Abstract
While iron plays an important role in many cellular functions, excess iron storage induces DNA damage by generating hydroxyl radicals and thus promotes carcinogenesis. However, it remains unclear whether body iron levels that are commonly observed in a general population are related to oxidative DNA damage. We examined the association between serum ferritin concentrations and levels of urinary 8‐hydroxydeoxyguanosine (8‐OHdG), a biomarker of systemic oxidative DNA damage and repair, in 528 Japanese men and women aged 21–67 years. Men had much higher ferritin levels than in women, and the levels were significantly greater in women aged 50 years or older than in women aged less than 50 years. Urinary 8‐OHdG concentrations were significantly and positively associated with serum ferritin levels in all the subgroups. The Spearman rank correlation coefficients were 0.47, 0.76, and 0.73 for men overall, women aged less than 50 years, and women aged 50 years or older, respectively. These associations were materially unchanged after adjustment for potential confounding variables. In men, a more pronounced association was observed in nonsmokers than in smokers. Our results suggest body iron storage is a strong determinant of levels of systemic oxidative DNA damage in a healthy population. (Cancer Sci 2009)
Iron plays an important role in cellular metabolism and aerobic respiration. In healthy adults, 1 to 2 mg of dietary iron is absorbed a day, and body iron is distributed between blood (∼3000 mg, mostly as hemoglobin), liver (∼1000 mg, mostly as ferritin), skeletal muscle (∼300 mg), and macrophages (∼600 mg).( 1 ) Besides, iron generates hydroxyl radicals according to the Fenton reaction in vivo,( 2 ) and thus has been hypothesized to promote carcinogenesis through lipid peroxidation and oxidative DNA and protein damage.( 3 ) In experimental animals,( 4 ) excess intake of heme iron induces the formation of radicals and the occurrence of colon cancer. In humans, high dietary intake of heme iron( 5 , 6 ) and blood measurements of iron( 7 , 8 , 9 , 10 ) have been shown to be associated with an increased risk of cancer. More recently, a randomized control trial found that phlebotomy, accompanied by a considerable reduction in serum ferritin levels, significantly decreased risk of cancer in men with a peripheral arterial disease.( 11 ) Such evidence suggests that cancer risk may vary according to body iron status even at levels commonly observed among the general population who do not have iron metabolic disorders. However, epidemiologic evidence regarding iron and cancer is far from consistent( 12 , 13 ) and the finding from the above‐mentioned trial should be interpreted cautiously because cancer was not the primary outcome.( 14 ) Investigations linking body iron to biomarkers of carcinogenesis may provide data to support or refute whether iron level currently admitted as normal influences cancer risk.
Urinary 8‐hydroxydeoxyguanosine (8‐OHdG) is a reliable biomarker of systemic oxidative DNA damage.( 2 ) Further, epidemiologic studies have shown that urinary 8‐OHdG concentrations can predict cancer risk.( 15 , 16 , 17 ) However, few studies have been performed to quantitate 8‐OHdG levels in association with body iron status. Nakano et al. reported a positive correlation between serum ferritin concentrations and urinary 8‐OHdG levels in 2507 healthy men and women.( 18 ) However, they did not control for smoking and body mass index, factors known to be associated with 8‐OHdG levels.( 19 , 20 ) In a small study of 48 mild dyslipidemic men, Tuomainen et al. demonstrated a linear, positive relationship between serum ferritin and urinary 8‐OHdG with adjustment for smoking, body mass index, and physical activity.( 21 ) To further explore this issue, the present study examined the association between serum ferritin concentrations, a marker of body iron storage( 22 ) and urinary 8‐OHdG levels in healthy men and women while adjusting for potential confounding factors.
Materials and Methods
Study participants. In 2006, a health survey was conducted among employees of two municipal offices in north‐eastern Kyushu, Japan.( 23 ) At the time of routine health check‐up, all full‐time workers (n = 601) except those on long sick‐leave or maternity‐leave were invited; of these, 547 subjects (323 men and 224 women aged 21–67 years) participated (response rate, 91%). Prior to the examination, participants completed a questionnaire on lifestyle including smoking, alcohol drinking, diet, and exercise, which was then checked by research staff for completeness and, where necessary, clarified by asking the subject. Participants were also asked to donate blood and urine specimens. We excluded 13 subjects with missing information on 8‐OHdG and ferritin concentrations, body mass index, and smoking status. Furthermore, those who reported they had cancer (one with thyroid cancer and two with breast cancer) or other diseases that affect serum ferritin levels (one with anemia and two with chronic liver disorder) were also excluded. Finally, 528 subjects (313 men and 215 women) remained for the present analyses. The ethics committee of the International Medical Center of Japan approved the protocol of the study, and written informed consent was obtained from each participant.
