Abstract
Molecular‐targeting drugs with fewer severe adverse effects are attracting great attention as the next wave of cancer treatment. There exist, however, populations of cancer cells resistant to these drugs that stem from the instability of tumor cells and/or the existence of cancer stem cells, and thus specific toxicity is required to destroy them. If such selectivity is not available, these targets may be sought out not by the cancer cell types themselves, but rather in their adjacent cancer microenvironments by means of hypoxia, low pH, and so on. The anaerobic conditions present in malignant tumor tissues have previously been regarded as a source of resistance in cancer cells against conventional therapy. However, there now appears to be a way to make use of these limiting factors as a selective target. In this review, we will refer to several trials, including our own, to direct attention to the utilizable anaerobic conditions present in malignant tumor tissues and the use of bacteria as carriers to target them. Specifically, we have been developing a method to attack solid cancers using the non‐pathogenic obligate anaerobic bacterium Bifidobacterium longum as a vehicle to selectively recognize and target the anaerobic conditions in solid cancer tissues. We will also discuss the existence of low oxygen pressure in tumor masses in spite of generally enhanced angiogenesis, overview current cancer therapies, especially the history and present situation of bacterial utility to treat solid tumors, and discuss the rationality and future possibilities of this novel mode of cancer treatment. (Cancer Sci 2010)
There are many well‐known orthodox methods to treat cancers, including surgery, radiation therapy, and chemotherapy, that have not always been effective despite considerable scientific advances. This is due mainly to the existence of metastases when the primary cancer is detected and/or recurrence with acquired resistance of cancer cells to anticancer reagents. These troublesome situations have spurred the emergence of new therapeutic methods, such as gene therapy and new drugs and delivery systems, but these new approaches have not been sufficient to overcome the deadlocks in cancer treatment. In the face of such circumstances, there are those who believe it is necessary to adopt ideas divergent from common sense to fight cancer, even if the idea might appear to be an abnormal or dangerous one.
The stigma of the word “bacteria” quickly stirs notions of something dangerous and pathogenic. Injection of bacteria into the blood is generally regarded as an irrational test that would at best induce artificial bacteriemia or septicemia, even if the bacteria were non‐pathogenic. However, through systematic accumulation of experimental results, we have become convinced that the treatment of solid cancer tissues with the non‐pathogenic obligate anaerobic bacterium Bifidobacterium longum can be safe, rational, and effective.
Human life is dependent on its coexistence with microorganisms. Intestinal bacteria are one such example, along with the use of gene‐engineering techniques to provide recombinant medicines through microorganisms and plasmids as effective and inexpensive drug producers. The recent emergence of probiotics has been seen not only for intestinal disorders, but also for maintenance of the skin and oral cavity.( 1 , 2 ) We have been further trying to expand the utility of probiotics to systemically treat solid cancers using intravenous administration by directing our attention to the particular anaerobic conditions within malignant tumors.( 3 , 4 ) As such, we applaud current efforts with toxicity‐attenuated Salmonella and Clostridium to treat solid cancers in the USA,( 5 , 6 , 7 ) using ideas similar to our own but with increased risk. Those studies have been already been evaluated in phase I clinical trials.( 5 , 6 ) More information is available from URL: http://www.clinicaltrials.gov/ct.
In the current review, the authors will overview the cancer therapies of today, then describe their findings and potential use of anaerobic bacteria for systemic delivery to solid cancer tissues based on the documented existence of low oxygen conditions in malignant tumors.
Conventional Cancer Therapies
In terms of complete cytoreduction, the best treatment for cancer is surgery, if there are no metastases and if the patient’s condition permits. Furthermore, advances in medical engineering and surgical techniques have developed minimally‐invasive treatment that is advantageous for the recovery and quality of life of patients. Radiotherapy for cancer is also a localized treatment, but it is limited by the radioresistance of cancer cells and concomitant damage to normal tissues. Novel targets uncovered by molecular approaches will hopefully be exploited to overcome the phenomenon of radioresistance. However, despite the inevitable progress of surgery and radiotherapy, neither will be completely effective in cases with distant dissemination and/or cancer metastasis. In such instances, there will be no choice but to use systemic therapies, such as chemotherapy and immunotherapy.
Conventional cytotoxic chemotherapy was developed based on the enhanced proliferation of malignant cells. As the epithelial cell cycle of the gastrointestinal tract and hematopoietic progenitors are generally more rapid than that of cancer cells, such chemotherapies may also cause damage to gastrointestinal and immunological defense functions. Such adverse effects not only reduce the quality of life of cancer patients, but also impose limits on the dose of therapeutic antitumor treatment.
Development of Molecular Targeted Therapies
Recent concepts of cancer treatment have been changing from conventional cytotoxic chemotherapy to molecular targeted cancer therapeutics directed towards the molecular specificity of cancer cells. The idea of this therapy is to target tumor cell receptors or signaling events that are critical and unique to tumor growth and progression, thus reducing the side‐effects to patients by discriminating between normal cells and tumor cells.( 8 , 9 , 10 ) Molecular targeted agents, to date, include small molecular compounds, monoclonal antibodies, siRNA, and permeable peptides that cause apoptosis, senescence, or cell cycle arrest.
