Abstract
With the aim of developing more efficient gastric cancer screening programs for use in Japan, we studied a new screening program that combines serum pepsinogen (PG) testing and barium digital radiography (DR). A total of 17 647 middle‐aged male subjects underwent workplace screening over a 7‐year period using a combination of PG testing and DR. This program's effectiveness, as well as other characteristics of the program, was analyzed. Forty‐nine cases of gastric cancer were detected (comprising 88% early cancer cases). The detection rate was 0.28%, and the positive predictive value was 0.85%. The PG test detected 63.3% of cases, DR detected 69.4% of cases, and both tests were positive in 32.7% of cancer cases. The two methods were almost equally effective, and were considerably more effective than conventional screening using photofluorography. Each screening method detected a distinct gastric cancer subgroup; the PG test efficiently detected asymptomatic small early cancer with intestinal type histology, while DR was efficient at detecting cancers with depressed or ulcerated morphology and diffuse type histology. The cost for the detection of a single cancer was much less than that for conventional screening. In fact, it is possible to further reduce the cost of detecting a single cancer to a cost comparable to that of surgically resecting a single gastric cancer. Thus, it is probable that a highly efficient gastric cancer screening system can be implemented by combining the two screening methods. Such a screening program would be beneficial in a population at high risk for gastric cancer. (Cancer Sci 2005; 96: 713 – 720)
Gastric cancer has been one of the leading causes of cancer‐related deaths worldwide and in Japan.( 1 , 2 , 3 , 4 ) The Japanese population is at especially high risk for gastric cancer, with a prevalence that is markedly higher than that in other industrialized nations. In Japan, 49 213 people died from gastric cancer in 2002.( 5 ) To cope with this serious public health problem, a gastric cancer screening system using double contrast barium X‐ray was introduced in the 1960s throughout Japan. Annually, more than five million people undergo screening and, as a result, thousands of gastric cancers are detected every year.( 6 , 7 , 8 , 9 ) However, the present gastric cancer screening system leaves much to be desired; the number of subjects screened has recently been reported to be decreasing, and the screening program itself covers less than 10% of the at‐risk population.( 10 ) Furthermore, conventional barium X‐ray by photofluorography with a 10 cm × 10 cm sized film is used to screen nearly 80% of the subjects screened annually, so its resolution is by no means high; in fact, it is able to detect no more than 39% of early gastric cancers.( 11 ) Thus, low cost‐effectiveness and the risk of X‐ray exposure have become issues of concern. Therefore, a more efficient screening system has been sought.
In high incidence areas, such as Japan, stomach carcinogenesis is considered to begin with gastritis that proceeds to extensive atrophy together with intestinal metaplasia, then to dysplasia, and finally to cancer.( 12 , 13 ) We have previously reported that serum pepsinogen (PG) levels provide a precise measure of the extent of gastric atrophy, and that the serum pepsinogen test is useful in identifying subjects with widespread atrophic gastritis, which is presumed to be a precancerous lesion, especially for intestinal type gastric cancer.( 14 ) The serum PG test is considered to be suitable for cancer screening because it is low‐cost, easy to perform, provides quick results, and produces no patient discomfort.( 15 , 16 ) Recently, serological cancer screening using the PG test followed by endoscopy was introduced on an experimental basis by some local communities and workplaces in Japan.( 17 , 18 , 19 , 20 ) This screening strategy has received widespread attention because of its significantly increased cancer detection rate. In addition, the number of subjects undergoing screening has also increased dramatically in the target populations. However, although the PG test is a reliable test for extensive atrophy, it does not directly detect cancer, but only identifies individuals who need further screening by endoscopy. As suggested by various pathological and epidemiological data, the association between atrophic gastritis and intestinal type gastric cancer is very strong, whereas there is only a weak association, if any, between atrophic gastritis and diffuse‐type gastric cancer.( 21 , 22 ) Thus, it is probable that non‐gastritis based cancers, especially of the diffuse type, escape detection by the PG test. Indeed, as several preliminary reports published by us and others have indicated, quite a few cancers, including those in the advanced stage, tend to be missed by serological cancer screening.( 23 , 24 , 25 ) Thus, it is essential that the design of a stable and efficient cancer screening system includes a strategy that deals with such PG test‐negative cancers. Recent reports have indicated that barium X‐ray with digital radiography (DR) has a high diagnostic value in the detection of gastric cancers. Therefore, it is considered to be a good alternative to conventional barium X‐ray.( 26 , 27 , 28 ) Thus, to overcome the drawbacks of gastric cancer screening using the PG test, we introduced gastric cancer screening using a combination of the PG test and DR as the first‐step screening tool. In the present study, we analyzed the results of 7 years of screening, and we evaluated not only the validity of the screening system itself, but also the particular characteristics of each of the two screening methods.
