Abstract
c‐Ski, originally identified as a proto‐oncogene product, is an important negative regulator of transforming growth factor (TGF)‐β family signaling through interaction with Smad2, Smad3, and Smad4. High expression of c‐Ski has been found in some cancers, including gastric cancer. We previously showed that disruption of TGF‐β signaling by dominant‐negative TGF‐β type II receptor in a diffuse‐type gastric carcinoma model accelerated tumor growth through induction of tumor angiogenesis by decreased expression of the anti‐angiogenic factor thrombospondin (TSP)‐1. Here, we examined the function of c‐Ski in human diffuse‐type gastric carcinoma OCUM‐2MLN cells. Overexpression of c‐Ski inhibited TGF‐β signaling in OCUM‐2MLN cells. Interestingly, c‐Ski overexpression resulted in extensive acceleration of the growth of subcutaneous xenografts in BALB/c nu/nu female mice (6 weeks of age). Similar to tumors expressing dominant‐negative TGF‐β type II receptor, histochemical studies revealed less fibrosis and increased angiogenesis in xenografted tumors expressing c‐Ski compared to control tumors. Induction of TSP‐1 mRNA by TGF‐β was attenuated by c‐Ski in vitro, and expression of TSP‐1 mRNA was decreased in tumors expressing c‐Ski in vivo. These findings suggest that c‐Ski overexpression promotes the growth of diffuse‐type gastric carcinoma through induction of angiogenesis. (Cancer Sci 2009; 100: 1809–1816)
Gastric cancer is one of the most prevalent malignancies throughout the world, particularly in Asian countries.( 1 ) Despite marked advances in diagnosis and treatment, the prognosis of advanced gastric cancer remains poor. In the Laurén classification, which is widely used for gastric cancer,( 2 ) gastric cancer is divided into two main histological types that are dissimilar in clinical and epidemiological features: intestinal‐type, well‐differentiated tumors with cohesive neoplastic cells forming gland‐like tubular structures, and diffuse‐type, poorly differentiated tumors in which individual cells infiltrate and thicken the stomach wall. Diffuse‐type gastric carcinoma includes scirrhous gastric cancer, and is characterized by remarkable fibrosis, rapid invasive progression, and a high frequency of metastasis to the peritoneum and lymph nodes. Although the incidence of intestinal‐type gastric carcinoma has continuously decreased, that of the diffuse type has progressively increased during the last three decades, and diffuse‐type gastric carcinoma now comprises approximately one‐third of gastric carcinomas in the USA.( 3 ) Recent investigations have improved understanding of the molecular mechanisms of gastric carcinogenesis. Although a number of molecular changes have been described in gastric carcinoma, substantial differences appear to exist in the pathways leading to intestinal‐ and diffuse‐type gastric carcinoma.( 4 , 5 ) Reduction or loss of E‐cadherin and catenins and amplification of K‐sam and c‐met are important for the development and progression of poorly differentiated gastric carcinomas. Hereditary diffuse‐type gastric carcinoma is induced by germline mutations in the E‐cadherin (CDH1) gene.( 6 )
Transforming growth factor (TGF)‐β is a potent growth‐inhibitory cytokine for many types of cells. TGF‐β binds to type II (TβRII) and type I (TβRI) serine‐threonine kinase receptors, and transduces signals through phosphorylation of receptor‐regulated Smads (Smad2 and Smad3). Smad2 and Smad3 form complexes with common‐partner Smad (Smad4) and regulate transcription of various target genes in the nucleus.( 7 , 8 ) Defects in the TGF‐β signal transduction pathway are observed in many gastrointestinal tumors.( 9 ) In earlier studies, mutations of TβRII and a signaling component of TGF‐β, Smad4 (originally termed DPC4), were identified in hereditary non‐polyposis colon cancer and pancreatic cancer, respectively.( 10 , 11 ) In the setting of gastric carcinogenesis, loss of Smad4 expression is frequently observed and correlated with poor clinical outcome.( 12 , 13 , 14 ) Consistent with this, it has been shown that haploid loss of Smad4 initiates gastric carcinogenesis in mice.( 15 ) In addition, reduced Smad4 expression is observed more frequently in diffuse‐type than in intestinal‐type gastric carcinomas.( 13 ) Moreover, RUNX3, a major tumor suppressor of gastric cancer, appears to play an important role in TGF‐β‐mediated tumor suppressive activity.( 16 )
Ski was originally identified as the oncogene of the avian Sloan–Kettering retrovirus (v‐Ski). Overexpression of v‐Ski induces morphological transformation and anchorage independence of fibroblasts.( 17 ) c‐Ski, a cellular counterpart of v‐Ski, and SnoN comprise a gene family of proto‐oncogenes, and c‐Ski and SnoN proteins act as negative regulators of TGF‐β signaling through interaction with Smad2 and Smad3 as well as with Smad4. The mechanism of Ski‐mediated suppression of TGF‐β signaling is thought primarily to involve transcriptional repression through recruitment of the nuclear co‐repressor and histone deacetylases to Smad complexes, interference of recruitment of the transcriptional co‐activator p300 or CREB‐binding protein (CBP), and disruption of R‐Smad–co‐Smad complexes.( 18 , 19 ) As c‐Ski interacts with Smad4, it also represses bone morphogenetic protein (BMP) signaling. High expression of c‐Ski has been reported in many human cancers, including melanoma, esophageal cancer, pancreatic cancer, colorectal cancer, and leukemia, and c‐Ski functions as an oncoprotein in these malignancies.( 20 , 21 , 22 , 23 , 24 ) Recently, Takahata et al. demonstrated high expression of c‐Ski in gastric carcinoma cells and tissues.( 25 ) In contrast to these observations, c‐Ski and SnoN have been shown to exert not only oncogenic but also anti‐oncogenic effects, depending on target cells and conditions,( 26 , 27 ) similar to the bidirectional functions of TGF‐β in oncogenesis.( 28 ) The function of c‐Ski in gastric carcinogenesis has, however, not been fully elucidated.