Small molecular compounds have been developed to inhibit enzyme activity associated with cancer malignancy. Imatinib,( 11 ) for example, is a tyrosine kinase inhibitor that inhibits abl‐specific phosphorylation and is approved for use in patients with chronic myelogenous leukemia. There are other representative examples of small molecular compounds in clinical practice, such as gefitinib,( 12 ) erlotinib,( 13 ) and bortezomib.( 14 ) The epidermal growth factor receptor/HER2 dual tyrosine kinase inhibitor lapatinib has also been approved for clinical use as a HER2‐targeted therapy.( 15 )
By directing attention to membrane proteins that regulate cell proliferation signals, an antibody against the protein can be used to shut down tumoral growth signals or induce cellular cytotoxicity. Rituximab,( 16 ) trastuzumab,( 17 ) cetuximab,( 18 ) and bevacizumab( 19 ) are the representative examples of mAbs in clinical use. Of the humanized antibodies, anti‐HER2/neu trastuzumab has been approved and widely used for patients with metastatic breast cancer expressing HER2/neu proteins. Rituximab is currently used for the treatment of patients with follicular lymphoma. Anti‐epidermal growth factor receptor (cetuximab or panitumumab) therapies are approved for the treatment of metastatic colorectal carcinoma, but are believed to only affect patients with the wild‐type KRAS gene.( 20 )
Similarly, siRNAs are expected to be used for cancer therapies, but the drug delivery systems for these remain to be developed.( 21 ) Permeable peptides that modify protein–protein interactions will also be effective to suppress malignant phenotypes of cancer cells.( 22 ) Other reports on targeted agents for various types of cancer have been released as well.( 23 , 24 , 25 , 26 , 27 , 28 )
In addition to anticancer drugs, cancer vaccines have attracted attention as a new generation of cancer therapy using the intrinsic immune response of cancer patients.( 29 ) Oncoantigens, which are highly expressed in cancer cells, can be immunogens that stimulate a patient’s immune‐survey system.( 30 , 31 ) Although there has been limited success so far, many clinical trials are ongoing worldwide to evaluate the effect of this therapy. Recently, it has been found that CCR‐4 is expressed on most adult T‐cell leukemia/lymphoma cells. Based on this finding, humanized anti‐CCR‐4 therapeutic monoclonal antibodies were developed and phase I testing has been carried out with promising results.( 32 ) Regardless of the enormous progress made in molecular targeted therapies, including that made in cancer vaccines, the heterogeneity of cancer cells remains an obstacle against complete cytoreduction.
Drug Delivery Systems for Cancer Therapy
Once an anticancer drug is systemically given to a patient, it circulates throughout the body, being quickly metabolized in the liver and excreted through the kidney. Thus, the amount of drug reaching the cancer tissue is very limited, and the portion of the drug reaching normal tissues causes various side‐effects. Ideally, the drug should be maintained at a high concentration for a long enough duration in the cancer tissue to kill cancer cells, but at a low dose and for a short duration in normal tissues.
The agents requiring delivery to tumor tissues include general anticancer drugs, anti‐angiogenic factors, genes coding enzymes to convert a pro‐drug into an active one, and shRNA to suppress cancer cell growth signals.( 33 ) However, selective and effective methods to deliver these materials to tumor tissues remain to be developed. One novel drug delivery system (DDS) is the macromolecular carrier system, which is designed to permeate tumor blood vessels and accumulate in solid tumors by making use of the enhanced permeability and retention effect.( 34 ) In this system, the carrier should be small enough (<100 nm) to effectively extravasate from the blood into solid tumor tissues. Such nanotechnology holds great promise for this purpose. There are reports of nano particles forming aggregates of micrometer particles.( 35 ) As seen with asbestos, the danger of mesothelioma occurrence is of concern in the use of nanotechnology; it has been reported that mesothelioma in p53+/− mice was induced by i.p. application of a multi‐wall carbon nanotube and that nano particles actually caused malignant progression of tumor cells.( 36 , 37 ) Recently, selective uptake of the agent into target cancer cells has been attempted to exploit cell surface receptors by installing moieties onto carriers.( 38 , 39 )
To cope with the heterogeneity of cancer populations, a new type of DDS remains to be developed in which the anticancer drug can selectively reach tumor tissues at a sufficiently large dose to kill the resistant population of cancer cells. In addition to heterogeneity, another deadlock to complete cytoreduction by DDS is attributed to the anaerobic condition of solid tumors, which imparts the same resistance to chemotherapy and radiotherapy.
Therapeutic Trials to Make Use of the Anaerobic Conditions in Malignant Tumors
There are several ongoing studies that focus on attacking solid tumors through their unique anaerobic conditions. One of them is based on interfering with the activity of hypoxia‐inducible transcription factors (HIFs).( 40 ) HIF‐1 is an essential component in changing the transcriptional repertoire when oxygen levels drop and has been regarded as a very important target for cancer drug development. There is also a chemotherapeutic idea that might have beneficial effects in hypoxic tumor regions using a bioreductive drug like mitomycin C.( 41 ) The third area of study, which is our own, is to specifically target hypoxic tumor regions using bacteria.
Anaerobic Conditions and Angiogenesis in Solid Cancer Tissues
The principle of using anaerobic bacteria as specific transporters to cancer tissues lies in the fact that tumor masses have unique anaerobic environments. It has been widely reported that while oxygen pressure is 3–5% and 20–100 mmHg in normal tissues, an anaerobic environment exists (<1% oxygen and 0–20 mmHg) within solid cancer tissues.( 42 , 43 ) How is such a low oxygen condition created despite evident enhancement of angiogenesis in malignant tumors? One explanation is that angiogenesis is outpaced by the growth of cancer cells, another is that the blood vessels formed by angiogenesis in solid tumors are insufficient to supply oxygen to all areas of the tumor.
