Abstract
Low consumption of vegetables and fruits, which leads to insufficient folate intake, is associated with increased risk of several types of cancer, including head and neck squamous cell carcinoma (HNSCC). Functional polymorphisms in genes encoding one‐carbon metabolism enzymes, such as methylenetetrahydrofolate reductase (MTHFR C677T and A1298C), methionine synthase (MTR A2756G), methionine synthase reductase (MTRR A66G) and thymidylate synthase (TS), influence folate metabolism and thus might impact on HNSCC risk. We conducted a case‐control study with 237 HNSCC cases newly and histologically diagnosed and 711 age‐ and sex‐matched non‐cancer controls to clarify associations with these five polymorphisms. Gene–environment interactions between polymorphisms and smoking and drinking habit and folate consumption were also evaluated by logistic regression analysis. Dietary folate intake was inversely associated with HNSCC risk. None of the polymorphisms showed any significant impact on HNSCC risk by genotype alone, but we found interactions between drinking habit and MTHFR C667T (P = 0.04), MTR A2756G (P = 0.04) and MTRR A66G (P = 0.03) polymorphisms. The results suggest that there may be interactions between one‐carbon metabolism‐related polymorphisms and alcohol drinking for HNSCC risk. (Cancer Sci 2007; 98: 1439–1446)
Head and neck squamous cell carcinoma (HNSCC) is common in several regions of the world in which there is extensive use of tobacco and high consumption of alcohol.( 1 ) Mortalities of oral and pharyngeal cancers in Japan have increased constantly from 1960 to 2000 and reached those of western countries in the late 1990s.( 2 ) Therefore, efforts toward primary prevention in addition to early detection have come under the spotlight.
Low dietary intake of vegetables and fruits is recognized as a risk factor for HNSCC,( 3 , 4 , 5 , 6 , 7 ) suggesting that folate, which is plentiful in vegetables and fruits, is a protective nutrient.( 8 , 9 , 10 , 11 ) Biological functions of folate within so‐called ‘one‐carbon metabolism’ are to facilitate de novo deoxynucleoside triphosphate synthesis and to provide methyl groups required for intracellular methylation reactions. Folate deficiency is thought to increase the risk of cancer through impaired DNA repair synthesis and disruption of DNA methylation that may lead to protooncogene activation.( 12 , 13 , 14 )
Methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), methionine synthase reductase (MTRR) and thymidylate synthetase (TS) play important and interrelated roles in folate metabolism (Fig. 1). The MTHFR reduces 5,10‐methylenetetrahydrofolate (5,10‐methylene THF) to 5‐methyl THF, the primary circulating form of folate.( 15 ) The TS catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) using 5,10‐methylene THF.( 16 ) The MTHFR product, 5‐methyl THF, is the methyl group donor for the remethylation of homocysteine to methionine catalyzed by MTR.( 17 ) MTR activity is maintained by MTRR.( 18 )
Figure 1.
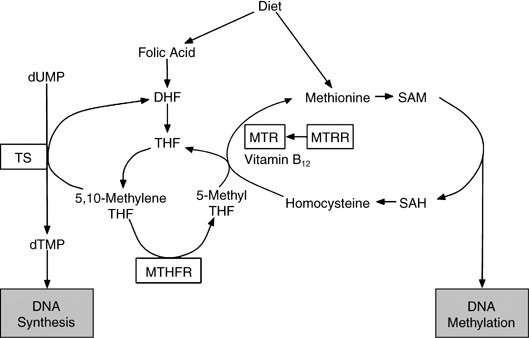
Overview of folate metabolism. Enzymes with polymorphisms investigated in the present study are boxed. DHF, dihydrofolate; dTMP, deoxythymidine monophoshate; dUMP, deoxyuridine monophoshate; SAH, S‐adenosylhomocysteine; SAM, S‐adenosylmethionine; THF, tetrahydrofolate.
Polymorphisms in the genes for MTHFR C677T and A1298C, MTR A2756G, MTRR A66G and TS 28‐bp variable number of tandem repeat (VNTR) are known to have functional relevance.( 19 ) Variant alleles of the MTHFR C677T and A1298C polymorphisms generate an enzyme with reduced activity compared with wild type.( 20 , 21 ) MTR A2756G heterozygote and homozygous variant genotypes are associated with increased plasma folate levels, and MTRR A66G variant alleles generate an enzyme with lower affinity for MTR.( 22 , 23 ) TS with triple repeat alleles is associated with greater TS expression compared with the double repeat.( 24 ) Thus they might play roles in the etiology of HNSCC in combination with environmental factors. A previous case‐control study in the USA reported that the MTHFR A1298C, MTR A2756G and MTRR A66G polymorphisms were associated with HNSCC risk,( 25 , 26 ) whereas the MTHFR C677T and TS polymorphisms demonstrated no link.( 25 , 27 ) Because information on HNSCC is limited regarding these polymorphisms,( 25 , 26 , 27 , 28 ) we conducted the present case‐control study, taking into consideration alcohol drinking, tobacco smoking and intake of folate.