We recently showed that disruption of TGF‐β signaling by dominant‐negative TβRII (dnTβRII) results in acceleration of the growth of diffuse‐type gastric carcinoma in vivo.( 29 ) c‐Ski has been shown to be overexpressed in some gastric carcinoma cells, including MKN28 cells, which are relatively resistant to the effects of TGF‐β.( 25 ) As the diffuse‐type gastric carcinoma cell line OCUM‐2MLN still responds to TGF‐β,( 29 ) we examined the function of c‐Ski in OCUM‐2MLN cells in vitro and in vivo. Overexpression of c‐Ski resulted in inhibition of TGF‐β signaling in diffuse‐type gastric carcinoma cells. Interestingly, the growth advantage conferred by c‐Ski was more prominent in vivo than in vitro. Similar to the effects of dnTβRII,( 29 ) expression of c‐Ski increased tumor angiogenesis and downregulated expression of the anti‐angiogenic factor thrombospondin (TSP)‐1. These findings suggested that c‐Ski overexpression promotes tumor proliferation in vivo through alterations of the microenvironment, especially angiogenesis.
Materials and Methods
Cell lines. The OCUM‐2M, OCUM‐2MD3, and OCUM‐2MLN cell lines were established as described previously.( 30 , 31 , 32 ) Cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 100 U/mL penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA).
Antibodies and reagents. The antibodies used were as follows: rat monoclonal anti‐hemagglutinin (HA) epitope tag antibody 3F10 (Roche Diagnostics, Basel, Switzerland), mouse monoclonal anti‐green fluorescent protein (GFP) antibody (#048‐3; MBL, Nagoya, Japan), mouse monoclonal anti‐tubulin antibody DM‐1A (Sigma, St Louis, MO, USA), and rat monoclonal platelet‐endothelial cell adhesion molecule (PECAM)‐1 antibody (#555024; BD Bioscience, Franklin Lakes, NJ, USA). For immunohistochemistry, Alexa 594‐conjugated secondary antibodies were purchased from Invitrogen Molecular Probes (Eugene, OR, USA). TGF‐β1 (1 ng/mL; R&D Systems, Minneapolis, MN, USA) was used for TGF‐β1 stimulation of OCUM‐2MLN cells in vitro.
Lentiviral production and infection. We used a lentiviral infection system to establish OCUM‐2MLN cells stably expressing c‐Ski (2MLN‐c‐Ski).( 33 ) Briefly, cDNA encoding c‐Ski with an N‐terminal HA epitope tag( 34 ) was inserted into the multicloning site of the lentiviral vector construct pCSII‐EF‐RfA‐IRES2‐Venus using pENTR according to a standard protocol (Invitrogen). For production of lentiviral vectors, 293FT cells (Invitrogen) were transfected using Lipofectamine 2000 (Invitrogen) with vector constructs pCAG‐HIVgp (packaging construct) and pCMV‐VSV‐G‐RSV‐Rev (VSV‐G‐ and Rev‐expressing construct). The culture supernatants were collected 48 h after transfection, and viral particles were concentrated by ultracentrifugation and used for transduction of OCUM‐2MLN cells. Successfully infected cells were enriched by Venus expression using fluorescence‐activated cell sorting. OCUM‐2MLN cells expressing GFP (2MLN‐GFP)( 29 ) were used as a control.
Cell proliferation assay. Cells (1 × 103 cells) were seeded in 96‐well plates for 24 h prior to TGF‐β addition, and cell growth was quantified for 3 days by tetrazolium‐based colorimetric assay using 4‐[3‐(2‐methoxy‐4‐nitrophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazolio]‐1,3‐benzene disulfonate sodium salt (WST‐8) assay (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's protocol.
Immunoblotting. Cultured cells were lysed in Triton lysis buffer containing 50 mM Tris‐HCl, pH 8.0, 150 mM NaCl, phosphatase inhibitors (β‐glycerophosphate, sodium orthovanadate, and sodium fluoride), 1% Triton X‐100 (Nacalai Tesque), 1% protease inhibitor cocktail containing 4‐(2‐aminoethyl) benzenesulfonyl fluoride hydrochloride, aprotinin, E‐64, leupeptin, EDTA, bestatin, and pepstatin A (#25955‐11; Nacalai Tesque). The cell lysates were boiled in SDS sample buffer (100 mM Tris‐HCl, pH 8.8, 0.01% bromophenol blue, 36% glycerol, 4% SDS, and 10 mM dithiothreitol) and subjected to SDS‐PAGE. Proteins were electrotransferred to PALL Fluorotrans W membranes (PALL, East Hills, NY, USA), immunoblotted with antibodies, and detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
RNA isolation and quantitative real‐time RT‐PCR. Total RNA from gastric carcinoma cells and excised subcutaneous tumors were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). First‐strand cDNAs were synthesized using the Quantitect Reverse Transcription kit (Qiagen) with random hexamer primers. Quantitative real‐time RT‐PCR analysis was carried out using the 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) using the following primers: human hypoxanthine phosphoribosyltransferase (HPRT) 1, forward 5′‐tttgctttccttggtcaggc‐3′, reverse 5′‐gcttgcgaccttgaccatct‐3′; human c‐Ski, forward 5′‐cgacgtgaaggagaaattcg‐3′, reverse 5′‐ggactgggaagaggtgtcat‐3′; human Smad7, forward 5′‐ccttagccgactctgcgaacta‐3′, reverse 5′‐ccagataattcgttccccctgt‐3′; human plasminogen activator inhibitor (PAI)‐1, forward 5′‐aatcagacggcagcactgtct‐3′, reverse 5′‐ggcagttccaggatgtcgtagt‐3′; human TGF‐β1, forward 5′‐agtggacatcaacgggttcac‐3′, reverse 5′‐catgagaagcaggaaaggcc‐3′; human TSP‐1, forward 5′‐aacaaccccacaccccagtttg‐3′, reverse 5′‐ttgaagcaggcatcaggcac‐3′; human p21, forward 5′‐gcgactgtgatgcgctaatg‐3′, reverse 5′‐ccagtggtgtctcggtgaca‐3′; human growth arrest and DNA‐damage‐inducible 45β (GADD45β), forward 5′‐acagtgggggtgtacgagtc‐3′, reverse 5′‐ggatgagcgtgaagtggatt‐3′; and human c‐myc, forward 5′‐ccacacatcagcacaactacg‐3′, reverse 5′‐cggttgttgctgatctgtctc‐3′. All samples were run in triplicate in each experiment. Values were normalized to those for human HPRT1.