Materials and Methods
The subjects were male employees who underwent gastric cancer screening in a workplace in Wakayama City, which is in the western part of Japan. In 2000, the gastric cancer mortality rate for the area was 45.7/100 000 for men and 18.0/100 000 for women, compared to 39.1/100 000 for men and 15.3/100 000 for women for the whole country. In fact, Wakayama ranks third among the 47 administrative divisions of Japan in terms of gastric cancer mortality.
From April 1995 through to the end of March 2002, a total of 17 647 male subjects (mean age [± SD]: 50.4 ± 5.4 years old; range: 40–60 years old) were screened with a PG test and barium DR. The number of those screened for the first time in 1995 was 3068, and among them, 2160 were screened repeatedly with the two screening tests during the following years. If either or both of the tests were positive, the subjects were further examined by upper gastrointestinal endoscopy (XQ‐200, Olympus, Tokyo, Japan). Subjects who required prompt medical care or who had previously undergone gastric resection were excluded and were analyzed separately. Serum PG levels were measured using a modification of the RIA method (Riabeads Kit, Dainabott Co., Tokyo), which we reported on previously.( 29 ) Subjects were screened on the basis of the following PG‐test positive criteria: PG I level of less than 50 µg/L and PG I/II ratio of less than 3.0.( 30 , 31 ) While taking into account the amount of manpower required for conducting thorough endoscopic examinations, the criteria were established so that the sensitivity would be maximized with a positive rate of approximately 20%.
The criteria had a sensitivity of 69% and a specificity of 80%.( 30 ) In the analysis of the PG test results, subjects who had been prescribed any medications that might affect gastrointestinal function (e.g. proton pump inhibitors or non‐steroidal anti‐inflammatory drugs) prior to the examination were excluded. For upper‐gastrointestinal barium X‐ray, remote controlled X‐ray fluoroscopy (TU‐230XB, Hitachi Medico, Japan) and real‐time digital radiography (DR‐2000H) were used. A total of 150 mL of high concentration barium (200%) was used for the double contrast upper‐gastrointestinal X‐ray series, and 11 films were taken for each subject. All subjects were followed for the duration of the study period to determine gastric cancer‐related deaths. Gastric cancer‐related deaths were determined on the basis of information collected about the terminal event. Standardized mortality ratios (SMR) of gastric cancer among participants during the 7‐year observation period were calculated based on sex, age, and year‐specific gastric cancer mortality, using the male population aged 40–60 years in Wakayama city as the standard population. For the standard population, the expected number of deaths for each 10‐year age category was calculated for each of the screening years and then combined to obtain the overall expected number of deaths. Then, the ratio of the observed number of deaths to the expected number of deaths (SMR) during the study period was calculated. The 95% confidence intervals of the SMR were calculated using Poisson regression models.
Data were analyzed using SPSS 11.0 (SPSS Inc., Chicago, Illinois, USA). Differences were tested for statistical significance using Student's t‐test and analysis of variance for comparisons among multiple values.
The ethics committee of Wakayama Medical University approved the protocol, and informed consent was obtained from all participating subjects.