Materials and Methods
Subjects. The cases were 237 patients who were newly and histologically diagnosed as having HNSCC between January 2001 and June 2005 at Aichi Cancer Center Hospital in Japan and who did not have any earlier history of cancer. Malignant neoplasms of the salivary glands, nasal and paranasal were excluded from the present study as they have quite distinct natural histories and poorly understood etiologies. Controls (n = 711) were selected randomly and matched by age (±3 years) and sex to cases with a 1:3 case‐control ratio. All of the subjects were recruited in the framework of the Hospital‐based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), as described elsewhere.( 29 , 30 ) In brief, information on lifestyle factors was collected using a self‐administered questionnaire, checked by a trained interviewer. Outpatients were also asked to provide blood samples. Each patient was asked about his or her lifestyle when healthy or before the current symptoms developed. Approximately 95% of eligible subjects complete the questionnaire and 60% provided blood samples. Our previous study showed that the lifestyle patterns of first‐visit outpatients accorded with those in a randomly selected sample of the general population of Nagoya City.( 31 ) The data were loaded into a HERPACC database and linked routinely with the hospital‐based cancer registry system to update the data on cancer incidence. All participants gave written informed consent and the study was approved by the Ethical Committee of Aichi Cancer Center.
Genotyping of MTHFR, MTR, MTRR and TS. DNA of each subject was extracted from the buffy coat fraction using BioRobot EZ1 and an EZ1 DNA Blood 350 mL Kit (Qiagen, Tokyo, Japan). The genotyping method was described in our previous reports with the polymerase chain reaction (PCR) TaqMan method using the GeneAmp PCR System 9700 or the 7500 Fast Real‐time PCR system (Applied Biosystems, Foster City, CA, USA). Briefly, for the MTHFR C677T (dbSNP ID: rs677) and A1298C (rs1801131), as well as MTR A2756G (rs1805087) and MTRR A66G (rs1801394) polymorphisms, extracted DNA was amplified with validated probes (assay ID: C__11975651–10, C__850486–20, C__12005959–10, and C__3068176–10, respectively; Applied Biosystems). The TS VNTR polymorphism was defined by PCR using 5′‐CGT GGC TCC TGC GTT TCC‐3′ and 5′‐GAG CCG GCC ACA GGC AT‐3′ primers. In our laboratory, the quality of genotyping was routinely assessed statistically using the Hardy–Weinberg test. When allelic distributions for controls departed from the Hardy–Weinberg frequency, genotyping was assessed using DNA sequencing.
Intake assessment for folate and other nutrients. The consumption of folate and other nutrients were determined using a food frequency questionnaire (FFQ), described in detail elsewhere.( 32 , 33 ) Briefly, the FFQ consisted of 47 single food items with frequencies in the following eight categories: never or seldom, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, once/day, twice/day, and 3+ times/day. We estimated the average daily intake of nutrients by multiplying the food intake (in g) by the nutrient content per 100 g of food as listed in standard tables of food composition.( 34 , 35 , 36 ) Consumption of folate and other vitamins from supplements was not able to be considered in total consumption because the questionnaire for multivitamins was not quantitative. Energy‐adjusted intake of nutrients was calculated using the residual method.( 37 ) The FFQ was validated by referring to a 3‐day weighed dietary record as a standard, which showed reproducibility and validity to be acceptable.( 38 ) The deattenuated correlation coefficients for energy‐adjusted intakes of folate were 0.36 in men and 0.38 in women.
Consumption of tobacco and alcohol. Cumulative smoking dose was evaluated as pack‐years, the product of the number of packs consumed per day and years of smoking. Smoking habit was entered under the following four categories: never, former, current smokers of <40 pack‐years, and current smokers of ≥40 pack‐years. Former smokers were defined as those who quit smoking at least 1 year before the survey. Consumption of each type of beverage (Japanese sake, beer, shochu, whiskey and wine) was determined by the average number of drinks per day, which was then converted into a Japanese sake (rice wine) equivalent. One drink equates to one ‘go’ (180 mL) of Japanese sake, which contains 23 g ethanol, equivalent to one large bottle (633 mL) of beer, two shots (60 mL) of whiskey and two and a half glasses of wine (200 mL). One drink of ‘Shochu’ (distilled spirit), which contains 25% ethanol, was rated as 108 mL. Total amount of alcohol consumption was estimated as the summarized amount of pure alcohol consumption (g/drink) of Japanese sake, beer, shochu, whiskey and wine among current regular drinkers. Drinking habit was entered in the following four categories: never, former, current moderate and heavy drinkers. Heavy drinkers were defined as those currently drinking alcoholic beverages 5 days or more per week in a daily amount of 46 g (two Japanese drinks) or more, whereas moderate drinkers were defined as those currently consuming less frequently than 5 days per week, in lower amounts, or both. Former drinkers were defined as those who quit drinking at least 1 year before the survey.
Statistical analysis. To assess the strength of the associations between polymorphic genes involved in folate metabolism and risk of HNSCC, odd ratios (OR) with 95% confidence intervals (CI) were estimated using age‐ and sex‐matched conditional logistic models adjusted for potential confounders. Folate and other nutrient intakes were categorized into three groups as follows: first, second, and third tertiles of dietary intake among controls. Potential confounders considered in the multivariate analyses were age, sex, smoking habit (never smokers, former smokers, current smokers of <40 pack‐years or current smokers of ≥40 pack‐years), drinking habit (never drinkers, former drinkers, moderate drinkers or heavy drinkers), multivitamin use (at least once per week for 1 year or longer: yes or no), total non‐alcohol energy intake (as a continuous variable), dietary carotene intake (µg/day, tertiles), dietary vitamin C intake (mg/day, tertiles), dietary vitamin E intake (mg/day, tertiles), dietary folate intake (µg/day, tertiles), dental brushing (at least once/day: yes or no),( 39 ) and referral pattern (self recommendation, recommendation by family or friends, referral by physicians, secondary screening, or others). The internal validity of this hospital‐based study is a potential threat to causal inference in this population. We used non‐cancer patients at our hospital as controls, given the likelihood that our cases arose within this population base. To account for the difference between cases and controls, we adjusted for referral pattern to our hospital. Missing values for each covariate were excluded in the logistic model. Differences in categorized demographic variables and mean values between the cases and controls were tested using the Mantel–Haenszel method. Accordance with the Hardy–Weinberg equilibrium was checked for controls using the χ2‐test and the exact P‐value was used to assess any discrepancies between genotype and allele frequencies. As a basis for the trend test, the median values of each tertile of folate intake consumption were included in the model. Gene–environment interactions between drinking and smoking habit and folate intake and genotypes in each polymorphism were evaluated under the multiplicative assumption. Products of scores for genotype (0, referent allele; and 1, non‐referent allele) and drinking habit (0, never drinker; 1, moderate drinker; and 2, heavy drinker), smoking habit (0, never smoker; 1, current smoker of <40 pack‐years; and 2, current smokers of ≥40 pack‐years) and folate intake (0, tertile 1; and 1, tertile 2 + 3) were included as interaction terms. A P‐value less than 0.05 was considered statistically significant. All analyses were carried out using STATA version 9 (Stata Corp., College Station, TX, USA).