In vivo cancer models and quantification of tumors. BALB/c nu/nu female mice (6 weeks of age) were obtained from Sankyo Labo Service Corporation (Tokyo, Japan). All animal experimental protocols were carried out in accordance with the policies of the Animal Ethics Committee of the University of Tokyo. A total of 1 × 107 cells in 200 µL PBS were injected into female nude mice (n > five mice/group) and allowed to grow for 1 week to reach proliferative phase. Subcutaneous xenografts were measured externally every second day until the end of evaluation periods, and tumor volume was approximated using the equation vol. = (a × b 2)/2, where vol. is volume, a the length of the major axis, and b the length of the minor axis. Relative tumor volume was then calculated by dividing tumor volume by that on day 7 (the day of initiation of evaluation). The results were further analyzed statistically by multivariate ANOVA using JMP6 software (SAS Institute, Raleigh, NC, USA) where applicable.
Histochemistry and immunohistochemistry. Samples excised from the animals were fixed for 1 h in 10% neutral‐buffered formalin at room temperature, washed overnight in PBS containing 10% sucrose at 4°C, and embedded in optimal cutting temperature compound (Tissue‐Tek; Sakura Finetek, Tokyo, Japan). The samples were then snap‐frozen in dry‐iced acetone for immunohistochemistry, or fixed overnight in 4% paraformaldehyde and then paraffin‐embedded for hematoxylin and eosin (H&E) or AZAN staining. Frozen samples were further sectioned at 10 µm thickness in a cryostat, briefly fixed with 10% formalin, and then incubated with primary and secondary antibodies. Samples were observed using a Zeiss LSM510 Meta confocal microscope (Zeiss, Thornwood, NY, USA) for immunohistochemistry and GFP or Venus fluorescence, and using an Olympus AX80 microscope (Olympus, Tokyo, Japan) for H&E and AZAN staining. Quantification of AZAN‐staining‐positive regions and PECAM‐1‐stained areas was carried out in four fields on five specimens using ImageJ 1.36b software (freeware distributed by the National Institutes of Health, Bethesda, MD, USA).
Statistical analyses. Results were compared by Student's t‐test. Differences were considered significant when P < 0.05. All statistical tests were two‐sided.
Results
c‐Ski overexpression inhibits TGF‐β signaling in diffuse‐type gastric carcinoma cells. The diffuse‐type gastric carcinoma cell line OCUM‐2M was previously established from a 49‐year‐old woman with diffuse‐type gastric carcinoma.( 30 ) OCUM‐2MD3 and OCUM‐2MLN cell lines were obtained from peritoneal metastasis induced by intraperitoneal inoculation and lymph node metastasis induced by orthotopic implantation of OCUM‐2M cells in nude mice, respectively.( 31 , 32 ) These cell lines were examined for expression of c‐Ski by RT‐PCR analysis, and all three cell lines were found to express c‐Ski at similar levels (data not shown).
To examine the function of c‐Ski in diffuse‐type gastric carcinoma cells, we next established OCUM‐2MLN cells expressing HA‐tagged c‐Ski (2MLN‐c‐Ski) using a lentiviral vector system. OCUM‐2MLN cells expressing GFP (2MLN‐GFP) were used as a control.( 29 ) Quantitative real‐time RT‐PCR analysis and immunoblot analysis confirmed the expression of GFP and c‐Ski in the 2MLN‐GFP and 2MLN‐c‐Ski cells, respectively (Fig. 1a,b). Consistent with previous findings, TGF‐β induced the expression of TGF‐β‐target genes, Smad7 and PAI‐1, in 2MLN‐GFP cells. c‐Ski inhibited TGF‐β signaling and suppressed the induction of Smad7 and PAI‐1 by TGF‐β (Fig. 1c).
Figure 1.

c‐Ski inhibits transforming growth factor (TGF)‐β signaling in OCUM‐2MLN cells. (a,b) Overexpression of c‐Ski in OCUM‐2MLN cells. OCUM‐2MLN cells stably expressing hemagglutinin (HA)‐tagged c‐Ski (2MLN‐c‐Ski) were generated by lentivirus‐mediated gene transfer. OCUM‐2MLN cells expressing green fluorescent protein (GFP) (2MLN‐GFP) were used as a control in subsequent experiments. (a) c‐Ski expression in 2MLN‐c‐Ski cells was confirmed by quantitative real‐time RT‐PCR analysis. (b) Cell lysates of parental OCUM‐2MLN cells, 2MLN‐GFP, and 2MLN‐c‐Ski cells were subjected to immunoblotting with anti‐GFP antibody and anti‐HA antibody. GFP and Venus are detected by the anti‐GFP antibody. (c) Inhibition of TGF‐β‐mediated Smad7 and plasminogen activator inhibitor (PAI)‐1 induction by c‐Ski. 2MLN‐GFP and 2MLN‐c‐Ski cells were treated with TGF‐β1 (1 ng/mL) and expression levels of human Smad7 (left) and human PAI‐1 (right) were determined by quantitative real‐time RT‐PCR analysis at the indicated time points.