The blood vessels formed by angiogenesis in cancer tissues have been reported to be unstable, disorganized with numerous intravascular connections and shunts, and thus unable to deliver fresh blood to the distal regions of malignant tumors.( 43 ) It is well known that angiogenesis is induced by vascular endothelial growth factor produced from cancer cells through HIF‐1 and by cytokines from inflammatory cells in tumor tissues.( 43 ) Endothelial cells recruited from pre‐existing normal blood vessels or endothelial progenitors from the bone marrow grow, form vacuoles, and in turn develop into neovascular systems in tumors. These blood vessels are expected to deliver oxygen and nutrients to tumor tissues. It has been reported, however, that endothelial cells that grow in this way are apt to die by apoptosis, such that newly formed tumoral blood vessels usually exhibit repeating cycles of appearance and disappearance.( 42 , 43 ) Furthermore, the remaining vascularization is immature and tends to form branches and intravascular shunts, and the tips of the vessels are very often closed, resulting in fluctuations and stoppages in blood flow.( 42 , 43 ) Thus, the delivery of oxygen and nutrition is considered to be inadequate to every corner of the tumor, leading to the appearance of anaerobic conditions in spite of enhanced neovascularization.
The fact that anaerobic bacteria can survive in the low oxygenic conditions of solid cancers is one of the important factors in the localization of anaerobic bacteria to malignant tumors. If bacteria are systemically introduced through a venous blood vessel, they will penetrate into the tissue through the fragile regions of blood vessels and/or will remain in tumoral blood vessels that are isolated from proximal normal blood vessels. In particular, B. longum does not possess flagellin for motility and invasion, and the bacteria appear to remain, survive, and grow in anaerobic tumor conditions.
As the cancer cells in these regions are generally thought to be resistant to radiation therapy and anticancer drugs, our trials using anaerobic bacteria make use of a usually troublesome region that is a sanctuary for cancer cells to protect themselves from attack. It has been reported that tumor hypoxia diminishes apoptotic potential and induces malignant progression of cancer cells; in other words, hypoxia increases the invasiveness and metastasis of cancer cells.( 44 , 45 , 46 ) It has also been shown that a highly tumorigenic fraction of human neuroblastoma was found in the hypoxic regions of tumor tissue, indicating that hypoxia enhances tumor stemness.( 47 ) Therefore, using anaerobic bacteria for solid cancer treatment seems ideal, as they are able to attack evasive, metastatic cell populations that are resistant to conventional chemotherapy and radiotherapy.
Bacterial Therapy for Cancer
Cancer therapy with bacteria was purportedly triggered by early observations of spontaneous tumor regression in cancer patients: tumors inexplicably regressed in gas gangrene caused by clostridial infection.( 6 , 48 ) In 1890, William Coley documented several trials using bacteria and began a large‐scale effort for cancer treatment by inoculating Streptococcus pyogenes into his patients with inoperable tumors.( 6 ) This work seemed to stimulate the emergence of bacterial tumor therapy and tumor immunology. Unfortunately, oncolysis by Clostridium itself was once regarded to be of limited, if any, clinical usefulness,( 49 ) but genetic manipulation of bacteria has continuously progressed the field of bacterial oncolysis. Now, the pre‐clinical and clinical data supporting the use of bacteria as a tumor‐targeting tool are accumulating, and bacteria are expected to constitute a promising method in clinical treatment.( 50 )
In addition to bacterial treatment, biological therapy with viruses is also being studied by modern research.( 51 , 52 ) To discriminate between cancer cells and non‐cancerous cells, genetically engineered oncolytic viruses have been constructed to target tumors by exploiting each cancer’s unique biology. The efficacy can be enhanced by carrying therapeutic genes to induce cellular apoptosis/suicide and/or facilitate tumor/virus imaging. Some viruses are armed with genes to affect the tumor microenvironment, such as genes encoding for angiostatic factors, inflammatory cytokines, or proteases that modulate the extracellular matrix to suppress tumor vascularization, or to enhance antitumor immune responses and viral spread throughout the solid tumor.( 51 , 52 )
Non‐Pathogenic Anaerobic Bifidobacterium to Treat Solid Cancers
Malmgren and Flanigan( 53 ) carried out experiments by injecting Clostridium tetani spores into tumor‐bearing and non‐tumor‐bearing mice and showed that only the former died, which indicated that the spores had germinated and produced toxins in the anaerobic conditions existing only in the tumor tissues. Their report inspired our study to target solid cancer tissues with the use of non‐pathogenic anaerobic bacteria.
Bifidobacterium was considered as a safe candidate carrier because the bacterial medicine, Bifidobacterium bifidum (LacB; Nikken Kagaku, Tokyo, Japan), has long been prescribed for infant patients in Japan. Indeed, when LacB suspended in saline or PBS was injected into tumor‐bearing mice through the tail vein, the animals showed no visible adverse symptoms. It has been repeatedly observed that the bacteria disappear from normal tissues and organs, such as the liver, spleen, kidney, lung, blood, and bone marrow, within 48–96 h, and that the bacteria grow only in tumor tissues (Fig. 1).( 54 ) The bone marrow was of particular concern for us because of its relatively low oxygen pressure, but complete clearance was seen.
Figure 1.
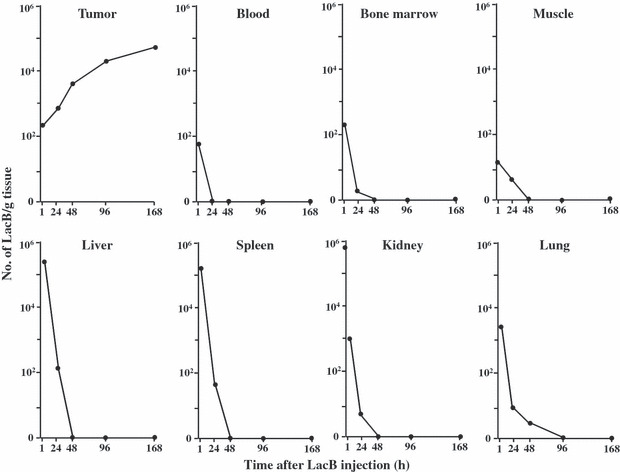
Specific distribution of Bifidobacterium bifidum (LacB) in tumor tissues( 54 ) following a single i.v. injection of 5 × 106 viable bacilli into Ehlich solid tumor‐bearing mice. Each point represents the mean of the number of bacilli per gram tissue of eight mice.