Measurement of urinary 8‐OHdG. Urinary 8‐OHdG and creatinine were determined by a method previously described.( 24 ) In short, a human urine sample was mixed with the same volume of a dilution solution containing the ribonucleoside marker 8‐hydroxyguanosine. A 20‐μL aliquot of the diluted urine sample was injected into HPLC‐1 (MCI GEL CA08F, 7 μm, 1.5 × 120 mm; elution, 2% acetonitrile in 0.3 mm sulfuric acid, 50 μL/min, 65°C), via the guard column (1.5 × 40 mm), and the chromatograms were recorded by a Gilson UV detector (UV/VIS‐155 with 0.2 mm light path cell). Creatinine was detected at 245 nm. The 8‐OHdG fraction was collected, depending on the relative elution position from the peak of the added marker, 8‐hydroxyguanosine, and was automatically injected into the HPLC‐2 column. The 8‐OHdG fraction was fractionated by the HPLC‐2 column (Capcell Pak C18, Shiseido, Tokyo, Japan; 5 μm, 4.6 × 250 mm; elution, 10 mm sodium phosphate buffer [pH 6.7] containing 5% methanol and an antiseptic Reagent MB [100 μL/L], 1 mL/min, 40°C). The 8‐OHdG was detected by a Coulochem II EC detector (ESA, Chelmsford, MA, USA) with a guard cell (5020) and an analytical cell (5011) (applied voltage: guard cell, 350 mV; E1, 170 mV; E2, 300 mV). The accuracy of the measurement, estimated from the recovery of an added 8‐OHdG standard, was 90–98%. When the same urine sample was analyzed three times, the variation of the data was within 7%. 8‐OHdG levels were adjusted for urinary creatinine levels before statistical analysis.
Measurement of serum ferritin. From each individual, 9 mL of venous blood was drawn in a vacuum blood collection tube and carried to our laboratory in a cooled box. Blood was centrifuged for 15 min and the serum separated was stored in a maximum of six tubes (0.5 mL each) at −20°C until analysis. Serum ferritin concentrations were measured by chemiluminescence immunoassay on the Bayer ADVIA Centaur at an external laboratory (Mitsubishi Chemical Medicine, Tokyo, Japan).
Other variables. Body height was measured to the nearest 0.1 cm with the subject standing without shoes. Body weight in light clothes was measured to the nearest 0.1 kg. Body mass index (BMI) was calculated as the body weight (kg) divided by the square of body height (m). Smoking status and alcohol intake were self‐reported in the lifestyle questionnaire. Participants were asked about weekly hours of leisure‐time physical activity engaged in for each of the four activities: strolling or walking; mild exercise; moderate intensity exercise; strong intensity exercise. Weekly minutes for walking or cycling while commuting to and from the work were also ascertained. Average metabolic equivalent task (MET) values were assigned to each level of activity according to the intensity of exercise, and total MET‐hours per week was estimated by summing all of the values for each participant. Dietary habit for the preceding month was assessed with a brief self‐administered diet history questionnaire.( 25 ) Intakes of iron, vitamin C, and vitamin E were estimated by an ad hoc computer algorithm, and their energy‐adjusted values (per 1000 kcal) were used for analysis. Blood hemoglobin was measured by sodium lauryl sulfate–hemoglobin method, serum iron was determined by colorimetric assay, and red blood cells were counted by automated blood counting machine at an external laboratory.
Statistical analysis. Median and inter‐quartile range of serum ferritin, urinary 8‐OHdG, and blood hemoglobin concentrations were calculated according to age (<35, 35–49, or ≥50 years), smoking status (nonsmoker, quitter, smoking 1–19 cigarettes/day, or smoking ≥20 cigarettes/day), BMI (tertile), ethanol consumption (0, 0.1–19.9, 20–39.9, or ≥40 g/day), physical activity (0, 0.1–4.9, 5–9.9, or ≥10 MET‐h/week), vitamin C (tertile), and vitamin E (tertile), and the difference between groups was assessed using the Wilcoxon two‐sample test. The Spearman rank correlation coefficient was calculated to assess the association between serum ferritin and urinary 8‐OHdG concentrations. In women, because serum ferritin concentrations considerably increase after menopause,( 26 ) analyses were done separately for those aged less than 50 years, and 50 years or older, with reference to data regarding the mean age of menopause in Japanese women (48.3 years old).( 27 )
Both ferritin and 8‐OHdG concentrations were log‐transformed for the following parametric analyses. The geometric mean and its 95% confidence interval of urinary 8‐OHdG concentrations were calculated for each tertile of the serum ferritin levels for the three groups: men, women aged less than 50 years, and women aged 50 years or older. To control the effects of potential confounding variables, we performed three types of analysis. In Model 1, we adjusted for age (continuous), smoking status (nonsmoker or smoker), and BMI (continuous). In Model 2, we additionally adjusted for hemoglobin levels (continuous). In Model 3, we adjusted for alcohol consumption (0, 0.1–19.9, 20–39.9, or ≥40 g/day), physical activity (0, 0.1–4.9, 5–9.9, or ≥10 MET‐h/week), vitamin C intake (tertile), and vitamin E intake (tertile) in addition to the covariates in Model 1. Trend association was evaluated by assigning 1–3 to the lowest through highest tertile categories of ferritin concentrations. Because smoking is a known, consistent determinant of urinary 8‐OHdG concentrations,( 28 ) analysis was repeated by smoking status in men. Statistical tests were two‐sided and regarded as statistically significant at P‐value <0.05. Analysis was done with STATA SE version 10.0 (Lakeway Drive College Station, TX, USA).