c‐Ski enhances the growth of OCUM‐2MLN cells in vivo. We explored the effects of c‐Ski on proliferation of OCUM‐2MLN cells. Although we found that 2MLN‐c‐Ski cells tended to proliferate faster than 2MLN‐GFP cells in vitro (Fig. 2a), the magnitude of proliferation induced by c‐Ski was not statistically significant. Among TGF‐β‐responsive genes, cyclin‐dependent kinase inhibitor, p21, and c‐myc are key participants in the TGF‐β cytostatic response,( 35 , 36 ) and p21 is induced whereas c‐myc is repressed by TGF‐β. GADD45β, a transcriptional mediator of stress responses to DNA damage( 37 ) and TGF‐β,( 38 ) is also involved in TGF‐β‐mediated cell cycle arrest and apoptosis. p21 and GADD45β were strongly induced by TGF‐β in control 2MLN‐GFP cells, whereas induction of these genes by TGF‐β was abolished in 2MLN‐c‐Ski cells, and basal expression of GADD45β was also downregulated by c‐Ski expression (Fig. 2b). Expression of c‐myc mRNA was reduced in 2MLN‐c‐Ski cells compared to the control 2MLN‐GFP cells, but was not affected by TGF‐β in either cell type in vitro (Fig. 2b). Thus, c‐Ski overexpression did not exert a significant impact on in vitro proliferation of OCUM‐2MLN cells. Weak enhancement of in vitro proliferation of 2MLN‐c‐Ski cells may be induced by alteration of signals other than those participating in the TGF‐β signaling pathway.
Figure 2.
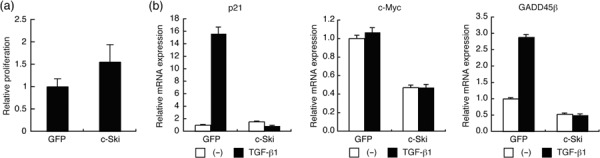
Effects of c‐Ski on in vitro proliferation of OCUM‐2MLN cells and the expression of p21, c‐myc, and growth arrest and DNA‐damage‐inducible 45β (GADD45β). (a) Proliferation of 2MLN‐green fluorescent protein (GFP) and 2MLN‐c‐Ski cells was compared by tetrazolium‐based colorimetric assay using 4‐[3‐(2‐methoxy‐4‐nitrophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazolio]‐1,3‐benzene disulfonate sodium salt (WST‐8) assay. Relative cell growth rate was quantified 3 days after seeding. Results are the mean ± SE of triplicate determinations. (b) 2MLN‐GFP and 2MLN‐c‐Ski cells were treated with or without transforming growth factor (TGF)‐β1 (1 ng/mL) for 24 h. Total RNA was extracted, and levels of expression of p21 (right), c‐myc (middle), and GADD45β (left) were examined by quantitative real‐time RT‐PCR analysis. Each value is normalized to the expression of hypoxanthine phosphoribosyltransferase 1 and represents the mean of triplicate determination.
Next, we subcutaneously transplanted 2MLN‐GFP or 2MLN‐c‐Ski cells into nude mice (n = 5–7) and measured tumor growth. 2MLN‐c‐Ski tumor volumes were 5.7‐fold larger than 2MLN‐GFP tumor volumes, and the difference was statistically significant (2MLN‐c‐Ski vs 2MLN‐GFP, P = 0.0345) (Fig. 3a,b). Quantitative real‐time RT‐PCR analysis using in vivo samples confirmed overexpression of c‐Ski in 2MLN‐c‐Ski tumors (Supplementary Fig. 1), and revealed that levels of expression of c‐myc were significantly upregulated, whereas those of p21 and GADD45β were downregulated in 2MLN‐c‐Ski tumors (Fig. 3c). As the expression of c‐myc mRNA was suppressed by c‐Ski in vitro, the upregulation of c‐myc in 2MLN‐c‐Ski tumors may result from signaling by stromal cells.
Figure 3.
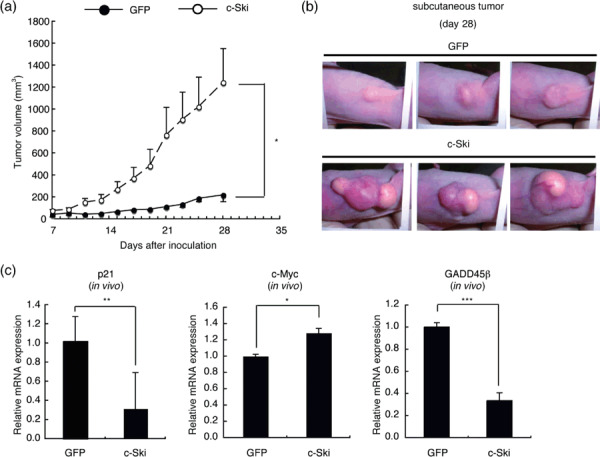
Effects of c‐Ski on the growth of OCUM‐2MLN subcutaneous tumors. (a) Growth curves of 2MLN‐green fluorescent protein (GFP) and 2MLN‐c‐Ski tumors in vivo. 2MLN‐GFP or 2MLN‐c‐Ski cells (1 × 107 cells/mouse) were subcutaneously injected into BALB/c nu/nu female mice (6 weeks of age), and subsequent tumor growth was monitored after 1 week. Results are shown as a mean ± SE (*P < 0.05). (b) Gross appearance of 2MLN‐GFP and 2MLN‐c‐Ski tumors. Representative pictures of 2MLN‐GFP and 2MLN‐c‐Ski tumors at day 28 are shown. Note that 2MLN‐c‐Ski tumors exhibit development of neovasculature on the surface. (c) Expression of growth‐regulatory genes in OCUM‐2MLN subcutaneous tumors. Total RNA was extracted from subcutaneous tumors, and the expression levels of p21, c‐myc, and growth arrest and DNA‐damage‐inducible 45β (GADD45β) were examined by quantitative real‐time RT‐PCR analysis. Each value is normalized to the expression of hypoxanthine phosphoribosyltransferase 1 and represents the mean of triplicate determination (*P < 0.05, **P < 0.01, ***P < 0.001).
Histological changes induced by c‐Ski expression. c‐Ski overexpression resulted in a significant and potent acceleration of the growth of subcutaneously transplanted tumors in vivo (Fig. 3a), and gross examination of 2MLN‐c‐Ski tumors revealed the development of subcutaneous neovasculature on the surface of tumors (see Fig. 3b). We hypothesized that, similar to dnTβRII, c‐Ski may induce alterations of the tumor microenvironment contributing to acceleration of in vivo tumor growth. We therefore examined the histological changes induced by c‐Ski expression.