Later, the development of an expression vector consisting of a promoter from a gene coding a histone‐like protein and a replication region in Bifidobacterium greatly promoted the study of a delivery system to treat solid cancer.( 55 , 56 , 57 , 58 ) The cytosine deaminase of Escherichia coli (e‐CD) was inserted into the plasmid under the promoter region of the plasmid, which converts low‐toxic 5‐fluorocytosine (5FC), a commercially available antifungal reagent, to active 5‐fluorouracil (5FU), a popular anticancer drug specifically for tumor tissues (Fig. 2).
Figure 2.
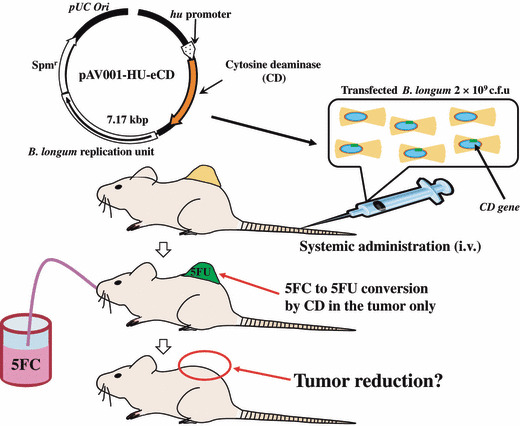
Concept of cancer treatment by combining cytosine deaminase of Escherichia coli (e‐CD)‐transformed Bifidobacterium longum (i.v.) with the prodrug 5‐fluorocytosine (5FC) (given orally). 5FU, 5‐fluorouracil.
In early cancer treatment experiments, autochthonous tumors of rat breast cancer were developed with the carcinogen 7,12‐dimethylbenz(a)anthracene. Suppression of tumor growth was observed in the group treated with i.v. injection of bacteria transformed by e‐CD‐expression vectors, and the prodrug 5FC given orally (Fig. 3a).( 58 ) The same treatment system has been successfully confirmed as effective on several human cancers transplanted into immunologically‐deficient nude mice. For example, KPL‐1 (human breast cancer) tumor‐bearing immunodeficient nude mice were treated (Fig. 3b) in a way similar to the rat experiment described above, and the antitumor effect of this treatment has also been verified in human stomach cancer (data not shown).
Figure 3.
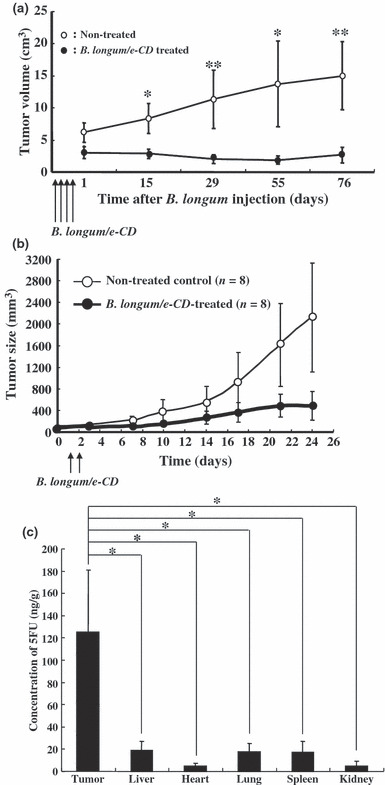
Antitumor effects of i.v.‐injected cytosine deaminase of Escherichia coli (e‐CD)‐transformed Bifidobacterium longum (B. longum/e‐CD) combined with 5‐fluorocytosine (5FC) (given orally). (a) Comparison of the tumor volumes of non‐injected rats (n = 5) with those of B. longum/e‐CD i.v. injected rats (n = 15).( 58 ) Rats bearing 7,12‐dimethylbenz(a)anthracene‐induced mammary tumors received i.v. B. longum/e‐CD and 500 mg/kg/day of 5FC.*P < 0.05; **P < 0.01. (b) Antitumor assessment of B. longum/e‐CD in nude mice transplanted with KPL‐1 human mammary tumor cells. Tumor‐bearing nude mice (n = 8) were given a dose of transformed bacteria cells i.v. (5.9 × 109 c.f.u./mouse), followed by 5FC (orally) for 21 days. (c) Measurement of 5‐fluorouracil (5FU) concentration in various tissues( 58 ) in rats bearing MRMT‐1 mammary gland carcinoma. Rats were given B. longum/e‐CD at 1.1 × 1010 c.f.u./rat i.v. and 5FC by intragastric gavage for 4 days starting from day 4 after bacterium injection. The concentration of 5FU in normal tissues and tumor tissues was measured. A rat given 5FC without injection of B. longum/e‐CD was used as the control. *P < 0.05.
In safety studies, toxicity experiments preformed with our model on cynomolgus monkeys have shown no severe adverse effects. Further anaphylaxis tests were conducted with guinea pigs, which are considered to be the most sensitive models to antigens, but no serious symptoms were observed (Table 1). The mechanism explaining non‐anaphylaxis in animals immunized with B. longum remains to be investigated.
Table 1.