Results
The results of gastric cancer screening from 1995 through to 2002 were analyzed and are summarized in Table 1. The effectiveness of the two primary screening methods was compared. During the 7‐year study period, 17 647 middle‐aged male subjects underwent screening. The PG test results were positive in 3441 subjects (19.5%), and DR was positive in 3971 subjects (22.5%). Endoscopy was performed in 2771 (78.8%) of the PG test‐positive cases and 3341 (84.1%) of the DR‐positive cases. During the study period, 49 cases (52 lesions) of gastric cancer were detected, a detection rate of 0.28%. The positive predictive value of the two combined cancer screening tests was 0.85%. Of the patients with cancer, 63.3% (31 cases) were detected by the PG test, 69.4% (34 cases) by DR, and 32.7% (16 cases) were positive for both screening tests. The two screening methods were almost equally effective, with similar detection rates (PG test, 0.18%; DR, 0.19%) and positive predictive values (PG test, 1.14%; DR, 1.02%).
Table 1.
Results of gastric cancer screening
| Method | Two screening methods (PG plus DR) | PG | DR |
|---|---|---|---|
| A | 17 647 | ||
| B | 7147 (40.5%) | 3441 (19.5%) | 3971 (22.5%) |
| C | 5802 (81.2%) | 2711 (78.8%) | 3341 (84.1%) |
| D | 49 | 31 | 34 |
| D/A (%) | 0.28 | 0.18 | 0.19 |
| D/B (%) | 0.69 | 0.9 | 0.86 |
| D/C (%) | 0.85 | 1.14 | 1.02 |
A, total number of subjects screened; B, total number of subjects requiring further tests (% of the total screened subjects, A); C, total number of the subjects who underwent endoscopy (% of the total subjects who required further tests, B); D, number of cancers detected; PG, pepsinogen test; DR, barium X‐ray with digital radiography.
The clinicopathological profiles of the detected cancers based on the results of the initial screening method are summarized in Table 2. There was no significant difference in the mean age among the three cancer subgroups (the single PG test‐positive group, the single DR‐positive group, and the two test‐positive group). Of the 52 detected cancer lesions, 46 (88%) were in the early stage (confined to the mucosa or submucosa). The cases detected by the PG test alone were all asymptomatic early cancers, while the cases detected by DR were by no means asymptomatic. In fact, 22% of the cases had some abdominal symptoms. There tended to be a smaller proportion of early cancer among the cases detected by DR alone (83%), and among the cases in whom both tests were positive (81%); the difference from those detected by the PG test was not statistically significant. We found that 89% of the cancers (16 lesions) detected by the PG test alone were localized to the mucosal layer, whereas 56% of those detected by DR alone and 50% of those in whom both screening tests were positive were mucosal cancers. As expected given these findings, the cancers detected by the PG test alone were considerably smaller in size. In particular, the mean (SD) diameter of the cancers detected by a positive PG test was 12.2 (7.4) mm, and for those detected by a positive DR test the mean diameter was 18.8 (13.9) mm, and with both screening methods positive the mean diameter was 23 (15.9) mm; the difference in the diameters between the PG test‐positive group and the two test‐positive group was statistically significant (P < 0.05). Overall, most of the cancers were located in the middle third of the stomach, irrespective of the screening method. However, the cancers detected by DR alone tended to be located more frequently in the upper third of the stomach, where the degree of atrophic change is less severe. According to macroscopic classification, most cancer cases were of the depressed type. Of the 46 early cancer lesions, 37 (80.4%) were IIc or IIc + III, and all but one of the advanced cancers were either Borrmann 2 or 3. The remaining case was a IIc‐like advanced cancer. Elevated IIa lesions were detected in 22% of the cases that were PG test‐positive alone, 0% of the cases that were DR positive alone, and 6% of cases in which both tests were positive. Of the two histological types of gastric cancer, the intestinal type was the most prevalent detected on screening. This tendency was quite pronounced in the cases detected by the PG test alone, as 83% of these cancers were of the intestinal type, while the proportion of the diffuse type cancers was the highest in those identified by DR alone (44%). Therefore, the PG test is good at detecting small, early, intestinal type cancers among asymptomatic subjects, which tend to escape screening by barium X‐ray.