On H&E staining, the subcutaneous tumors generated by 2MLN‐GFP cells exhibited marked fibrosis with infiltration of poorly differentiated adenocarcinoma cells, reflecting the original nature of scirrhous gastric carcinoma (Fig. 4a, left panels). Fibrotic areas were reduced and vasculature was more abundantly developed in 2MLN‐c‐Ski than in control tumors (Fig. 4a, right panels). We determined the degree of fibrosis in tumor tissues by AZAN staining, in which collagen fibers appear blue (Fig. 4b). Area of fibrosis was significantly decreased in 2MLN‐c‐Ski tumors compared with 2MLN‐GFP tumors (2MLN‐c‐Ski vs 2MLN‐GFP, P < 0.01; Fig. 4b). The extensive fibrosis in diffuse‐type gastric carcinoma has been reported to be associated with TGF‐β production by cancer cells or cancer‐associated fibroblasts.( 39 ) Expression of TGF‐β1 was induced by TGF‐β in a positive feedback fashion in OCUM‐2MLN cells. c‐Ski expression repressed this positive feedback loop and abolished the TGF‐β‐induced expression of TGF‐β1 mRNA (Fig. 4c). Moreover, c‐Ski reduced the expression of TGF‐β1 mRNA in subcutaneous tumors (Fig. 4d), suggesting that attenuation of TGF‐β1 production by c‐Ski may be responsible for reduced fibrosis in 2MLN‐c‐Ski tumors.
Figure 4.
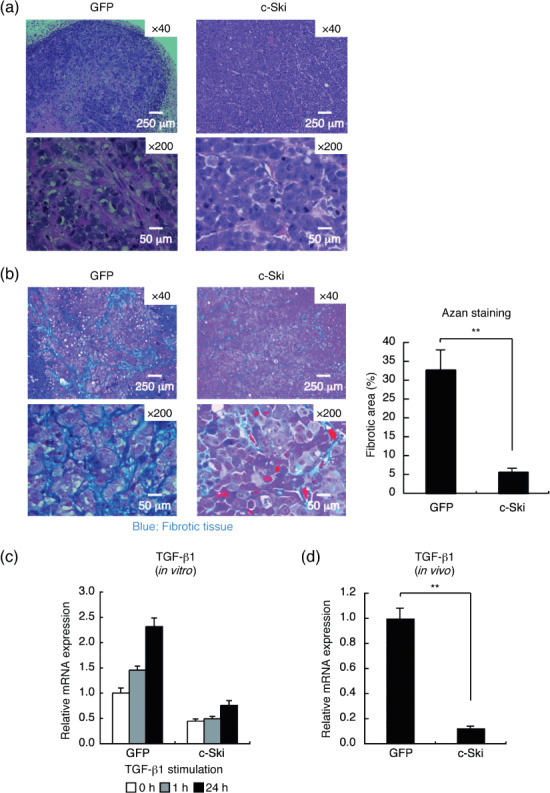
Histological changes in OCUM‐2MLN tumors induced by c‐Ski expression. (a) H&E staining of tissue sections of 2MLN‐green fluorescent protein (GFP) (left) and 2MLN‐c‐Ski (right) tumors at day 28. Representative images are shown. Scale bars = 250 µm in upper panels and 50 µm in lower panels. (b) Fibrotic areas of 2MLN‐GFP (left) and 2MLN‐c‐Ski (right) tumors were determined by AZAN staining of sections of subcutaneous tumors. Representative images are shown in the left panels. Scale bars = 250 µm in upper panels and 50 µm in lower panels. Percentage fibrotic area is shown in the right panel. Results are the mean of quantification of five fields (**P < 0.01). (c) Effects of c‐Ski on the transcription of transforming growth factor (TGF)‐β1 in vitro (left). 2MLN‐GFP and 2MLN‐c‐Ski cells were treated with TGF‐β1 (1 ng/mL), and expression of TGF‐β1 was examined by quantitative real‐time RT‐PCR analysis at the indicated time points (left). (d) Expression of TGF‐β1 in OCUM‐2MLN subcutaneous tumors. Total RNA was extracted from subcutaneous tumors, and expression of human TGF‐β1 was examined by quantitative real‐time RT‐PCR analysis. Results represent the mean of triplicate determination (**P < 0.01).
c‐Ski suppresses expression of TSP‐1 and enhances tumor angiogenesis. H&E staining of 2MLN‐c‐Ski tumors revealed that vascular components are more abundant in 2MLN‐c‐Ski than in 2MLN‐GFP tumors (Fig. 4a). We next compared the degree of tumor angiogenesis in OCUM‐2MLN tumors. We determined vascular density using a specific marker of vascular endothelium, PECAM‐1. PECAM‐1‐positive area was significantly increased in 2MLN‐c‐Ski tumors compared with 2MLN‐GFP tumors (2MLN‐c‐Ski vs 2MLN‐GFP, P < 0.01; Fig. 5a).
Figure 5.