Anaphylaxis symptoms( 58 ) of actively immunized guinea pigs injected i.v. with cytosine deaminase of Escherichia coli (e‐CD)‐transformed Bifidobacterium longum (B. longum/e‐CD) or OVA 14 days after final sensitization.
| Group | Sensitized antigen | Cause antigen | No. of animals | Antigen challenge outcome | ||||
|---|---|---|---|---|---|---|---|---|
| (−) | (+/−) | (+) | (++) | (+++) | ||||
| A | B. longum/e‐CD | B. longum/e‐CD | 5 | 4 | 1 | 0 | 0 | 0 |
| B | B. longum/e‐CD + FCA | B. longum/e‐CD | 5 | 5 | 0 | 0 | 0 | 0 |
| C | OVA + FCA | OVA | 5 | 0 | 0 | 5 | 5 | 4 |
| D | Saline + FCA | B. longum/e‐CD | 5 | 5 | 0 | 0 | 0 | 0 |
Anaphylaxis symptoms were quantified by the following criteria: −, no symptoms; +/−, scrub of face or ear and/or scratch of nose; +, coughing or locomotion ataxia; ++, convulsion or roll, but no death observed within 1 h; and +++, death observed within 1 h. FCA, Freund’s complete adjuvant; OVA, ovalbumine.
Recently, the production efficiency of 5FU has been increased to more than 100 times that of the primary vector (120 ng/g; Fig. 3c). This was achieved by mutating the active center region of cytosine deaminase at the 324th amino acid Asp to Ala to better fit 5FC than the natural ligand, cytosine.( 59 ) In a representative experiment, when 20 mg/kg 5FU alone was given orally, the concentrations of 5FU detected in the tumor and liver were 43.6 ng/g tissue and 253.5 ng/g tissue, respectively. However, when a revised recombinant B. longum was given i.v. with 5FC (750 mg/kg, given orally), 5FU concentrations were 13,196 ng/g tissue in the tumor and only 10.6 ng/g tissue in the liver. It was reported that when capecitabine, a well‐known prodrug of 5FU, was given orally at 1255 mg/m2, 5FU concentration was 510 ng/g in tumor tissue and 296 ng/g in the liver.( 60 ) Thus, our method seems to be both more selective and effective than presently available prodrug systems of 5FU.
Non‐Induction of Inflammatory Cytokines by Genetically Modified B. longum
In further tests to examine immunological toxicity, the blood levels of various inflammatory cytokines were examined after i.v. injection of B. longum carrying the e‐CD expression vector or non‐pathogenic E. coli as a control. As shown in Figure 4, no inflammatory cytokines were induced by inoculation with B. longum, whereas E. coli clearly induced cytokines such as interleukin (IL)‐1β, IL‐18, and IL‐6. These results indicated that genetically modified B. longum does not induce septicemia. Similar results have been reported, showing that Bifidobacterium induces low levels of IL‐12 and tumor necrosis factor‐α production independently of the strain, and various strains of Lactobacillus differed substantially in their capacities to induce IL‐12 and tumor necrosis factor‐α.( 61 )
Figure 4.
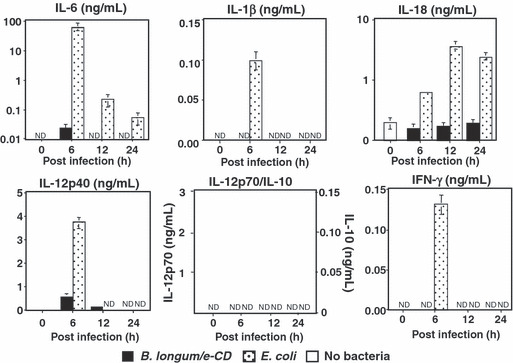
Production of inflammatory cytokines in C57BL/6 mice injected i.v. with cytosine deaminase of Escherichia coli (e‐CD)‐transformed Bifidobacterium longum (B. longum/e‐CD) or non‐pathogenic E. coli (control). Cytokines were assessed with ELISA 6 h after injection. Closed bars, blood of mice injected with B. longum/e‐CD; dotted bars, blood of mice injected with non‐pathogenic E. coli; open bars, normal blood. IFN, interferon; IL, interleukin; ND, not detected.
To evaluate the toxicity of genetically modified B. longum, a number of preclinical studies have been also carried out in several animal species, including normal mice, nude mice, normal rats, nude rats, and monkeys. Both pharmacological and preliminary general toxicity studies were done, none of which revealed serious unfavorable toxicities.
Other Recent Examples Using Bacteria in Clinical Applications
In the USA, Salmonella VNP20009 was recently used to treat metastases of melanoma and kidney cancers in phase I testing.( 6 , 62 ) The minimum dose tolerable to toxicity was 3 × 108/body surface area, and confirmation of the existence of the bacteria was done by biopsy or fine needle assay of tumor tissue. Shrinking of the tumor was not observed in any case, and the existence of bacteria in the tumor was detected in only three patients. In four patients, bacteriosis was observed but the bacteria were not detected in the tumors. This was noted to be due to the insensitivity of fine needle assay, as the bacteria were later confirmed in the excised whole tumor tissue. The research group speculated that one factor determining the frequency of bacterial detection in tumors is the time the bacteria can exist in the blood; it was observed that VNP20009 disappeared from the bloodstream of humans much more quickly than in monkeys.