Results
Data from 237 HNSCC cases, comprising 119 (50%) oral cancers, 86 (36%) pharyngeal cancers and 32 (14%) laryngeal cancers, and 711 controls were available for analysis. Table 1 shows the distribution of cases and controls by background characteristics. Age and sex were matched. Heavy drinkers comprised 26.2% of the cases and this was significantly higher than for the controls, at 11.7%. Smoking habits also differed to a large extent between cases and controls, with 26.2 and 15.2% smokers of <40 pack‐years or ≥40 pack‐years, respectively. Total non‐alcohol energy intake was lower in the case group. Lower intakes of dietary antioxidant vitamins, such as carotene, vitamins C and E, and folate were found among the cases. Multivitamin supplementation was more prevalent in the controls. The proportion of subjects without dental brushing at least once per day was significantly higher in the case group, although the number of subjects was low. With regard to referral pattern, referral by physicians was more frequent, whereas self recommendation, recommendation by family or friends and secondary screening were less common among the case group than the control group.
Table 1.
Characteristics of cases and controls
| Characteristic | No. cases (n = 237) | No. controls (n = 711) | P‐value |
|---|---|---|---|
| Age (years) | |||
| <50 | 53 (22.4%) | 138 (19.4%) | |
| 50–59 | 67 (28.3%) | 217 (30.5%) | |
| 60–69 | 69 (29.1%) | 213 (30.0%) | |
| 70–79 | 48 (20.3%) | 143 (20.1%) | |
| Mean age (SD) | 57.9 (12.4) | 58.4 (12.0) | |
| Sex | |||
| Male | 188 (79.3%) | 564 (79.3%) | |
| Female | 49 (20.7%) | 147 (20.7%) | |
| Drinking | |||
| Never | 56 (23.6%) | 235 (33.1%) | |
| Former † | 14 (5.9%) | 37 (5.2%) | |
| Current | |||
| Moderate ‡ | 96 (40.5%) | 345 (48.5%) | |
| Heavy § | 62 (26.2%) | 83 (11.7%) | <0.01 |
| Unknown | 9 (3.8%) | 11 (1.5%) | |
| Smoking | |||
| Never | 56 (23.6%) | 256 (36.0%) | |
| Former † | 64 (27.0%) | 210 (29.5%) | |
| Current (pack years) | |||
| 0–39 | 52 (21.9%) | 134 (18.8%) | |
| ≥40 | 62 (26.2%) | 108 (15.2%) | <0.01 |
| Unknown | 3 (1.3%) | 3 (0.4%) | |
| Mean total energy (kcal/day) (SD) | 1652.0 (381.6) | 1689.1 (333.0) | 0.20 |
| Mean total non‐alcohol energy (kcal/day) (SD) | 1545.1 (358.4) | 1622.5 (327.1) | <0.01 |
| Carotene (mean ± SD, µg/day) | |||
| Tertile 1 (1829.8 ± 209.8) | 104 (43.9%) | 236 (33.2%) | |
| Tertile 2 (2667.3 ± 314.0) | 73 (30.8%) | 236 (33.2%) | |
| Tertile 3 (4394.3 ± 1317.4) | 55 (23.2%) | 236 (33.2%) | <0.01 |
| Unknown | 5 (2.1%) | 3 (0.4%) | |
| Vitamin C (mean ± SD, mg/day) | |||
| Tertile 1 (53.9 ± 9.7) | 113 (47.7%) | 237 (33.3%) | |
| Tertile 2 (82.5 ± 8.2) | 72 (30.4%) | 237 (33.3%) | |
| Tertile 3 (129.0 ± 32.9) | 47 (19.8%) | 236 (33.2%) | <0.01 |
| Unknown | 5 (2.1%) | 1 (0.1%) | |
| Vitamin E (mean ± SD, total α‐mg/day) | |||
| Tertile 1 (3.9 ± 0.6) | 96 (40.5%) | 237 (33.3%) | |
| Tertile 2 (5.5 ± 0.5) | 88 (37.1%) | 237 (33.3%) | |
| Tertile 3 (8.1 ± 1.8) | 50 (21.1%) | 236 (33.2%) | <0.01 |
| Unknown | 3 (1.3%) | 1 (0.1%) | |
| Folate (mean ± SD, µg/day) | |||
| Tertile 1 (218.5 ± 31.5) | 111 (46.8%) | 237 (33.3%) | |
| Tertile 2 (305.3 ± 23.4) | 67 (28.3%) | 237 (33.3%) | |
| Tertile 3 (435.3 ± 91.7) | 54 (22.8%) | 236 (33.2%) | <0.01 |
| Unknown | 5 (2.1%) | 1 (0.1%) | |
| Multivitamin use (at least once per week for 1 year or longer) | |||
| Yes | 32 (13.5%) | 162 (22.8%) | |
| No | 191 (80.6%) | 511 (71.9%) | 0.01 |
| Unknown | 14 (5.9%) | 38 (5.3%) | |
| Dental brushing (at least once per day) | |||
| Yes | 227 (95.8%) | 703 (98.9%) | |
| No | 10 (4.2%) | 8 (1.1%) | <0.01 |
| Referral pattern to our hospital | |||
| Self recommendation | 26 (11.0%) | 202 (28.4%) | |
| Recommendation by family or friends | 19 (8.0%) | 143 (20.1%) | |
| Referral by physicians | 177 (74.7%) | 211 (29.7%) | |
| Secondary screening | 11 (4.6%) | 148 (20.8%) | |
| Others | 0 (0%) | 4 (0.6%) | <0.01 |
| Unknown | 4 (1.7%) | 3 (0.4%) | |
Former smokers and drinkers were defined as subjects who had quit smoking and drinking at least 1 year previously.