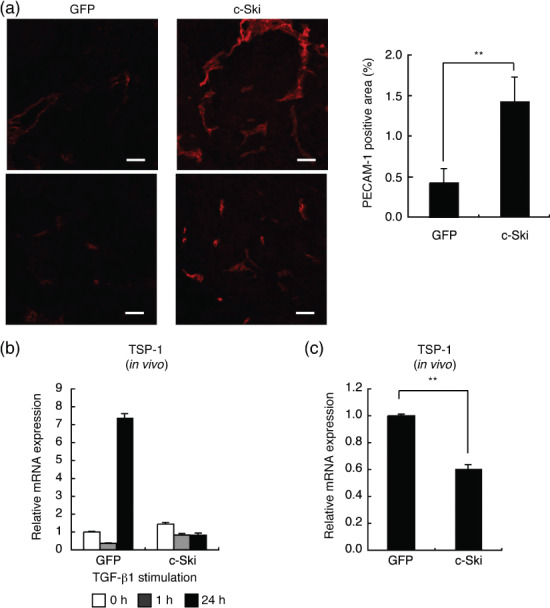
c‐Ski promotes tumor angiogenesis. (a) Enhancement of tumor angiogenesis in 2MLN‐c‐Ski tumors. Degree of vascular density was examined by platelet‐endothelial cell adhesion molecule (PECAM)‐1 immunostaining of sections of subcutaneous tumors (left panels, scale bars = 50 µm). Representative images of two different regions are shown. PECAM‐1‐positive areas were quantified (right panel). Results are the mean of quantification of five fields (**P < 0.01). (b) Effects of c‐Ski on expression of TSP‐1 in vitro. 2MLN‐green fluorescent protein (GFP) and 2MLN‐c‐Ski cells were treated with transforming growth factor (TGF)‐β1 (1 ng/mL) for 24 h, and expression of thrombospondin (TSP)‐1 was examined by quantitative real‐time RT‐PCR analysis. (c) Expression of TSP‐1 in OCUM‐2MLN subcutaneous tumors was examined by quantitative real‐time RT‐PCR analysis. Results represent the mean of triplicate determination (**P < 0.01).
To elucidate the mechanism of increase in tumor vasculature, we examined whether c‐Ski alters the levels of mRNA expression of several genes controlling angiogenesis. Among the genes involved in angiogenesis, TSP‐1, an inhibitor of angiogenesis, has been shown to be induced by TGF‐β( 40 ) and to mediate in part the tumor‐suppressive function of TGF‐β.( 41 ) We found that TSP‐1 was strongly induced by TGF‐β in 2MLN‐GFP cells in vitro, and that its induction was abolished in 2MLN‐c‐Ski cells (Fig. 5b). Consistent with this, expression of TSP‐1 was significantly suppressed in the xenografted 2MLN‐c‐Ski tumors compared with 2MLN‐GFP tumors (2MLN‐c‐Ski vs 2MLN‐GFP, P < 0.01; Fig. 5b), similar to the 2MLN tumors expressing dnTβRII.( 29 )
Discussion
In the present study, we showed that c‐Ski inhibits TGF‐β signaling and promotes tumor growth and angiogenesis in diffuse‐type gastric cancer cells. c‐Ski is expressed in almost all adult and embryonic tissues at low levels, and its expression increases during certain stages of embryonic development and later in some human malignancies. We confirmed that c‐Ski is expressed in the diffuse‐type gastric cancer cell lines OCUM‐2M, OCUM‐2MD3, and OCUM‐2MLN, although they still respond to TGF‐β (our unpublished data).
Cancer cells frequently acquire resistance to TGF‐β‐mediated growth inhibition through various mechanisms. Impairment of TGF‐β signaling has been reported to be due to inactivation of TβRII and Smad4 in colorectal and pancreatic cancers.( 10 , 11 ) However, according to a systematic analysis of the TGF‐β signaling pathway in gastrointestinal cancer cell lines, functional TGF‐β signaling was not recovered by restoration of TβRII or Smad genes in gastric cancer cell lines.( 9 ) Increased expression of certain molecules inhibiting TGF‐β signaling pathways, for example, c‐Ski and/or SnoN, may thus play a more important role in the escape by gastric carcinoma from TGF‐β‐induced growth suppression in the process of carcinogenesis. Interestingly, chromosomal co‐amplification of two distinct transcriptional co‐repressors for TGF‐β, c‐Ski and MDS1/EVI1‐like gene 1 (MEL1), which are located at chromosome position 1p36.32, was recently found in MKN28 gastric cancer cells, and knockdown of c‐Ski and MEL1 synergistically conferred TGF‐β responsiveness to these cells.( 25 ) Moreover, 22% of primary gastric cancers had a gain in copy number at 1p36,( 42 ) suggesting that c‐Ski and MEL1 could be co‐amplified in some portions of gastric cancer.
The oncogeneic function of c‐Ski has for the most part been assumed to involve relief of TGF‐β‐mediated growth inhibition.( 43 ) Importantly, we found that, similar to dnTβRII,( 29 ) c‐Ski promotes overall tumor formation with increased angiogenesis and downregulation of TSP‐1. As dnTβRII lacks the intracellular kinase domain, it inhibits the TGF‐β signaling mediated by both Smad and non‐Smad signaling pathways. In contrast, c‐Ski does not affect the kinase activities of TβRII and TβRI, and thus does not inhibit non‐Smad signaling pathways activated by TGF‐β. c‐Ski inhibits TGF‐β signaling by repression of transcriptional activity of Smads through recruitment of transcriptional co‐repressors to Smad complexes and disruption of them.( 18 , 19 ) c‐Ski has also been shown to inhibit BMP signaling pathways via interaction with Smad4, as Smad4 is shared by both TGF‐β‐ and BMP‐dependent Smad signaling pathways. In support of our findings, restoration of Smad4 in a Smad4‐deficient pancreatic cancer model resulted in inhibition of angiogenesis through induction of TSP‐1,( 44 ) indicating that the effect of overexpression of c‐Ski may in part mimic that of loss of Smad4. Collectively, c‐Ski may induce growth and angiogenesis of diffuse‐type gastric tumors primarily through inhibition of the TGF‐β–Smad signaling pathway.