Results
Table 1 presents medians of urinary 8‐OHdG and serum ferritin concentrations according to age, smoking, and BMI for women and men. There was no significant difference in 8‐OHdG concentration between women and men (2.95 vs 3.10 μg/g creatinine, P = 0.45), although women showed a greater variation of 8‐OHdG concentrations than did men. In women, those aged 50 years or older had significantly higher 8‐OHdG levels than those aged less than 50 years (3.35 vs 2.90, P = 0.043). Median serum ferritin concentration markedly differed among the three groups (P < 0.001): 24.9, 51.2, and 130 ng/mL for women under 50 years, women aged 50 years or older, and men, respectively. In men, smokers had significantly higher 8‐OHdG (P < 0.001) and ferritin concentrations (P = 0.042) than nonsmokers. Blood hemoglobin levels did not appreciably differ according to demographic and lifestyle factors except smoking; heavy smokers showed a higher mean of hemoglobin levels than nonsmokers in men. In both women and men, ferritin concentrations tended to increase with BMI. In men, 8‐OHdG levels decreased as BMI increased (P for trend = 0.01) but tended to increase with increasing intake of vitamin C. In women, both serum ferritin and 8‐OHdG levels were significantly higher in the highest category of vitamin C intake or physical activity than in the lowest category of the respective variable.
Table 1.
Description of study participants (n = 528)
| n | Urinary 8‐OHdG concentrations (μg/g creatinine) | Serum ferritin concentrations (ng/mL) | n | Blood hemoglobin levels (g/dL)† | |
|---|---|---|---|---|---|
| Women (n = 215) | |||||
| Age (years) | |||||
| <35 | 72 | 2.84 (2.17–3.85) | 24.4 (12.3–44.3) | 72 | 13.2 (12.5–14.0) |
| 35–49 | 82 | 3.09 (2.04–4.03) | 25.5 (12.5–54.8) | 82 | 13.2 (12.7–13.6) |
| ≥50 | 61 | 3.35 (2.37–4.84) | 51.2 (20.8–119.0)** | 61 | 13.3 (12.8–14.1) |
| Smoking | |||||
| Nonsmoker | 208 | 2.99 (2.20–4.20) | 28.9 (14.0–56.9) | 208 | 13.2 (12.7–13.8) |
| Quitter | 3 | 2.75 (2.58–2.77) | 24.0 (11.8–57.5) | 3 | 13.1 (12.7–14.4) |
| Current smoker | 4 | 2.76 (1.76–5.60) | 24.3 (9.1–82.3) | 4 | 14.3 (13.4–15.2) |
| BMI (kg/m2) | |||||
| <18.5 | 46 | 2.64 (2.10–3.43) | 22.5 (11.6–46.6) | 46 | 13.1 (12.5–13.9) |
| 18.5–21.9 | 106 | 3.13 (2.32–4.22) | 29.1 (16.4–54.9) | 106 | 13.1 (12.5–13.6) |
| ≥22 | 63 | 3.05 (2.15–4.42) | 35.5 (13.8–93.7) | 63 | 13.5 (12.9–14.1) |
| Alcohol (ethanol consumption, g/day) | |||||
| 0 | 69 | 2.91 (2.12–4.42) | 29.3 (10.9–57.3) | 69 | 13.1 (12.4–14.0) |
| 0.1–19.9 | 131 | 2.95 (2.20–4.00) | 27.9 (13.9–56.4) | 131 | 13.2 (12.7–13.7) |
| 20–39.9 | 15 | 3.07 (2.83–3.95) | 32.7 (19.7–129.0) | 15 | 13.6 (12.8–14.0) |
| Physical activity involved in leisure time exercise and commuting (MET‐h/week) | |||||
| 0 | 110 | 2.90 (2.20–4.07) | 29.1 (13.9–52.7) | 110 | 13.2 (12.7–13.9) |
| 0.1–4.9 | 64 | 3.13 (2.17–4.02) | 29.1 (14.3–68.7) | 64 | 13.2 (12.7–13.8) |
| 5–9.9 | 23 | 2.82 (2.26–3.95) | 19.8 (10.0–43.9) | 23 | 13.0 (12.1–13.8) |
| ≥10 | 17 | 3.16 (2.76–4.59) | 67.2 (20.8–115.0)* | 17 | 13.4 (12.8–13.6) |
| Vitamin C consumption (mg/1000 kcal)‡ | |||||
| <62 | 71 | 2.89 (2.39–3.80) | 29.3 (13.8–52.7) | 71 | 13.1 (12.4–13.6) |
