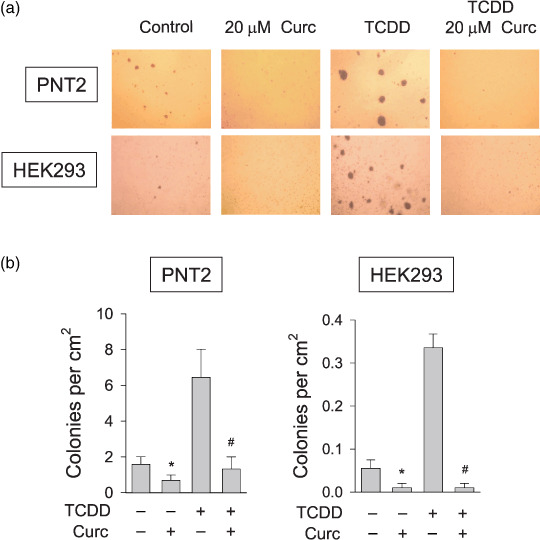
Effects of curcumin (Curc) on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced colony formation in two non‐cancerous cell‐lines. (a) Effects of curcumin on TCDD‐induced colony formation. Equal numbers of PNT2 or HEK293 cells were mixed with 0.4% soft agar and laid on top of 0.8% agar in dishes. The indicated drugs were administered twice a week for 2 weeks. Colonies were stained with crystal violet and photographed at 40×. (b) Statistical analyses of colony numbers. Stained colonies were counted and their numbers were presented as numbers per cm2. Bars represent mean ± SD of three separate experiments. *P < 0.05 versus the untreated control; #P < 0.05 versus the TCDD control.
Discussion
This study demonstrated that curcumin induced the proteasomal degradations of AhR and ARNT by increasing oxidative stress and subsequently abolished the TCDD‐induced expressions of CYP1A1 and CYP1B1. Furthermore, curcumin prevented non‐cancerous cells from being transformed to cancerous cells as characterized by cell density‐ and anchorage‐independent proliferation. Even though the correlation between CYP expression and malignant transformation was not investigated in the present study, it is speculated that the CYP suppression could be one of mechanisms responsible for the transformation inhibition by curcumin. These results imply that curcumin could be used to prevent dioxin‐induced carcinogenesis and that it could be utilized to further understand cellular responses to dioxin.
TCDD has been reported to activate various transformation‐related signalings, e.g. the dysregulation of growth factors,( 19 ) the production of arachidonic acids by cyclooxygenase induction,( 20 ) and the up‐regulation of proto‐oncogenes.( 21 , 22 ) However, these signaling pathways are not primarily activated by TCDD. TCDD primarily acts by activating AhR, which leads to the induction of CYP1 subfamily members. Currently, CYP1 induction is believed to be positively associated with cancer risk in humans and rodents.( 23 ) For example, CYP1A1 overexpression produces oxidative DNA damage and subsequently induces genomic mutations.( 24 ) Likewise, CYP1B1, which is highly expressed in cancer cells, is also known to cause cancerous transformation.( 25 ) Furthermore, several clinical studies have demonstrated that the CYP1B1 polymorphism having gain‐of‐function could be a sufficient risk factor in renal cell carcinoma,( 26 , 27 ) and it has been revealed that CYP1B1 expression in prostate cancer is significantly higher than that in the normal prostate.( 28 ) In the present study, CYP1A1 and CYP1B1 expressions in non‐cancerous cell lines were undetectable by western blotting. However, after TCDD treatment, CYP1A1 and 1B1 were both highly induced at the transcriptional level and cell transformation was concomitantly initiated.