Moderate drinker means <46 g ethanol/drink and/or <5 days/week.
Heavy drinker means ≥46 g ethanol/drink and ≥5 days/week.
Table 2 shows the impact of dietary folate consumption on HNSCC risk. A significant inverse association was observed (trend P = 0.02).
Table 2.
Adjusted odds ratios (OR) relating associations with dietary folate intake for risk of head and neck squamous cell carcinoma
| Folate intake (µg/day) | Cases/controls | OR † (95% CI) | P‐value |
|---|---|---|---|
| Tertile 1 (133.0–265.0) | 111/237 | 1.00 (reference) | |
| Tertile 2 (265.9–345.2) | 67/237 | 0.55 (0.34–0.88) | |
| Tertile 3 (345.2–975.1) | 54/236 | 0.53 (0.32–0.89) | 0.02 |
| Unknown | 5/1 |
OR was matched for age and sex and adjusted for drinking habit, smoking habit, non‐alcohol energy intake, multivitamin use, dental brushing and referral pattern to our hospital. CI, confidence interval.
Table 3 shows genotype distributions for MTHFR, MTR, MTRR and TS, and their OR and 95% CI for HNSCC risk. The genotype frequencies for all of the polymorphisms were in accordance with the Hardy–Weinberg law in controls: MTHFR C677T (P = 0.29), MTHFR A1298C (P = 0.57), MTR A2756G (P = 0.87), MTRR A66G (P = 0.38) and TS VNTR (P = 0.51). None of the polymorphisms showed any significant impact on HNSCC risk by genotype alone. When two polymorphisms were evaluated together (MTHFR C677T and MTHFR A1298C, MTR A2756G and MTRR A66G), no significant association with HNSCC risk was found (data not shown).
Table 3.
MTHFR, MTR, MTRR and TS genotype distributions, and odds ratios (OR) for head and neck squamous cell carcinoma
| Polymorphism | No. cases | No. controls | OR1 † (95% CI) | P‐value | OR2 ‡ (95% CI) | P‐value |
|---|---|---|---|---|---|---|
| MTHFR (C677T) | ||||||
| CC | 88 (37.1%) | 252 (35.4%) | 1.00 (reference) | 1.00 (reference) | ||
| CT | 113 (47.7%) | 331 (46.6%) | 0.98 (0.71–1.36) | 0.98 (0.63–1.52) | ||
| TT | 36 (15.2%) | 128 (18.0%) | 0.80 (0.51–1.25) | 0.39 | 0.77 (0.42–1.41) | 0.46 |
| MTHFR (A1298C) | ||||||
| AA | 149 (62.9%) | 453 (63.7%) | 1.00 (reference) | 1.00 (reference) | ||
| AC | 79 (33.3%) | 222 (31.2%) | 1.09 (0.78–1.51) | 1.07 (0.69–1.66) | ||
| CC | 8 (3.4%) | 31 (4.4%) | 0.79 (0.36–1.75) | 0.96 | 0.82 (0.28–2.46) | 0.97 |
| UK § | 1 (0.4%) | 5 (0.7%) | ||||
| MTR (A2756G) | ||||||
| AA | 151 (63.7%) | 496 (69.8%) | 1.00 (reference) | 1.00 (reference) | ||
| AG | 75 (31.6%) | 195 (27.4%) | 1.26 (0.91–1.73) | 1.18 (0.76–1.83) | ||
| GG | 11 (4.6%) | 20 (2.8%) | 1.83 (0.84–3.97) | 0.06 | 1.30 (0.49–3.44) | 0.38 |
| MTRR (A66G) | ||||||
| AA | 108 (45.6%) | 332 (46.7%) | 1.00 (reference) | 1.00 (reference) | ||
| AG | 100 (42.2%) | 315 (44.3%) | 0.97 (0.71–1.33) | 0.85 (0.55–1.31) | ||
| GG | 29 (12.2%) | 64 (9.0%) | 1.39 (0.85–2.26) | 0.38 | 1.25 (0.64–2.44) | 0.89 |
| TS VNTR | ||||||
| Non‐2R/Non‐2R | 168 (70.9%) | 502 (70.6%) | 1.00 (reference) | 1.00 (reference) | ||
| 2R/Non‐2R | 64 (27.0%) | 188 (26.4%) | 1.02 (0.73–1.42) | 1.14 (0.72–1.78) | ||
| 2R/2R | 5 (2.1%) | 21 (3.0%) | 0.71 (0.26–1.91) | 0.78 | 0.66 (0.15–2.90) | 0.84 |
OR1 was matched for age and sex.