| 62–83.9 | 70 | 2.74 (1.89–4.06) | 21.2 (8.9–44.0) | 70 | 13.1 (12.5–13.7) |
| ≥84 | 72 | 3.22 (2.31–4.40) | 40.8 (21.2–101.1)** | 72 | 13.4 (12.9–14.0) |
| Vitamin E consumption (mg/1000 kcal) | |||||
| <4.13 | 72 | 2.84 (2.06–3.80) | 27.6 (12.2–52.1) | 72 | 13.1 (12.4–14.0) |
| 4.13–4.949 | 71 | 3.10 (2.15–4.18) | 29.3 (14.7–57.3) | 71 | 13.2 (12.7–13.8) |
| ≥4.95 | 70 | 3.09 (2.29–4.26) | 29.8 (13.9–79.4) | 70 | 13.2 (12.7–13.8) |
| Men (n = 313) | |||||
| Age (years) | |||||
| <35 | 76 | 3.19 (2.55–3.86) | 130.5 (78.4–191.0) | 57 | 15.5 (15.1–16.1) |
| 35–49 | 111 | 3.33 (2.41–4.28) | 139.0 (90.2–232.0) | 111 | 15.6 (15.1–16.2) |
| ≥50 | 126 | 3.05 (2.43–3.72) | 124.0 (75.7–196.0) | 126 | 15.3 (14.8–16.1) |
| Smoking (cigarettes/day) | |||||
| Nonsmoker | 113 | 2.92 (2.24–3.92) | 120.0 (71.0–205.0) | 102 | 15.4 (14.9–16.0) |
| Quitter | 62 | 3.00 (2.37–3.60) | 124.5 (83.3–181.0) | 59 | 15.4 (14.9–15.9) |
| 1–19 | 43 | 3.30 (2.79–4.27)* | 128.0 (74.6–188.0) | 38 | 15.4 (14.9–16.2) |
| ≥20 | 95 | 3.38 (2.71–4.19)** | 153.0 (93.0–249.0)* | 95 | 15.7 (15.0–16.3)* |
| BMI(kg/m2) | |||||
| <22 | 110 | 3.36 (2.63–4.12) | 113.0 (79.2–184.0) | 97 | 15.5 (14.9–16.1) |
| 22–24.9 | 102 | 3.16 (2.43–3.96) | 135.0 (78.0–198.0) | 100 | 15.3 (14.9–16.0) |
| ≥25 | 101 | 2.97 (2.35–3.77)* | 156.0 (84.3–261.0)* | 97 | 15.6 (14.9–16.2) |
| Alcohol (ethanol consumption, g/day) | |||||
| 0 | 44 | 2.95 (2.33–3.78) | 112.0 (65.5–183.0) | 42 | 15.5 (14.9–16.1) |
| 0.1–19.9 | 166 | 3.09 (2.52–4.14) | 135.0 (78.4–195.0) | 154 | 15.5 (14.9–16.1) |
| 20–39.9 | 71 | 3.29 (2.43–3.96) | 134.0 (98.1–249.0)* | 67 | 15.4 (14.9–16.3) |
| ≥40 | 32 | 3.24 (2.47–3.77) | 135.0 (92.9–233.0)* | 31 | 15.7 (15.3–16.3) |
| Physical activity involved in leisure time exercise and commuting (MET‐h/week) | |||||
| 0 | 108 | 3.32 (2.47–3.99) | 131.5 (85.3–210.0) | 101 | 15.5 (15.0–16.2) |
| 0.1–4.9 | 82 | 2.89 (2.41–4.16) | 143.5 (83.9–206.0) | 79 | 15.6 (14.9–16.2) |
| 5–9.9 | 42 | 3.01 (2.48–3.54) | 146.0 (78.1–280.0) | 40 | 15.6 (14.8–16.3) |
| ≥10 | 79 | 3.19 (2.29–4.14) | 120.0 (76.0–186.0) | 72 | 15.3 (14.8–16.1) |
| Vitamin C consumption (mg/1000 kcal)‡ | |||||
| <41 | 100 | 3.06 (2.40–3.67) | 121.0 (83.5–189.0) | 93 | 15.5 (14.9–16.1) |
| 41–59.9 | 102 | 3.00 (2.37–3.90) | 135.0 (88.4–211.0) | 99 | 15.4 (14.9–16.3) |
| ≥60 | 108 | 3.32 (2.60–4.18)* | 134.0 (76.4–214.0) | 99 | 15.5 (15.0–16.1) |
| Vitamin E consumption (mg/1000 kcal) | |||||
| <3.42 | 102 | 3.18 (2.42–3.90) | 125.0 (83.7–212.0) | 97 | 15.6 (14.9–16.2) |
| 3.42–4.119 | 103 | 3.05 (2.43–4.06) | 127.0 (83.6–187.0) | 94 | 15.5 (15.0–16.2) |
| ≥4.12 | 105 | 3.13 (2.50–3.99) | 139.0 (76.0–224.0) | 100 | 15.5 (14.9–16.0) |
Values are median (inter‐quartile range) unless otherwise stated. *P < 0.05, **P < 0.01 as compared with those in the lowest tertile (age, BMI, alcohol, physical activity, vitamin C and E) or with nonsmokers (smoking). †Blood hemoglobin has 19 missing values for men less than 35 years old. ‡Vitamin C and vitamin E have two missing values for women and three missing values in men. 8‐OHdG, 8‐hydroxydeoxyguanosine; BMI, body mass index; MET, metabolic equivalent task.