Another current pilot study is a prodrug treatment combined with VNP20009. The first report described that attenuated VNP20009 carrying cytosine deaminase (TAPET‐CD) was given directly to tumors and showed a 38–79% antitumor growth effect. For refractory cancers, intratumoral inoculation with VNP20009‐CD and oral 5FC was performed safely without any adverse effects, and the bacteria did not spread throughout the body.( 63 )
Lastly, when spores of the anaerobic bacterium Clostridium novyi‐NT were systemically injected into animals, they germinated exclusively within the hypoxic cancer regions. Approximately 30% of mice treated with such spores were cured of their cancers despite the viable tumor rim initially remaining after spore germination. The mechanism underlying this effect was shown to be immune‐mediated because the cured animals rejected a subsequent challenge of the same tumor. It was noted that the induced immune response, when combined with the bacteriolytic effects of C. novyi‐NT, could eradicate large established tumors. Clinical trials of this technique are now underway.( 6 )
Future Prospects and Closing Remarks
The rationality and promise of targeting the anaerobic conditions in solid cancers was addressed in this review in terms of the selective delivery and potential capability to cope with the resistance of cancer cell populations in hypoxic regions to radiation and chemotherapy. It was also noted in this review that the non‐pathogenic and obligate anaerobic Bifidobacterium is a seemingly safe and effective carrier for selective drug delivery to anaerobic regions in solid cancers based on the authors’ substantial experimental data. To cope with cases that have dissemination of cancer cells in the peritoneal cavity, for which this system will not be effective, further development of effective therapy regimens are required.( 64 )
Presently, in the USA, clinical trials are being carried out to treat cancer with bacteria, but it is our concern that the attenuated Salmonella and Clostridium being used may revert to their original toxic natures; the vegetative anaerobicity of Salmonella targets normal organs as well as tumors, and a considerable amount of bacteria were detected in the livers of mice treated with Salmonella.( 65 , 66 ) In addition, the flagellin of Salmonella imparts cell motility and invasiveness, which is effective for diffusion inside the tumor but can also lead to invasion into other important organs, such as the gallbladder. Clostridium is being used by a group of John Hopkins School of Medicine (http://www.clinicaltrials.gov/ct), whose trials are now in phase I testing.
In contrast, B. longum bacteria are inherently non‐pathogenic and seem to be much safer than those being trialed abroad. Furthermore, a variety of enzymes, cytokines, and prodrug‐activating enzymes can be produced by inserting the corresponding genes into vectors. Combination trials of our present treatment using the e‐CD‐carrying Bifidobacterium and 5FC system with anti‐angiogenic drugs, and the development of a carrier which helps the product to easily diffuse inside the tumor, are both underway with positive results (data not shown). As the drugs produced by the bacteria destroy adjacent tumor cells, it will be necessary to attack the residual rim of well‐oxygenated tumor cells that may subsequently expand. Obviously, shRNA delivered by a bifidobacterium bacterial system( 33 , 67 ) is being considered, however, the means of effectively transferring shRNA into cancer cells remain to be developed.
In future generations of bacterial agents, insertion of a fluorescence protein into the expression vector( 68 ) will make it possible to observe the real time in vivo spread of the bacteria. Additionally, why Bifidobacterium longum exhibits no serious anaphylaxis is a very intriguing issue from the immunological point of view; the mechanism by which the bacteria are scarcely recognized as exogenous antigens, and how sepsis does not occur with these bacteria, will be interesting issues for innate and adoptive immunologists in relation to toll‐like receptors( 69 ) and NLRPs.( 70 , 71 )
The most important and pressing problem in cancer therapy is how to cope with the heterogeneity of cancer cells, which causes metastasis and resistance to cancer treatment including even the most sophisticated molecular target therapies. Recently, the concept of cancer stem cells has emerged and suggests that the removal of stem cells, from which heterogeneous cell populations are produced, is enough to cure cancer. Accordingly, considerable attention has been directed to the molecular targets of such cancer stem cells. It remains unclear, however, if the concept is applicable to all types of cancer disease and if the molecular specificity of cancer stem cells can be discriminated from normal stem cells. As a prospective cancer therapy, it will also be important to direct our attention to the specificity in cancer microenvironments to deliver a sufficient dose of agents to kill resistant populations, including cancer stem cells. For this purpose, a DDS using non‐pathogenic bacteria fortified with expression vectors for various proteins, such as enzymes to produce more cytocidal drugs and cytokines, may be key for cancer therapy.
In conclusion, Malmgren’s report originally led us to study a new DDS using non‐pathogenic anaerobic bacteria through the idea that troubles in the human, a bioorganism, could be treated with the help of other bioorganisms. As very sophisticated scientific systems are not always effective for cancer treatment, a very primitive and simple system using Bifidobacterium may be essential for the future of cancer therapy.
Acknowledgments
We thank all of our colleagues at Anaeropharma Science Inc. for their technical support and useful discussions. This work was supported by grants from the Japan Science and Technology Agency and the New Energy and Industrial Technology Development Organization.