‡ OR2 was matched for age and sex and adjusted for drinking habit, smoking habit, non‐alcohol energy intake, carotene intake, vitamin C intake, vitamin E intake, folate intake, multivitamin use, dental brushing and referral pattern to our hospital.
§ UK denotes genotype unknown. CI, confidence interval.
Table 4 summarizes interactions between polymorphisms and drinking and smoking habits. There were interactions between drinking habit and MTHFR C677T (P = 0.04), MTR A2756G (P = 0.04) and MTRR A66G (P = 0.03). Among subjects with the MTHFR 677 TT or the MTR 2756 GG genotypes, OR were not increased in heavy drinkers compared with never drinkers, whereas the GG genotype of MTRR had a more increased risk among heavy drinkers compared with AA/AG genotype (OR = 12.39, 95% CI 2.51–61.25). No obvious associations were observed for the MTHFR A1298C and TS polymorphisms regarding combinations with alcohol drinking. Associations between polymorphisms examined and smoking habit were less clear.
Table 4.
Interaction between MTHFR, MTR, MTRR or TS polymorphisms and drinking and smoking habit for head and neck squamous cell carcinoma risk
| Polymorphism | Drinking habit | Interaction P § | Smoking habit | Interaction P § | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never drinker | Moderate drinker ‡ | Heavy drinker ‡ | Never smoker | Current smoker pack years 0–39 | Current smoker pack years ≥ 40 | |||||||||
| Cases/ controls | OR † (95% CI) | Cases/ controls | OR † (95% CI) | Cases/ controls | OR † (95% CI) | Cases/ controls | OR † (95% CI) | Cases/ controls | OR † (95% CI) | Cases/ controls | OR† (95% CI) | |||
| MTHFR (C677T) | ||||||||||||||
| CC + CT | 45/193 | 1.00 (ref.) | 85/286 | 1.48 (0.83–2.62) | 57/68 | 4.41 (2.06–9.44) | 48/211 | 1.00 (ref.) | 44/119 | 0.62 (0.27–1.43) | 52/82 | 1.57 (0.63–3.88) | ||
| TT | 11/42 | 1.94 (0.73–5.12) | 11/59 | 0.90 (0.34–2.38) | 5/15 | 1.61 (0.37–7.02) | 0.04 | 8/45 | 1.61 (0.51–5.01) | 8/15 | 0.98 (0.24–3.93) | 10/26 | 1.02 (0.26–3.99) | 0.30 |
| MTHFR (A1298C) | ||||||||||||||
| AA + AC | 55/223 | 1.00 (ref.) | 91/329 | 1.11 (0.64–1.92) | 60/80 | 3.15 (1.57–6.31) | 55/242 | 1.00 (ref.) | 52/127 | 0.57 (0.25–1.27) | 59/102 | 1.23 (0.50–3.03) | ||
| CC | 1/11 | 0.14 (0.01–1.46) | 4/14 | 1.09 (0.22–5.26) | 2/3 | 5.92 (0.17–206.63) | 0.13 | 1/12 | 0.07 (0.01–0.97) | 0/5 | NA ¶ | 3/6 | 2.75 (0.26–28.77) | 0.06 |
| MTR (A2756G) | ||||||||||||||
| AA + AG | 51/230 | 1.00 (ref.) | 91/337 | 1.20 (0.70–2.05) | 61/77 | 3.76 (1.84–7.69) | 54/251 | 1.00 (ref.) | 51/130 | 0.66 (0.29–1.48) | 59/106 | 1.35 (0.55–3.29) | ||
| GG | 5/5 | 4.49 (0.72–28.19) | 5/8 | 2.01 (0.47–8.60) | 1/6 | 0.62 (0.05–7.29) | 0.04 | 2/5 | 0.74 (0.08–6.64) | 1/4 | 0.26 (0.02–4.16) | 3/2 | 2.67 (0.07–106.29) | 0.75 |
| MTRR (A66G) | ||||||||||||||
| AA + AG | 51/213 | 1.00 (ref.) | 86/313 | 1.09 (0.63–1.87) | 52/78 | 2.61 (1.26–5.39) | 49/232 | 1.00 (ref.) | 45/123 | 0.68 (0.30–1.56) | 54/99 | 1.52 (0.61–3.81) | ||
| GG | 5/22 | 0.51 (0.14–1.91) | 10/32 | 1.76 (0.62–4.95) | 10/5 | 12.39 (2.51–61.25) | 0.03 | 7/24 | 1.68 (0.47–5.99) | 7/11 | 0.79 (0.15–4.13) | 8/9 | 1.41 (0.31–6.40) | 0.55 |
| TS VNTR | ||||||||||||||
| Non‐2R/Non‐2R | 37/172 | 1.00 (ref.) | 70/241 | 1.61 (0.86–3.00) | 44/55 | 4.23 (1.84–9.73) | 41/184 | 1.00 (ref.) | 37/93 | 0.77 (0.32–1.84) | 47/77 | 1.98 (0.75–5.23) | ||
| 2R/Non‐2R + 2R/2R | 19/63 | 1.92 (0.87–4.23) | 26/104 | 1.31 (0.61–2.81) | 18/28 | 4.02 (1.43–11.34) | 0.22 | 15/72 | 1.86 (0.77–4.53) | 15/41 | 0.62 (0.17–2.31) | 15/31 | 0.95 (0.26–3.47) | 0.08 |
Odds ratio (OR) was matched for age and sex and adjusted for drinking habit, smoking habit, non‐alcohol energy intake, carotene intake, vitamin C intake, vitamin E intake, folate intake, multivitamin use, dental brushing, and referral pattern to our hospital.