Serum ferritin concentrations were significantly and positively correlated with urinary 8‐OHdG concentrations in both women and men (Fig. 1), with the Spearman rank correlation coefficient being 0.47, 0.76, and 0.73 for men, women aged less than 50 years, and women aged 50 years or older, respectively. In men, the coefficient was 0.52, 0.52, 0.45, and 0.31 for nonsmokers, quitters, 1–19 cigarettes/day, and 20 or more cigarettes/day, respectively. Meanwhile, regression coefficients of log‐transformed ferritin (ng/mL) on log‐transformed 8‐OHdG (μg/g creatinine) were 0.748, 0.737, and 0.546, for women under 50 years, women aged 50 years or older, and men, respectively.
Figure 1.
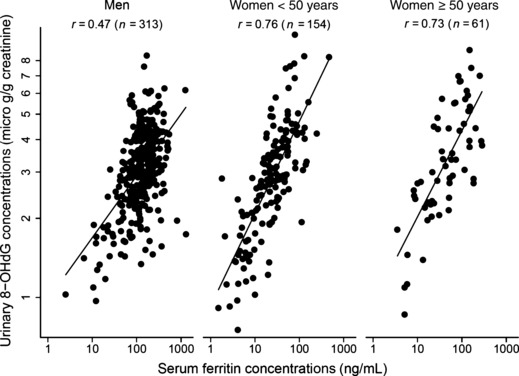
Correlation between serum ferritin and urinary 8‐hydroxydeoxyguanosine (8‐OHdG) concentrations.
As Table 2 shows, the geometric mean of urinary 8‐OHdG concentrations increased steadily as serum ferritin levels increased in all the three groups (P for trend <0.001), and this association was materially unchanged after adjustment of potential confounders. Of all the subgroups divided by sex, age, and serum ferritin levels, the highest unadjusted geometric mean of 8‐OHdG concentrations was recorded in the highest tertile of ferritin among women aged 50 years or older (4.87 μg/g creatinine), whereas the lowest mean was observed in the lowest tertile of ferritin among women aged less than 50 years (1.80 μg/g creatinine). Urinary 8‐OHdG concentrations were not significantly associated with red blood cell count, dietary iron intake, and serum iron concentrations (data not shown).
Table 2.