References
- 1. Gueniche A, Knaudt B, Schuck E et al. Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. Br J Dermatol 2008; 159: 1357–63. [DOI] [PubMed] [Google Scholar]
- 2. He X, Lux R, Kuramitsu HK, Anderson MH, Shi W. Achieving probiotic effects via modulating oral microbial ecology. Adv Dent Res 2009; 21: 53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taniguchi S. Anti‐cancer therapies with anaerobic bacteria (Japanese). Exp Med 2009; 27: 319–24. [Google Scholar]
- 4. Fujimori M, Amano J, Taniguchi S. The genus Bifidobacterium for cancer gene therapy. Curr Opin Drug Discov Devel 2002; 5: 200–3. [PubMed] [Google Scholar]
- 5. Toso JF, Gill VJ, Hwu P et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy‐dawn of a new era. Cancer Lett 2008; 259: 16–27. [DOI] [PubMed] [Google Scholar]
- 7. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A 2001; 98: 15155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan DS, Thomas GV, Garrett MD et al. Biomarker‐driven early clinical trials in oncology: a paradigm shift in drug development. Cancer J 2009; 15: 406–20. [DOI] [PubMed] [Google Scholar]
- 9. Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 2010; 10: 267–77. [DOI] [PubMed] [Google Scholar]
- 10. Brown P, Hunger SP, Smith FO, Carroll WL, Reaman GH. Novel targeted drug therapies for the treatment of childhood acute leukemia. Expert Rev Hematol 2009; 2: 145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carpiuc KT, Stephens JM, Botteman MF, Feng W, Hay JW. A review of the clinical and economic outcomes of imatinib in Philadelphia chromosome‐positive acute lymphoblastic leukemia. Expert Opin Pharmacother 2007; 8: 2775–87. Review. [DOI] [PubMed] [Google Scholar]
- 12. Cappuzzo F, Finocchiaro G, Metro G et al. Clinical experience with gefitinib: an update. Crit Rev Oncol Hematol 2006; 58: 31–45. [DOI] [PubMed] [Google Scholar]
- 13. Gridelli C, Rossi A, Maione P et al. Erlotinib in non‐small‐cell lung cancer. Expert Opin Pharmacother 2007; 8: 2579–92. [DOI] [PubMed] [Google Scholar]
- 14. Utecht KN, Kolesar J. Bortezomib: a novel chemotherapeutic agent for hematologic malignancies. Am J Health Syst Pharm 2008; 65: 1221–31. [DOI] [PubMed] [Google Scholar]
- 15. Nahta R, Shabaya S, Ozbay T, Rowe DL. Personalizing HER2‐targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens. Curr Pharmacogenomics Person Med 2009; 7: 263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schuster SJ, Venugopal P, Kern JC, McLaughlin P. GM‐CSF plus rituximab immunotherapy: translation of biologic mechanisms into therapy for indolent B‐cell lymphomas. Leuk Lymphoma 2008; 49: 1681–92. [DOI] [PubMed] [Google Scholar]
- 17. Ismael G, Rosa DD, De Azambuja E, Braga S, Piccart‐Gebhart M. Trastuzumab (herceptin) for early‐stage breast cancer. Hematol Oncol Clin North Am 2007; 21: 239–56. [DOI] [PubMed] [Google Scholar]
- 18. Rossi A, Bria E, Maione P, Palazzolo G, Falanga M, Gridelli C. The role of cetuximab and other epidermal growth factor receptor monoclonal antibodies in the treatment of advanced non‐small cell lung cancer. Rev Recent Clin Trials 2008; 3: 217–27. [DOI] [PubMed] [Google Scholar]
- 19. McCormack PL, Keam SJ. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs 2008; 68: 487–506. [DOI] [PubMed] [Google Scholar]
- 20. Stintzing S, Heinemann V, Moosmann N, Hiddemann W, Jung A, Kirchner T. The treatment of colorectal carcinoma with monoclonal antibodies: the importance of KRAS mutation analysis and EGFR status. Dtsch Arztebl Int 2009; 106: 202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashihara E, Kawata E, Maekawa T. Future prospect of RNA interference for cancer therapies. Curr Drug Targets 2010; 11: 345–60. [DOI] [PubMed] [Google Scholar]
- 22. Privé GG, Melnick A. Specific peptides for the therapeutic targeting of oncogenes. Curr Opin Genet Dev 2006; 16: 71–7. [DOI] [PubMed] [Google Scholar]
- 23. Mercer RW, Tyler MA, Ulasov IV, Lesniak MS. Targeted therapies for malignant glioma: progress and potential. BioDrugs 2009; 23: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duhoux FP, Machiels JP. Antivascular therapy for epithelial ovarian cancer. J Oncol 2010; 2010: 372547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilks CB. Molecular abnormalities in ovarian cancer subtypes other than high‐grade serous carcinoma. J Oncol 2010; 2010: 740968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang ZQ, Buchsbaum DJ. Monoclonal antibodies in the treatment of pancreatic cancer. Immunotherapy 2009; 1: 223–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jadvar H. Molecular imaging of prostate cancer: a concise synopsis. Mol Imaging 2009; 8: 56–64. [PMC free article] [PubMed] [Google Scholar]
- 28. Yin M, Guan X, Liao Z, Wei Q. Insulin‐like growth factor‐1 receptor‐targeted therapy for non‐small cell lung cancer: a mini review. Am J Transl Res 2009; 1: 101–14. [PMC free article] [PubMed] [Google Scholar]
- 29. Ho C, Ochsenbein AF, Gautschi O, Davies AM. Early clinical trial experience with vaccine therapies in non‐small‐cell lung cancer. Clin Lung Cancer 2008; 9(Suppl 1): S20–7. [DOI] [PubMed] [Google Scholar]
- 30. Chiarle R, Martinengo C, Mastini C et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med 2008; 14: 676–80. [DOI] [PubMed] [Google Scholar]
- 31. Romero P. Current state of vaccine therapies in non‐small‐cell lung cancer. Clin Lung Cancer 2008; 9(Suppl 1): S28–36. [DOI] [PubMed] [Google Scholar]
- 32. Ishii T, Ishida T, Utsunomiya A et al. Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res 2010; 16: 1520–31. [DOI] [PubMed] [Google Scholar]
- 33. Xiang S, Fruehauf J, Li CJ. Short hairpin RNA‐expressing bacteria elicit RNA interference in mammals. Nat Biotechnol 2006; 24: 697–702. [DOI] [PubMed] [Google Scholar]
- 34. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46(12 Pt 1): 6387–92. [PubMed] [Google Scholar]