Moderate drinker means <46 g ethanol/drink and/or <5 days/week. Heavy drinker means ≥46 g ethanol/drink and ≥5 days/week.
Interactions were modeled as a product of drinking habit in score (0, never; 1, moderate drinker; and 2, heavy drinker), smoking habit in score (0, never; 1, 0–39 pack years; and 2, pack years ≥ 40) and genotype in score.
NA indicates that OR was not available because of insufficient data. CI, confidence interval.
Table 5 shows data for combinations of the polymorphisms and folate intake with regard to HNSCC risk. There was no clear interaction between folate consumption and these polymorphisms, but a non‐significantly decreased risk of HNSCC was found among those reporting high folate intakes with the MTHFR 677TT genotype.
Table 5.
Interaction between MTHFR, MTR, MTRR or TS polymorphisms and folate intake for head and neck squamous cell carcinoma risk
| Polymorphism | Folate intake | ||||
|---|---|---|---|---|---|
| Tertile 1 (133.0–264.7 µg/day) | Tertile 2 + 3 (264.8–975.1 µg/day) | Interaction P ‡ | |||
| Cases/ controls | OR † (95% CI) | Cases/ controls | OR † (95% CI) | ||
| MTHFR (C677T) | |||||
| CC + CT | 93/203 | 1.00 (reference) | 104/379 | 1.00 (0.54–1.79) | |
| TT | 18/33 | 1.40 (0.58–3.21) | 17/95 | 0.51 (0.23–1.15) | 0.10 |
| MTHFR (A1298C) | |||||
| AA + AC | 105/224 | 1.00 (reference) | 118/450 | 0.80 (0.46–1.39) | |
| CC | 5/10 | 0.97 (0.21–4.46) | 3/21 | 0.70 (0.16–3.12) | 0.92 |
| MTR (A2756G) | |||||
| AA + AG | 105/227 | 1.00 (reference) | 116/463 | 0.80 (0.46–1.39) | |
| GG | 6/9 | 1.26 (0.34–4.70) | 5/11 | 1.14 (0.25–5.20) | 0.90 |
| MTRR (A66G) | |||||
| AA + AG | 99/218 | 1.00 (reference) | 105/428 | 0.81 (0.46–1.43) | |
| GG | 12/18 | 1.64 (0.59–4.59) | 16/46 | 0.96 (0.39–2.32) | 0.61 |
| TS VNTR | |||||
| Non‐2R/Non‐2R | 82/168 | 1.00 (reference) | 83/333 | 0.77 (0.42–1.42) | |
| 2R/Non‐2R + 2R/2R | 29/68 | 1.03 (0.51–2.10) | 38/141 | 0.88 (0.43–1.80) | 0.82 |
Odds ratio (OR) was matched for age and sex and adjusted for drinking habit, smoking habit, non‐alcohol energy intake, carotene intake, vitamin C intake, vitamin E intake, multivitamin use, dental brushing and referral pattern to our hospital.
Interaction was modeled as a product of folate intake in score (0, tertile 1; and 1, tertile 2 + 3) and genotype in score. CI, confidence interval.
Discussion
The results of the present study of associations between folate intake or one‐carbon metabolism‐related gene polymorphisms (MTHFR C677T and A1298C, MTR A2756G, MTRR A66G and TS VNTR) and HNSCC risk, combined with alcohol drinking, tobacco smoking and intake of folate, suggest that: (1) intake of folate is inversely associated with HNSCC risk; (2) none of the polymorphisms independently has any significant effect on HNSCC risk; and (3) MTHFR C677T, MTR A2756G and MTRR A66G may modify HNSCC risk by alcohol consumption.
Folate deficiency may increase the risk of cancer by inducing an imbalance in DNA precursors, leading to modified DNA synthesis and repair. It can also alter normal DNA methylation, which may contribute to loss of normal control of protooncogene expression.( 12 , 13 , 14 ) Numerous studies have shown a reduced risk of HNSCC with increased fruit or vegetable consumption,( 3 , 4 ) but few studies have examined the role of folate specifically. In two studies, folate intake was negatively associated with risk of head and neck cancer.( 8 , 9 ) Our finding, which adjusted for potential confounders, was in accordance with these results.
A high plasma level of folate has been found to decrease the risk of HNSCC in some case‐control studies.( 10 , 11 ) Functional polymorphisms of genes playing an important role in folate metabolism contribute to alterations in plasma levels of folate.( 19 ) It is therefore plausible that they have a substantial impact on risk; however, few studies have previously examined the relationships between these polymorphisms and HNSCC risk. Hospital‐based case‐control studies in the USA showed a decreased risk with MTHFR 1298 AC/CC and MTRR AA genotypes, an increased risk with MTR AG/GG genotypes and no association with MTHFR C677T and TS polymorphisms.( 25 , 26 , 27 ) A population‐based case‐control study in Puerto Rico reported that individuals with the MTHFR 677 TT genotype have a non‐significantly lower risk of developing oral cancer.( 28 )
We failed to find any association between any of the folate metabolism‐related polymorphisms alone and HNSCC risk; however, combined analysis suggested that the polymorphisms can modify the relationship between alcohol consumption and HNSCC risk. Risk reduction with drinking was seen among individuals with the MTHFR 677TT and MTR 2756 GG genotypes, whereas an increased risk was found for the MTRR 66 GG genotype. To our knowledge, the significant interaction between drinking status and these polymorphisms on HNSCC risk has not been reported previously, although the results from the combined analyses must be treated with caution due to reduced numbers of observations in the subgroups.