Urinary 8‐OHdG concentrations by serum ferritin levels
| Ferritin tertile (ng/mL) | Geometric mean (95% CI) of 8‐OHdG concentrations (μg/g creatinine) | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Unadjusted | Model 1† | n | Model 2‡ | n | Model 3§ | ||
| Women <50 years | <17 | 51 | 1.80 (1.61–2.00) | 1.90 (1.65–2.20) | 51 | 2.16 (1.82–2.56) | 51 | 1.91 (1.63–2.24) |
| 17–35 | 51 | 2.93 (2.69–3.19) | 3.04 (2.60–3.56) | 51 | 3.20 (2.73–3.75) | 51 | 3.00 (2.52–3.58) | |
| ≥36 | 52 | 4.19 (3.82–4.61) | 4.37 (3.76–5.08) | 52 | 4.57 (3.92–5.33) | 49 | 4.39 (3.71–5.19) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Women ≥50 years | <25 | 20 | 2.03 (1.69–2.43) | 1.66 (1.16–2.37) | 20 | 1.84 (1.22–2.76) | 20 | 1.44 (1.01–2.04) |
| 25–84 | 20 | 3.57 (3.05–4.19) | 2.87 (1.91–4.30) | 20 | 3.11 (2.02–4.77) | 20 | 2.24 (1.47–3.39) | |
| ≥85 | 21 | 4.87 (4.21–5.64) | 3.92 (2.49–6.16) | 21 | 4.19 (2.64–6.64) | 21 | 3.41 (2.21–5.28) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Men | <98 | 105 | 2.47 (2.30–2.65) | 2.47 (2.32–2.63) | 99 | 2.38 (2.21–2.56) | 105 | 2.45 (2.30–2.61) |
| 98–179 | 102 | 3.22 (3.03–3.43) | 3.17 (2.98–3.38) | 97 | 3.05 (2.83–3.28) | 98 | 3.24 (3.03–3.47) | |
| ≥180 | 106 | 3.56 (3.36–3.77) | 3.59 (3.35–3.83) | 98 | 3.39 (3.13–3.68) | 106 | 3.58 (3.34–3.84) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
†Adjusted for age, smoking status, and body mass index. ‡Adjusted for age, smoking status, body mass index, and hemoglobin. §Adjusted for age, smoking status, body mass index, alcohol consumption, physical activity, vitamin C and vitamin E intake. 8‐OHdG, 8‐hydroxydeoxyguanosine; CI, confidence interval.
Discussion
In this cross‐sectional biomarker study of healthy Japanese men and women, we found a strong positive association between serum ferritin and urinary 8‐OHdG concentrations. This association was robust in all the subgroups whose serum ferritin concentrations markedly differed, and were materially unchanged even after adjustment for potential confounding variables. Our finding underscores the importance of body iron store as a determinant of level of oxidative DNA damage in a population without iron metabolism disorders.
8‐OHdG, an oxidized nucleoside of DNA, is the most frequently detected DNA lesion in nuclear and mitochondrial DNA. Several reactive oxygen species, such as hydroxyl radical and singlet oxygen, attack on C‐8 of guanine in DNA and generate 8‐OHdG. 8‐OHdG and its oxidation products lead to GC→TA transversions in human cells since 8‐hydroxyguanine has hydrogen‐bonding ability to adenine.( 3 )Base excision repair by 8‐hydroxyguanine glycosylase1 is the major removal path of 8‐OHdG from DNA.( 1 ) Removed 8‐OHdG from the whole body is excreted in human urine,( 29 ) and thus urinary 8‐OHdG is considered to reflect oxidative DNA damage and repair from all cells in the organism.
Ferritin is an iron binding protein that can store up to 4500 Fe3+ions and is distributed throughout the body, and its concentrations in serum reflect the body’s iron store.( 30 ) Although ferritin itself has the potential to protect against oxidative stress by chelating free iron,( 31 ) it could also act as a mediator of oxidative stress by releasing free iron.( 32 ) This paradoxical behavior makes the role of ferritin in oxidative stress controversial.( 33 )
Previously, two studies have examined the association between serum ferritin and 8‐OHdG concentrations in persons with no known condition that might influence these measurements. In a study of mild dyslipidemic men,( 21 ) there was a significant positive association between the two measures (Spearman rank correlation coefficient, 0.36). However, this finding is limited due to the small sample size (n = 48) and subjects’ profile (dyslipidemic men), which may not represent the general population. In another study in a health checkup setting,( 18 ) the correlation coefficient between serum ferritin and urinary 8‐OHdG concentrations were 0.32 and 0.54 for men and women, respectively. However, the source population of the health checkup attendants was not clearly defined and no consideration was made for potentially important confounders including smoking. The observed association in our study (Spearman rank correlation coefficient, 0.47 and 0.76 for men and women, respectively) appears stronger than those documented previously. Our study participants shared a similar social background (municipal employees) and the survey was conducted in a short period of time (less than 10 days for each survey). We believe that such study design might have contributed to the minimization of the confounding effect of unmeasured variables.
Epidemiologic studies have reported an increased cancer risk associated with menopause( 34 ) or high body iron status( 35 ) in women. In the present study, women aged 50 years or older had markedly higher ferritin concentrations than did women aged less than 50 years, whereas hemoglobin levels did not differ. Although we did not obtain information regarding menopausal status from the study participants, given that the human body does not have a specific excretion route for stored iron( 36 ), this probably reflected an increase of body iron storage after menopause. Moreover, in accordance with the difference of ferritin concentrations, women aged 50 or older had significantly higher 8‐OHdG levels than women aged less than 50 years, suggesting an increased oxidative DNA damage after menopause. Because the regression line of serum ferritin concentrations on urinary 8‐OHdG levels was similar between the two women’s age groups, the increased levels of oxidative DNA damage among the older women could be ascribed to their elevated body iron storage, rather than to decreased physiologic function against oxidative stress after menopause.