Despite a growing body of epidemiological evidence, the molecular mechanism by which curcumin inhibits tumorigenesis is still unclear. One possible mechanisms is that curcumin inhibits the inductions of carcinogenic CYP1s,( 29 ) which raises questions as to how curcumin suppresses CYP1s. Some previous reports showed that curcumin directly competes with DMBA for AhR binding and then reduced the expression of CYP1A1.( 30 ) Likewise, curcumin prevents CYP1A1 induction by benzo[a]pyrene, which also acts as AhR ligand.( 31 ) In addition to competing with carcinogens, curcumin is known to functionally inhibit AhR by dephosphorylating AhR and ARNT.( 32 ) However, the effects of curcumin on the expressions of AhR and ARNT have not been extensively investigated. In the present study, curcumin was found to induce AhR degradation by generating oxidative stress. In terms of the mechanism underlying AhR degradation, it has been suggested that AhR is degraded via the ubiquitin‐proteasome pathway after ligand binding.( 33 , 34 ) Since curcumin binds to AhR like a ligand, it is possible that curcumin facilitates the proteasomal degradation of AhR, as do other ligands. However, since the ligand‐dependent degradation of AhR is not mediated by ROS, other ROS‐dependent processes may participate in AhR degradation, as has been shown for ARNT degradation.( 15 )
In AhR/ARNT complex, AhR functions as the main factor for transactivating CYP1 genes, and thus, is viewed as a potential target for cancer prevention. In contrast, ARNT has not been regarded as a target molecule. However, it was recently suggested that ARNT should be consider a target molecule, as Ito et al. concluded that whole‐body CYP1A1 induction by phytochemicals is controlled by a factor produced ARNT‐dependently in the gut.( 35 ) Moreover, BRCA‐1, whose gain‐of‐functional mutations predispose individuals to breast or ovarian cancer, regulates ARNT stability and subsequently modulates TCDD‐induced xenobiotic stress.( 36 ) Specifically, BRCA‐1 knock‐down was found to destabilize ARNT and attenuate the transcription of CYP1A1 in the presence of TCDD, and conversely, BRCA‐1 overexpression was found to stabilize ARNT and reinforce CYP 1A1 induction by TCDD. Thus, these results support the notion that ARNT should be viewed as a cancer chemopreventive target. Furthermore, in the present study, curcumin was found to suppress AhR and ARNT, which is likely to have a synergistic chemopreventive effect.
Curcumin is known to have anti‐inflammatory, antioxidant, antineoplastic, antiangiogenic, and antimicrobial activities. These various actions of curcumin may be due to many cellular pathways that include transcription factors, hormones, and growth factors and their associated receptors. In particular, in tumors, these curcumin‐targeted pathways might be associated with all stages of carcinogenesis, which is why curcumin and its analogs are currently being developed as chemopreventive and anticancer agents. Here, we propose another mechanism for the anticarcinogenic effect of curcumin, namely, that curcumin inhibits cellular transformations in response to TCDD by stimulating the degradations of AhR and ARNT. Hopefully, the findings of this study will be found helpful during the design stage of pharmacodynamic studies aimed at further developing the use of curcumin as a chemopreventive or anticancer agent.
Supporting information
Fig. S1. Effect of curcumin on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced focus formation.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (R01‐2006‐000‐10977‐0) and a grant from Seoul National University Cancer Research Institute (2007).
References
- 1. Bock KW, Köhle C. Ah receptor: dioxin mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol 2006; 72: 393–404. [DOI] [PubMed] [Google Scholar]
- 2. Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD: experimental, mechanistic, and epidemiologic evidence. Annu Rev Pharmacol Toxicol 1994; 34: 343–72. [DOI] [PubMed] [Google Scholar]
- 3. Burbach KM, Poland A, Bradfield CA. Cloning of the Ah receptor cDNA reveals a distinctive ligand‐activated transcription factor. Proc Natl Acad Sci USA 1992; 89: 8185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolwick KM, Swanson HI, Bradfield CA. In vitro analysis of Ah receptor domains involved in ligand activated DNA recognition. Proc Natl Acad Sci USA 1993; 90: 8566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estabrook RW. The remarkable P450s: a historical overview of these versatile hemeprotein catalysts. Faseb J 1996; 10: 202–20. [DOI] [PubMed] [Google Scholar]
- 6. Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 2000; 27: 67–73. [DOI] [PubMed] [Google Scholar]
- 7. Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 1998; 19: 1–27. [DOI] [PubMed] [Google Scholar]
- 8. Cavalieri EL, Stack DE, Devanesan PD et al . Molecular origin of cancer: catechol estrogen‐3,4‐quinones as endogenous tumor initiators. Proc Natl Acad Sci USA 1997; 94: 10937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17b‐estradiol hydroxylation catalyzed by human cytochrome P4501B1. Proc Natl Acad Sci USA 1996; 93: 9776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawamori T, Lubet R, Steele VE et al . Chemopreventive effect of curcumin, a naturally occurring anti‐inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 1999; 59: 597–601. [PubMed] [Google Scholar]