We found the MTRR 66GG genotype to be a significant HNSCC risk factor among heavy drinkers compared with the AA + AG genotype. Although the functional effects have not been fully established, the G allele of MTRR is considered to decrease the enzyme activity compared with the A allele.( 40 ) Subjects with MTRR 66GG may have reduced methionine levels compared with those who had other genotypes. However, MTR, which is maintained by MTRR, is responsible for the remethylation of homocysteine to methionine. Ethanol has been reported to reduce mRNA and activity of MTR.( 41 ) These findings suggest that hypomethylation of DNA, which results in a predisposition to cancer, occurs among heavy drinkers with MTRR 66GG.( 42 ) This speculation is consistent with our results, but further studies on the underlying mechanisms of the MTRR polymorphism are warranted.
A previous study showed a significant interaction between smoking status and the polymorphisms for HNSCC risk,( 26 ) but our results did not confirm the association. In addition, because risk of other site neoplasia associated with the folate metabolism‐related polymorphisms has been shown previously to differ by folate intake status,( 19 , 43 , 44 ) we stratified the analyses of genotype combinations by dietary intakes of folate. Although no clear interaction was seen in the present study, the MTHFR 677 TT genotype showed a non‐significantly reduced risk of HNSCC among subjects with an adequate intake of folate. This trend is consistent with previous studies.( 19 , 43 , 44 )
Several potential limitations of the present study warrant consideration. First, because this was a hospital‐based case‐control study, the source population from which cases arise may differ from that for controls. To account for the difference of background between cases and controls, we adjusted for referral pattern to our hospital. Second, as with other case‐control studies, this study may suffer from recall bias. Although the questionnaires were completed before the diagnosis in our hospital, in some cases patients referred to the hospital might have known the diagnosis. Third, we used a self‐administered questionnaire to evaluate folate intake. Data obtained from the FFQ may not reflect intake as accurately as those from other methods, such as biological markers. Nevertheless, the reproducibility and validity of the FFQ were acceptable.( 38 ) We could not consider consumption of folate from supplements in total consumption, but the proportion of folate‐containing supplement users is very low in Japan (0.1%).( 45 ) Last, our study had a modest sample size, and these influences may reach significance in a larger study.
In conclusion, our case‐control study suggested gene–environment interactions between alcohol drinking and the MTHFR C677T and MTRR A66G polymorphisms for HNSCC risk in the Japanese population.
Acknowledgments
The authors thank the assistant staff at the Division of Epidemiology and Prevention at Aichi Cancer Center Research Institute for their support of this study. This study was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan and by a Grant‐in‐Aid for the Third Term Comprehensive 10‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Parkin DM, Bray F, Ferlay J et al . Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka S, Sobue T. Comparison of oral and pharyngeal cancer mortality in five countries: France, Italy, Japan, UK and USA from the WHO Mortality Database (1960–2000). Jpn J Clin Oncol 2005; 35: 488–91. [DOI] [PubMed] [Google Scholar]
- 3. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991; 2: 325–57. [DOI] [PubMed] [Google Scholar]
- 4. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991; 2: 427–42. [DOI] [PubMed] [Google Scholar]
- 5. Winn DM, Ziegler RG, Pickle LW et al . Diet in the etiology of oral and pharyngeal cancer among women from the southern United States. Cancer Res 1984; 44: 1216–22. [PubMed] [Google Scholar]
- 6. McLaughlin JK, Gridley G, Block G et al . Dietary factors in oral and pharyngeal cancer. J Natl Cancer Inst 1988; 80: 1237–43. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki T, Wakai K, Matsuo K et al . Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci 2006; 97: 760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Negri E, Franceschi S, Bosetti C et al . Selected micronutrients and oral and pharyngeal cancer. Int J Cancer 2000; 86: 122–7. [DOI] [PubMed] [Google Scholar]
- 9. Pelucchi C, Talamini R, Negri E et al . Folate intake and risk of oral and pharyngeal cancer. Ann Oncol 2003; 14: 1677–81. [DOI] [PubMed] [Google Scholar]
- 10. Almadori G, Bussu F, Galli J et al . Serum folate and homocysteine levels in head and neck squamous cell carcinoma. Cancer 2002; 94: 1006–11. [DOI] [PubMed] [Google Scholar]
- 11. Raval GN, Sainger RN, Rawal RM et al . Vitamin B (12) and folate status in head and neck cancer. Asian Pac J Cancer Prev 2002; 3: 155–62. [PubMed] [Google Scholar]
- 12. Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 1999; 55: 578–92. [DOI] [PubMed] [Google Scholar]
- 13. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000; 130: 129–32. [DOI] [PubMed] [Google Scholar]
- 14. Wei Q, Shen H, Wang LE et al . Association between low dietary folate intake and suboptimal cellular DNA repair capacity. Cancer Epidemiol Biomarkers Prev 2003; 12: 963–9. [PubMed] [Google Scholar]
- 15. Bailey LB, Gregory JF 3rd. Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: metabolic significance, risks and impact on folate requirement. J Nutr 1999; 129: 919–22. [DOI] [PubMed] [Google Scholar]