In the tumor microenvironment, TSP‐1 has been shown to suppress tumor growth by inhibiting angiogenesis and by activating the latent form of TGF‐β. TSP‐1 inhibits angiogenesis through direct effects on endothelial cell migration and survival, as well as effects on the bioavailability of vascular endothelial growth factor (VEGF).( 45 ) TSP‐1 has been shown to be epigenetically downregulated through aberrant DNA methylation of the promoter region in gastric cancer.( 46 , 47 ) We recently showed that overexpression of TSP‐1 suppresses the angiogenesis and growth of OCUM‐2MLN cells, and that silencing of the expression of TSP‐1 results in acceleration of tumor growth.( 29 ) Our findings thus suggested that c‐Ski functions as an inducer of angiogenesis, at least through downregulation of TSP‐1, as in the case of dnTβRII.( 29 )
In addition to TSP‐1, VEGF‐A has been reported to be responsible for enhanced angiogenesis in the Smad4‐deficient pancreatic tumor model.( 44 ) Schwarte‐Waldhoff et al. showed that restoration of Smad4 in Smad4‐deficient pancreatic carcinoma cells resulted in downregulation of VEGF‐A and upregulation of TSP‐1. However, we observed no differences in the expression of VEGF‐A in 2MLN tumors expressing dnTβRII.( 29 ) In OCUM‐2MLN cells, we found that VEGF‐A was slightly upregulated by TGF‐β, whereas c‐Ski expression increased the expression of VEGF‐A in vitro (our unpublished data). However, we failed to observe an increase in VEGF‐A mRNA in 2MLN‐c‐Ski tumors in vivo. Whether c‐Ski enhances angiogenesis via induction of VEGF‐A as well as suppression of TSP‐1 remains to be determined.
In conclusion, we showed that c‐Ski overexpression promotes the growth of diffuse‐type gastric carcinoma cells in vivo through inhibition of TGF‐β–Smad signaling. Importantly, the growth advantage conferred by c‐Ski expression was accompanied by increased tumor angiogenesis and downregulation of the anti‐angiogenic factor TSP‐1. Enhancement of tumor angiogenesis may be an important and novel aspect of the oncogenic function of c‐Ski, and anti‐angiogenic strategies using various angiogenesis inhibitors, including TSP‐1 analogues,( 48 ) may prove useful for the treatment of advanced cancers with defects of TGF‐β signaling pathways.
Supporting information
Fig. S1. Expression of c‐Ski in OCUM‐2MLN subcutaneous tumors. Total RNA was obtained from subcutaneous tumors as described in Fig. 3(c), and expression of c‐Ski was examined by quantitative real‐time RT‐PCR analysis. Each value is normalized to the expression of hypoxanthine phosphoribosyltransferase 1 and represents the mean of triplicate determination (***P < 0.001).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
We thank Dr Masako Oka for discussion; S. Ichihara (University of Tokyo, Tokyo, Japan) for assistance with fluorescence‐activated cell sorting; and all members of the Department of Molecular Pathology, the University of Tokyo. This work was supported by KAKENHI (Grant‐in‐Aid for Scientific Research, no. 17016011) on Priority Areas ‘New strategies for cancer therapy based on advancement of basic research’ and the Global Center of Excellence Program for ‘Integrative Life Science Based on the Study of Biosignaling Mechanisms’ from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors declare no conflict of interest.
References
- 1. Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 2006; 20: 633–49. [DOI] [PubMed] [Google Scholar]
- 2. Laurén P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 3. Henson DE, Dittus C, Younes M, Nguyen H, Albores‐Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med 2004; 128: 765–70. [DOI] [PubMed] [Google Scholar]
- 4. Smith MG, Hold GL, Tahara E, El‐Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol 2006; 12: 2979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogiatzi P, Vindigni C, Roviello F, Renieri A, Giordano A. Deciphering the underlying genetic and epigenetic events leading to gastric carcinogenesis. J Cell Physiol 2007; 211: 287–95. [DOI] [PubMed] [Google Scholar]
- 6. Humar B, Guilford P. Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sci 2009; 100: 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heldin CH, Miyazono K, Ten Dijke P. TGF‐β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–71. [DOI] [PubMed] [Google Scholar]
- 8. Feng XH, Derynck R. Specificity and versatility in TGF‐β signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: 659–93. [DOI] [PubMed] [Google Scholar]
- 9. Ijichi H, Ikenoue T, Kato N et al . Systematic analysis of the TGF‐β‐Smad signaling pathway in gastrointestinal cancer cells. Biochem Biophys Res Commun 2001; 289: 350–7. [DOI] [PubMed] [Google Scholar]
- 10. Markowitz S, Wang J, Myeroff L et al . Inactivation of the type II TGF‐β receptor in colon cancer cells with microsatellite instability. Science 1995; 268: 1336–8. [DOI] [PubMed] [Google Scholar]
- 11. Hahn SA, Schutte M, Hoque AT et al . DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271: 350–3. [DOI] [PubMed] [Google Scholar]
- 12. Xiangming C, Natsugoe S, Takao S et al . Preserved Smad4 expression in the transforming growth factor β signaling pathway is a favorable prognostic factor in patients with advanced gastric cancer. Clin Cancer Res 2001; 7: 277–82. [PubMed] [Google Scholar]
- 13. Kim JY, Park DY, Kim GH et al . Smad4 expression in gastric adenoma and adenocarcinoma: frequent loss of expression in diffuse type of gastric adenocarcinoma. Histol Histopathol 2005; 20: 543–9. [DOI] [PubMed] [Google Scholar]
- 14. Wang LH, Kim SH, Lee JH et al . Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res 2007; 13: 102–10. [DOI] [PubMed] [Google Scholar]
- 15. Xu X, Brodie SG, Yang X et al . Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 2000; 19: 1868–74. [DOI] [PubMed] [Google Scholar]
- 16. Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF‐β superfamily signaling. Curr Opin Genet Dev 2003; 13: 43–7. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Turck CM, Teumer JK, Stavnezer E. Unique sequence, ski, in Sloan–Kettering avian retroviruses with properties of a new cell‐derived oncogene. J Virol 1986; 57: 1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF‐β signaling. Cell Res 2009; 19: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyazono K, Maeda S, Imamura T. Smad transcriptional co‐activators and co‐repressors. In: Ten Dijke P, Heldin CH, eds. Smad Signal Transduction. Netherlands: Springer Netherlands, 2006; 277–93. [Google Scholar]