- 35. Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 2006; 36: 189–217. [DOI] [PubMed] [Google Scholar]
- 36. Takagi A, Hirose A, Nishimura T et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi‐wall carbon nanotube. J Toxicol Sci 2008; 33: 105–16. [DOI] [PubMed] [Google Scholar]
- 37. Onuma K, Sato Y, Ogawara S et al. Nano‐scaled particles of titanium dioxide convert benign mouse fibrosarcoma cells into aggressive tumor cells. Am J Pathol 2009; 175: 2171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haag R. Supramolecular drug‐delivery systems based on polymeric core‐shell architectures. Angew Chem Int Ed Engl 2004; 43: 278–82. Review. [DOI] [PubMed] [Google Scholar]
- 39. Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther 2006; 112: 630–48. [DOI] [PubMed] [Google Scholar]
- 40. Giaccia A, Siim BG, Johnson RS. HIF‐1 as a target for drug development. Nat Rev Drug Discov 2003; 2: 803–11. [DOI] [PubMed] [Google Scholar]
- 41. Verweij J, Pinedo HM. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs 1990; 1: 5–13. [PubMed] [Google Scholar]
- 42. Brown JM. The hypoxic cell: a target for selective cancer therapy – eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 1999; 59: 5863–70. [PubMed] [Google Scholar]
- 43. Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008; 8: 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graeber TG, Osmanian C, Jacks T et al. Hypoxia‐mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91. [DOI] [PubMed] [Google Scholar]
- 45. Gort EH, Groot AJ, Van Der Wall E, Van Diest PJ, Vooijs MA. Hypoxic regulation of metastasis via hypoxia‐inducible factors. Curr Mol Med 2008; 8: 60–7. [DOI] [PubMed] [Google Scholar]
- 46. Sullivan R, Graham CH. Hypoxia‐driven selection of the metastatic phenotype. Cancer Metastasis Rev 2007; 26: 319–31. [DOI] [PubMed] [Google Scholar]
- 47. Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008; 26: 1818–30. [DOI] [PubMed] [Google Scholar]
- 48. Ryan RM, Green J, Lewis CE. Use of bacteria in anti‐cancer therapies. Bioessays 2006; 28: 84–94. [DOI] [PubMed] [Google Scholar]
- 49. Carey R. Clostridial oncolysis in man. Eur J Cancer, 1967; 3: 37–46. [Google Scholar]
- 50. Morrissey D, O’Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther 2010; 10: 3–14. [DOI] [PubMed] [Google Scholar]
- 51. Lu Y, Madu CO. Viral‐based gene delivery and regulated gene expression for targeted cancer therapy. Expert Opin Drug Deliv 2010; 7: 19–35. [DOI] [PubMed] [Google Scholar]
- 52. Kaur B, Cripe TP, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment. Curr Gene Ther 2009; 9: 341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res 1955; 15: 473–8. [PubMed] [Google Scholar]
- 54. Kimura NT, Taniguchi S, Aoki K, Baba T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res 1980; 40: 2061–8. [PubMed] [Google Scholar]
- 55. Yazawa K, Fujimori M, Nakamura T et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat 2001; 66: 165–70. [DOI] [PubMed] [Google Scholar]
- 56. Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors. Cancer Gene Ther 2000; 7: 269–74. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura T, Sasaki T, Fujimori M et al. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro‐drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem 2002; 66: 2362–6. [DOI] [PubMed] [Google Scholar]
- 58. Sasaki T, Fujimori M, Hamaji Y et al. Genetically engineered Bifidobacterium longum for tumor‐targeting enzyme‐prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci 2006; 97: 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamaji Y, Fujimori M, Sasaki T et al. Strong enhancement of recombinant cytosine deaminase activity in Bifidobacterium longum for tumor‐targeting enzyme/prodrug therapy. Biosci Biotechnol Biochem 2007; 71: 874–83. [DOI] [PubMed] [Google Scholar]
- 60. Schüller J, Cassidy J, Dumont E et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 2000; 45: 291–7. [DOI] [PubMed] [Google Scholar]
- 61. Zeuthen LH, Christensen HR, Frøkiaer H. Lactic acid bacteria inducing a weak interleukin‐12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram‐negative bacteria. Clin Vaccine Immunol 2006; 13: 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest 2000; 105: 1027–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nemunaitis J, Cunningham C, Senzer N et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther 2003; 10: 737–44. [DOI] [PubMed] [Google Scholar]
- 64. Taniguchi S. Suppression of cancer phenotypes through a multifunctional actin‐binding protein, calponin, that attacks cancer cells and simultaneously protects the host from invasion. Cancer Sci 2005; 96: 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zheng LM, Luo X, Feng M et al. Tumor amplified protein expression therapy: Salmonella as a tumor‐selective protein delivery vector. Oncol Res 2000; 12: 127–35. [DOI] [PubMed] [Google Scholar]
- 66. Low KB, Ittensohn M, Le T et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor‐targeting in vivo. Nat Biotechnol 1999; 17: 37–41. [DOI] [PubMed] [Google Scholar]
- 67. Xu DQ, Zhang L, Kopecko DJ et al. Bacterial delivery of siRNAs: a new approach to solid tumor therapy. Methods Mol Biol 2009; 487: 161–87. [DOI] [PubMed] [Google Scholar]
- 68. Zhao M, Yang M, Li XM et al. Tumor‐targeting bacterial therapy with amino acid auxotrophs of GFP‐expressing Salmonella typhimurium. Proc Natl Acad Sci U S A 2005; 102: 755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar H, Kawai T, Akira S. Toll‐like receptors and innate immunity. Biochem Biophys Res Commun 2009; 388: 621–5. [DOI] [PubMed] [Google Scholar]
- 70. Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol 2007; 29: 213–29. [DOI] [PubMed] [Google Scholar]
- 71. Taniguchi S, Sagara J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Semin Immunopathol 2007; 29: 231–8. [DOI] [PubMed] [Google Scholar]