Several epidemiologic studies have shown an increased risk of cancer associated with high dietary intake of heme iron( 5 , 6 ) and high blood levels of iron.( 7 , 8 , 9 , 10 ) Moreover, as a phlebotomy intervention study indicated,( 11 ) iron reduction may decrease cancer risk even among persons without iron metabolism disorders. Together with accumulating data for the usefulness of urinary 8‐OHdG as a marker of cancer risk,( 15 , 16 , 17 ) the strong positive association between serum ferritin concentrations and urinary 8‐OHdG concentrations in the present study supports the hypothesis that body iron storage increases cancer risk through oxidative DNA damage in humans.
Major strengths of the present study include high participation rate (91%), adjustment of potential confounding variables in the analysis, and use of a reliable method for 8‐OHdG measurement (HPLC). Our study has also some limitations. First, causality can not be inferred from any cross‐sectional study, like ours. Second, we measured biomarker levels only at a single point in time, which may not represent long‐term status. Third, other biomarkers of body iron including transferrin saturation or soluble transferrin receptor( 37 ) were not measured in the present study. However, ferritin is considered a preferred marker for the assessment of iron‐related oxidative stress.( 38 ) Finally, the study subjects were healthy municipal workers, and thus caution should be exercised when applying the result to populations with a different background.
In conclusion, we found a strong positive association between urinary 8‐OHdG levels and serum ferritin concentrations in Japanese workers. This finding suggests that body iron storage is an important determinant of oxidative DNA damage, and thus supports a significant role for iron in carcinogenesis in a general population. The observed cross‐sectional association requires confirmation in longitudinal studies.
Acknowledgments
This work was supported by a Grant‐in‐Aid for the Third Term Comprehensive 10‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan; and Grant‐in‐Aids for Scientific Research (C) (no. 18590601) and (B) (no. 21390213) from the Japan Society for the Promotion of Science. We are grateful to Professor Hideki Igisu and Professor Hatsumi Taniguchi (University of Occupational and Environmental Health, Japan) for their guidance. We thank Tamani Hatano, Yasumi Kimura, Akihoro Takana, and Yoko Ejima (Kyushu University); Mio Ozawa (Fukuoka Women’s University); and Akiko Hayashi and Kie Nagao (International Medical Center of Japan) for their help in data collection.
References
- 1. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 4th edn. New York: Oxford University Press, 2007; 220–36. [Google Scholar]
- 2. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress‐induced cancer. Chem Biol Interact 2006; 160: 1–40. [DOI] [PubMed] [Google Scholar]
- 3. Toyokuni S. Iron‐induced carcinogenesis: the role of redox regulation. Free Radic Biol Med 1996; 20: 553–66. [DOI] [PubMed] [Google Scholar]
- 4. Sawa T, Akaike T, Kida K, Fukushima Y, Takagi K, Maeda H. Lipid peroxyl radicals from oxidized oils and heme‐iron: implication of a high‐fat diet in colon carcinogenesis. Cancer Epidemiol Biomarkers Prev 1998; 7: 1007–12. [PubMed] [Google Scholar]
- 5. Lee DH, Anderson KE, Folsom AR, Jacobs DR, Jr . Heme iron, zinc and upper digestive tract cancer: the Iowa Women’s Health Study. Int J Cancer 2005; 117: 643–7. [DOI] [PubMed] [Google Scholar]
- 6. Zhou W, Park S, Liu G et al. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 2005; 16: 772–9. [DOI] [PubMed] [Google Scholar]
- 7. Hann HW, Kim CY, London WT, Blumberg BS. Increased serum ferritin in chronic liver disease: a risk factor for primary hepatocellular carcinoma. Int J Cancer 1989; 43: 376–9. [DOI] [PubMed] [Google Scholar]
- 8. Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliovaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer 1994; 56: 379–82. [DOI] [PubMed] [Google Scholar]
- 9. Selby JV, Friedman GD. Epidemiologic evidence of an association between body iron stores and risk of cancer. Int J Cancer 1988; 41: 677–82. [DOI] [PubMed] [Google Scholar]
- 10. Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer 1994; 56: 364–9. [DOI] [PubMed] [Google Scholar]
- 11. Zacharski LR, Chow BK, Howes PS et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst 2008; 100: 996–1002. [DOI] [PubMed] [Google Scholar]
- 12. Cross AJ, Gunter MJ, Wood RJ et al. Iron and colorectal cancer risk in the alpha‐tocopherol, beta‐carotene cancer prevention study. Int J Cancer 2006; 118: 3147–52. [DOI] [PubMed] [Google Scholar]