- 20. Reed JA, Bales E, Xu W, Okan NA, Bandyopadhyay D, Medrano EE. Cytoplasmic localization of the oncogenic protein Ski in human cutaneous melanomas in vivo: functional implications for transforming growth factor β signaling. Cancer Res 2001; 61: 8074–8. [PubMed] [Google Scholar]
- 21. Fukuchi M, Nakajima M, Fukai Y et al . Increased expression of c‐Ski as a co‐repressor in transforming growth factor‐β signaling correlates with progression of esophageal squamous cell carcinoma. Int J Cancer 2004; 108: 818–24. [DOI] [PubMed] [Google Scholar]
- 22. Heider TR, Lyman S, Schoonhoven R, Behrns KE. Ski promotes tumor growth through abrogation of transforming growth factor‐β signaling in pancreatic cancer. Ann Surg 2007; 246: 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buess M, Terracciano L, Reuter J et al . Amplification of SKI is a prognostic marker in early colorectal cancer. Neoplasia 2004; 6: 207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ritter M, Kattmann D, Teichler S et al . Inhibition of retinoic acid receptor signaling by Ski in acute myeloid leukemia. Leukemia 2006; 20: 437–43. [DOI] [PubMed] [Google Scholar]
- 25. Takahata M, Inoue Y, Tsuda H et al . SKI and MEL1 cooperate to inhibit transforming growth factor‐β signal in gastric cancer cells. J Biol Chem 2009; 284: 3334–44. [DOI] [PubMed] [Google Scholar]
- 26. Shinagawa T, Nomura T, Colmenares C, Ohira M, Nakagawara A, Ishii S. Increased susceptibility to tumorigenesis of ski‐deficient heterozygous mice. Oncogene 2001; 20: 8100–8. [DOI] [PubMed] [Google Scholar]
- 27. Zhu Q, Krakowski AR, Dunham EE et al . Dual role of SnoN in mammalian tumorigenesis. Mol Cell Biol 2007; 27: 324–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006; 6: 506–20. [DOI] [PubMed] [Google Scholar]
- 29. Komuro A, Yashiro M, Iwata C et al . Diffuse‐type gastric carcinoma: progression, angiogenesis, and transforming growth factor β signaling. J Natl Cancer Inst 2009; 101: 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer 1995; 72: 1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujihara T, Sawada T, Hirakawa K et al . Establishment of lymph node metastatic model for human gastric cancer in nude mice and analysis of factors associated with metastasis. Clin Exp Metastasis 1998; 16: 389–98. [DOI] [PubMed] [Google Scholar]
- 32. Yashiro M, Chung YS, Kubo T, Hato F, Sowa M. Differential responses of scirrhous and well‐differentiated gastric cancer cells to orthotopic fibroblasts. Br J Cancer 1996; 74: 1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibuya K, Shirakawa J, Kameyama T et al . CD226 (DNAM‐1) is involved in lymphocyte function‐associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med 2003; 198: 1829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akiyoshi S, Inoue H, Hanai J et al . c‐Ski acts as a transcriptional co‐repressor in transforming growth factor‐β signaling through interaction with Smads. J Biol Chem 1999; 274: 35 269–77. [DOI] [PubMed] [Google Scholar]
- 35. Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF‐β in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807–21. [DOI] [PubMed] [Google Scholar]
- 36. Yagi K, Furuhashi M, Aoki H et al . c‐myc is a downstream target of the Smad pathway. J Biol Chem 2002; 277: 854–61. [DOI] [PubMed] [Google Scholar]
- 37. Tran H, Brunet A, Grenier JM et al . DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 2002; 296: 530–4. [DOI] [PubMed] [Google Scholar]
- 38. Yoo J, Ghiassi M, Jirmanova L et al . Transforming growth factor‐β‐induced apoptosis is mediated by Smad‐dependent expression of GADD45β through p38 activation. J Biol Chem 2003; 278: 43 001–7. [DOI] [PubMed] [Google Scholar]
- 39. Mizoi T, Ohtani H, Miyazono K, Miyazawa M, Matsuno S, Nagura H. Immunoelectron microscopic localization of transforming growth factor β1 and latent transforming growth factor β1 binding protein in human gastrointestinal carcinomas: qualitative difference between cancer cells and stromal cells. Cancer Res 1993; 53: 183–90. [PubMed] [Google Scholar]
- 40. Nakagawa T, Li JH, Garcia G et al . TGF‐β induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int 2004; 66: 605–13. [DOI] [PubMed] [Google Scholar]
- 41. Filleur S, Volpert OV, Degeorges A et al . In vivo mechanisms by which tumors producing thrombospondin 1 bypass its inhibitory effects. Genes Dev 2001; 15: 1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakakura C, Mori T, Sakabe T et al . Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer 1999; 24: 299–305. [DOI] [PubMed] [Google Scholar]
- 43. Medrano EE. Repression of TGF‐β signaling by the oncogenic protein SKI in human melanomas: consequences for proliferation, survival, and metastasis. Oncogene 2003; 22: 3123–9. [DOI] [PubMed] [Google Scholar]
- 44. Schwarte‐Waldhoff I, Volpert OV, Bouck NP et al . Smad4/DPC4‐mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA 2000; 97: 9624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kazerounian S, Yee KO, Lawler J. Thrombospondins: from structure to therapeutics: thrombospondins in cancer. Cell Mol Life Sci 2008; 65: 700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res 2001; 61: 2847–51. [PubMed] [Google Scholar]
- 47. Miyamoto N, Yamamoto H, Taniguchi H et al . Differential expression of angiogenesis‐related genes in human gastric cancers with and those without high‐frequency microsatellite instability. Cancer Lett 2007; 254: 42–53. [DOI] [PubMed] [Google Scholar]
- 48. Hoekstra R, De Vos FY, Eskens FA et al . Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin‐1‐mimetic angiogenesis inhibitor ABT‐510 in patients with advanced cancer. J Clin Oncol 2005; 23: 5188–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of c‐Ski in OCUM‐2MLN subcutaneous tumors. Total RNA was obtained from subcutaneous tumors as described in Fig. 3(c), and expression of c‐Ski was examined by quantitative real‐time RT‐PCR analysis. Each value is normalized to the expression of hypoxanthine phosphoribosyltransferase 1 and represents the mean of triplicate determination (***P < 0.001).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