- 11. Singh SV, Hu X, Srivastava SK et al . Mechanism of inhibition of benzo[a]pyrene‐induced forestomach cancer in mice by dietary curcumin. Carcinogenesis 1998; 19: 1357–60. [DOI] [PubMed] [Google Scholar]
- 12. Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett 1997; 116: 197–203. [DOI] [PubMed] [Google Scholar]
- 13. Meht RG, Moon RC. Characterization of effective chemopreventive agents in mammary gland in vitro using and initiation‐promotion protocol. Anticancer Res 1991; 11: 593–6. [PubMed] [Google Scholar]
- 14. Soudamini NK, Kuttan R. Inhibition of chemical carcinogenesis by curcumin. J Ethnopharmacol 1989; 27: 227–33. [DOI] [PubMed] [Google Scholar]
- 15. Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia‐induced factor‐1 by degrading arylhydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol 2006; 70: 1664–71. [DOI] [PubMed] [Google Scholar]
- 16. Shin DH, Li SH, Chun YS, Huang LE, Kim MS, Park JW. CITED2 mediates the paradoxical responses of HIF‐1a to proteasome inhibition. Oncogene 2008; 20: 1939–44. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez‐Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl‐hydrocarbon receptor‐deficient mice are resistant to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin induced toxicity. Toxicol Appl Pharmacol 1996; 1: 173–9. [DOI] [PubMed] [Google Scholar]
- 18. Yoon CY, Park M, Kim BH et al . Gene expression profile by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin in the liver of wild‐type (AhR+/+) and aryl hydrocarbon receptor‐deficient (AhR–/–) mice. J Vet Medical Sci 2006; 7: 663–8. [DOI] [PubMed] [Google Scholar]
- 19. Yang JH, Vogel C, Abel J. A malignant transformation of human cells by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin exhibits altered expressions of growth regulatory factors. Carcinogenesis 1999; 20: 13–18. [DOI] [PubMed] [Google Scholar]
- 20. Wölfle D, Marotzki S, Dartsch D, Schäfer W, Marquardt H. Induction of cyclooxygenase expression and enhancement of malignant cell transformation by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin. Carcinogenesis 2000; 21: 15–21. [DOI] [PubMed] [Google Scholar]
- 21. Puga A, Nebert DW, Carrier F. Dioxin induces expression of c‐fos and c‐jun proto‐oncogenes and a large increase in transcription factor AP‐1. DNA Cell Biol 1992; 11: 269–81. [DOI] [PubMed] [Google Scholar]
- 22. Weiss C, Faust D, Schreck I et al . TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene 2008; 27: 2198–207. [DOI] [PubMed] [Google Scholar]
- 23. Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 2006; 6: 947–60. [DOI] [PubMed] [Google Scholar]
- 24. Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin or indolo (3,2‐b) carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA 1996; 93: 2322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue‐specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol 2001; 41: 297–316. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki M, Tanaka Y, Okino ST et al . Polymorphisms of the CYP1B1 gene as risk factors for human renal cell cancer. Clin Cancer Res 2004; 10: 2015–9. [DOI] [PubMed] [Google Scholar]
- 27. McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 CYP1B1 activity in renal cell carcinoma. Br J Cancer 2004; 91: 966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tokizane T, Shiina H, Igawa M et al . Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res 2005; 11: 5793–801. [DOI] [PubMed] [Google Scholar]
- 29. Thapliyal R, Maru GB. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo . Food Chem Toxicol 2001; 39: 541–7. [DOI] [PubMed] [Google Scholar]
- 30. Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF‐7 human breast carcinoma cells. Biochem Pharmacol 1998; 56: 197–206. [DOI] [PubMed] [Google Scholar]
- 31. Rinaldi AL, Morse MA, Fields HW et al . Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits(‐)‐benzo(a) pyrene‐7R‐trans‐7,8‐dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res 2002; 62: 5451–6. [PubMed] [Google Scholar]
- 32. Nishiumi S, Yoshida K, Ashida H. Curcumin suppresses the transformation of an aryl hydrocarbon receptor through its phosphorylation. Arch Biochem Biophys 2007; 466: 267–73. [DOI] [PubMed] [Google Scholar]
- 33. Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem 1999; 274: 28708–15. [DOI] [PubMed] [Google Scholar]
- 34. Ma Q, Baldwin KT. 2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin‐induced degradation of ArylHydrocarbon Receptor (AhR) by the ubiquitin–proteasome pathway. J Biol Chem 2000; 275: 8432–8. [DOI] [PubMed] [Google Scholar]
- 35. Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole‐body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator‐dependent system in gut. J Clin Invest 2007; 117: 1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang HJ, Kim HJ, Kim SK et al . BRCA1 modulates xenobiotic stress‐inducible gene expression by interacting with ARNT in human breast cancer cells. J Biol Chem 2006; 281: 14654–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of curcumin on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD)–induced focus formation.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