- 13. Kato I, Dnistrian AM, Schwartz M et al. Iron intake, body iron stores and colorectal cancer risk in women: a nested case‐control study. Int J Cancer 1999; 80: 693–8. [DOI] [PubMed] [Google Scholar]
- 14. Zacharski LR, Chow BK, Howes PS et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA 2007; 297: 603–10. [DOI] [PubMed] [Google Scholar]
- 15. Loft S, Svoboda P, Kasai H et al. Prospective study of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 2006; 27: 1245–50. [DOI] [PubMed] [Google Scholar]
- 16. Cooke MS, Osborne JE, Singh R et al. Evidence that oxidative stress is a risk factor for the development of squamous cell carcinoma in renal transplant patients. Free Radic Biol Med 2007; 43: 1328–34. [DOI] [PubMed] [Google Scholar]
- 17. Thanan R, Murata M, Pinlaor S et al. Urinary 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol Biomarkers Prev 2008; 17: 518–24. [DOI] [PubMed] [Google Scholar]
- 18. Nakano M, Kawanishi Y, Kamohara S et al. Oxidative DNA damage (8‐hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy people. Free Radic Biol Med 2003; 35: 826–32. [DOI] [PubMed] [Google Scholar]
- 19. Kasai H, Iwamoto‐Tanaka N, Miyamoto T et al. Life style and urinary 8‐hydroxydeoxyguanosine, a marker of oxidative dna damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res 2001; 92: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizoue T, Tokunaga S, Kasai H, Kawai K, Sato M, Kubo T. Body mass index and oxidative DNA damage: a longitudinal study. Cancer Sci 2007; 98: 1254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuomainen TP, Loft S, Nyyssonen K, Punnonen K, Salonen JT, Poulsen HE. Body iron is a contributor to oxidative damage of DNA. Free Radic Res 2007; 41: 324–8. [DOI] [PubMed] [Google Scholar]
- 22. Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol 2008; 88: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murakami K, Mizoue T, Sasaki S et al. Dietary intake of folate, other B vitamins, and omega‐3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition 2008; 24: 140–7. [DOI] [PubMed] [Google Scholar]
- 24. Kasai H, Svoboda P, Yamasaki S, Kawai K. Simultaneous determination of 8‐hydroxydeoyguanosine, a marker of oxidative stress, and creatinine, a standardization compound, in urine. Ind Health 2005; 43: 333–6. [DOI] [PubMed] [Google Scholar]
- 25. Sasaki S. Development and evaluation of dietary assessment methods using biomarkers and diet history questionnaires for individuals. (Japanese) In: Tanaka H, ed. Research for Evaluation Methods of Nutrition and Dietary Lifestyle Programs Held on Health Japan 21. Summary report. Tokyo: Ministry of Health, Welfare, and Labour, 2004: 10–44. [Google Scholar]
- 26. Whitfield JB, Treloar S, Zhu G, Powell LW, Martin NG. Relative importance of female‐specific and non‐female‐specific effects on variation in iron stores between women. Br J Haematol 2003; 120: 860–6. [DOI] [PubMed] [Google Scholar]
- 27. Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Age at menopause and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol 2006; 16: 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8‐hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992; 13: 2241–7. [DOI] [PubMed] [Google Scholar]
- 29. Pilger A, Rudiger HW. 8‐Hydroxy‐2’‐deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int Arch Occup Environ Health 2006; 80: 1–15. [DOI] [PubMed] [Google Scholar]
- 30. Cook JD, Lipschitz DA, Miles LE, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr 1974; 27: 681–7. [DOI] [PubMed] [Google Scholar]
- 31. Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood 2002; 99: 3505–16. [DOI] [PubMed] [Google Scholar]
- 32. Reif DW. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med 1992; 12: 417–27. [DOI] [PubMed] [Google Scholar]
- 33. Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem 2007; 14: 857–74. [DOI] [PubMed] [Google Scholar]
- 34. Van Asperen IA, Feskens EJ, Bowles CH, Kromhout D. Body iron stores and mortality due to cancer and ischaemic heart disease: a 17‐year follow‐up study of elderly men and women. Int J Epidemiol 1995; 24: 665–70. [DOI] [PubMed] [Google Scholar]
- 35. Hercberg S, Estaquio C, Czernichow S et al. Iron status and risk of cancers in the SU.VI.MAX cohort. J Nutr 2005; 135: 2664–8. [DOI] [PubMed] [Google Scholar]
- 36. Siah CW, Ombiga J, Adams LA, Trinder D, Olynyk JK. Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev 2006; 27: 5–16. [PMC free article] [PubMed] [Google Scholar]
- 37. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003; 101: 3359–64. [DOI] [PubMed] [Google Scholar]
- 38. Lee DH, Zacharski LR, Jacobs DR, Jr . Comparison of the serum ferritin and percentage of transferrin saturation as exposure markers of iron‐driven oxidative stress‐related disease outcomes. Am Heart J 2006; 151: 1247.e1,1247.e7. [DOI] [PubMed] [Google Scholar]