- 16. Radparvar S, Houghton PJ, Houghton JA. Characteristics of thymidylate synthase purified from a human colon adenocarcinoma. Arch Biochem Biophys 1988; 260: 342–50. [DOI] [PubMed] [Google Scholar]
- 17. Leclerc D, Campeau E, Goyette P et al . Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet 1996; 5: 1867–74. [DOI] [PubMed] [Google Scholar]
- 18. Leclerc D, Wilson A, Dumas R et al . Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA 1998; 95: 3059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol 2004; 159: 423–43. [DOI] [PubMed] [Google Scholar]
- 20. Frosst P, Blom HJ, Milos R et al . A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–13. [DOI] [PubMed] [Google Scholar]
- 21. Weisberg I, Tran P, Christensen B et al . A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 1998; 64: 169–72. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Stampfer MJ, Ma J et al . Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis 2001; 154: 667–72. [DOI] [PubMed] [Google Scholar]
- 23. Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry 2002; 41: 13 378–85. [DOI] [PubMed] [Google Scholar]
- 24. Horie N, Aiba H, Oguro K et al . Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′‐terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 1995; 20: 191–7. [DOI] [PubMed] [Google Scholar]
- 25. Neumann AS, Lyons HJ, Shen H et al . Methylenetetrahydrofolate reductase polymorphisms and risk of squamous cell carcinoma of the head and neck: a case‐control analysis. Int J Cancer 2005; 115: 131–6. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Z, Shi Q, Liu Z et al . Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case‐control analysis. Cancer Epidemiol Biomarkers Prev 2005; 14: 1188–93. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z, Shi Q, Sturgis EM et al . Thymidylate synthase 5′‐ and 3′‐untranslated region polymorphisms associated with risk and progression of squamous cell carcinoma of the head and neck. Clin Cancer Res 2004; 10: 7903–10. [DOI] [PubMed] [Google Scholar]
- 28. Weinstein SJ, Gridley G, Harty LC et al . Folate intake, serum homocysteine and methylenetetrahydrofolate reductase (MTHFR) C677T genotype are not associated with oral cancer risk in Puerto Rico. J Nutr 2002; 132: 762–7. [DOI] [PubMed] [Google Scholar]
- 29. Tajima K, Hirose K, Inoue M et al . A model of practical cancer prevention for out‐patients visiting a hospital: the hospital‐based epidemiologic research program at Aichi Cancer Center (HERPACC). Asian Pac J Cancer Prev 2000; 1: 35–47. [PubMed] [Google Scholar]
- 30. Hamajima N, Matsuo K, Saito T et al . Gene–environment interactions and polymorphism studies of cancer risk in the hospital‐based epidemiologic research program at Aichi Cancer Center II (HERPACC‐II). Asian Pac J Cancer Prev 2001; 2: 99–107. [PubMed] [Google Scholar]
- 31. Inoue M, Tajima K, Hirose K et al . Epidemiological features of first‐visit outpatients in Japan: comparison with general population and variation by sex, age, and season. J Clin Epidemiol 1997; 50: 69–77. [DOI] [PubMed] [Google Scholar]
- 32. Tokudome S, Ikeda M, Tokudome Y et al . Development of data‐based semi‐quantitative food frequency questionnaire for dietary studies in middle‐aged Japanese. Jpn J Clin Oncol 1998; 28: 679–87. [DOI] [PubMed] [Google Scholar]
- 33. Tokudome S, Goto C, Imaeda N et al . Development of a data‐based short food frequency questionnaire for assessing nutrient intake by middle‐aged Japanese. Asian Pac J Cancer Prev 2004; 5: 40–3. [PubMed] [Google Scholar]
- 34. Science and Technology Agency, Japan . Standard Tables of Food Composition in Japan, 4th edn. Tokyo: Ministry of Finance, 1982. (In Japanese.) [Google Scholar]
- 35. Science and Technology Agency . Fatty acids, Cholesterol, Vitamin E Composition Table of Japanese Foods. Tokyo: Ishiyaku Shuppan, 1989. (In Japanese.) [Google Scholar]
- 36. Science and Technology Agency, Japan . Standard Tables of Food Composition in Japan, 5th edn. Tokyo: Ministry of Finance, 1993. (In Japanese.) [Google Scholar]
- 37. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986; 124: 17–27. [DOI] [PubMed] [Google Scholar]
- 38. Tokudome Y, Goto C, Imaeda N et al . Relative validity of a short food frequency questionnaire for assessing nutrient intake versus three‐day weighed diet records in middle‐aged Japanese. J Epidemiol 2005; 15: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talamini R, Vaccarella S, Barbone F et al . Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer 2000; 83: 1238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson A, Platt R, Wu Q et al . A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab 1999; 67: 317–23. [DOI] [PubMed] [Google Scholar]
- 41. Barak AJ, Beckenhauer HC, Tuma DJ et al . Effects of prolonged ethanol feeding on methionine metabolism in rat liver. Biochem Cell Biol 1987; 65: 230–3. [DOI] [PubMed] [Google Scholar]
- 42. Goelz SE, Vogelstein B, Hamilton SR et al . Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 1985; 228: 187–90. [DOI] [PubMed] [Google Scholar]
- 43. Heijmans BT, Boer JM, Suchiman HE et al . A common variant of the methylenetetrahydrofolate reductase gene (1p36) is associated with an increased risk of cancer. Cancer Res 2003; 63: 1249–53. [PubMed] [Google Scholar]
- 44. Shrubsole MJ, Gao YT, Cai Q et al . MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev 2004; 13: 190–6. [DOI] [PubMed] [Google Scholar]
- 45. Imai T, Nakamura M, Ando F et al . Dietary supplement use by community‐living population in Japan: data from the National Institute for Longevity Sciences Longitudinal Study of Aging (NILS‐LSA). J Epidemiol 2006; 16: 249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