Table 2.
Clinicopathological profiles of gastric cancers detected by screening
| I | II | III | I | II | III | ||
|---|---|---|---|---|---|---|---|
| Age in years (mean ± SD) | 55.5 ± 3.4 | 54.1 ± 4.4 † | 53.4 ± 3.6 †† | Size of the cancer (mm) (mean ± SD) | 12.2 ± 7.4 | 18.8 ± 13.9 ‡ | 23 ± 15.9 ‡‡ |
| No. of cancer cases | 15* | 18 | 16 | ||||
| No. of lesions | 18 | 18 | 16 | Depth of cancer invasion (no. of lesions [%]) | |||
| Locus (no. of lesions [%]) | Early cancer | 18 (100%) | 15 (83%) | 13(81%) | |||
| C | 5 (28%) | 6 (33%) | 2 (13%) | Mucosa | 16 (89%) | 10 (56%) | 8(50%) |
| M | 8 (44%) | 9 (50%) | 8 (50%) | Submucosa | 2 (11%) | 5 (27%) | 5(31%) |
| A | 5 (28%) | 3 (17%) | 6 (37%) | Advanced cancer | 0 (0%) | 3 (17%) | 3(19%) |
| Macroscopic type (no. of lesions [%]) | Muscularis Propria | 0 (0%) | 0 (0%) | 1(6%) | |||
| IIa | 4 (22%) | 0 (0%) | 1 (6%) | Subserosa | 0 (0%) | 3 (17%) | 2(13%) |
| IIa+IIc | 1 (5%) | 0 (0%) | 1 (6%) | Histological type (no. of lesions [%]) | |||
| IIb | 0 (0%) | 1 (5%) | 0 (0%) | Intestinal type | 15 (83%) | 10 (56%) | 13(81%) |
| IIb+IIc | 0 (0%) | 1 (5%) | 0 (0%) | Diffuse type | 3 (17%) | 8 (44%) | 3(19%) |
| IIc | 10 (56%) | 10 (56%) | 10 (64%) | Treatment (no. of lesions [%]) | |||
| IIc+III | 3 (17%) | 3 (17%) | 1 (6%) | EMR | 8 (44%) | 4 (22%) | 2(12%) |
| IIc‐like advanced cancer | 0 (0%) | 0 (0%) | 1 (6%) | Surgery | 10 (56%) | 14 (78%) | 14(88%) |
| Borrmann 2 | 0 (0%) | 1 (5%) | 1 (6%) | ||||
| Borrmann 3 | 0 (0%) | 2 (12%) | 1 (6%) |
PG, pepsinogen test; DR, barium X‐ray with digital radiography; I, cancer cases detected only by the PG test; II, cancer cases detected only by barium X‐ray with DR; III, cancer cases detected by both methods. C, upper third of the stomach; M, middle third; A, lower third. IIa, superficial‐elevated type; IIb, superficial‐flat type; IIc, superficial‐depressed type. EMR, endoscopic mucosal resection. †Not significant (NS) vs I and III; ††NS vs I and II; ‡NS vs I; ‡‡ P < 0.05 vs I. *Two cases had synchronous mucosal cancers, and one case had metachronous mucosal and submucosal cancers.
With respect to cancer therapy, all the cancer cases were successfully treated either surgically or endoscopically. Since endoscopic mucosal resection (EMR) is less invasive and produces a better quality of life compared with surgical resection, it has been widely accepted in Japan as the primary therapeutic strategy for the treatment of early gastric cancer. EMR is particularly indicated for intestinal type mucosal cancers of the elevated type no larger than 2 cm in size and for cancers of the depressed type no larger than 1 cm without ulceration.( 32 ) Many cancer cases detected by the PG test alone were small mucosal cancers with no sign of lymph node or distant metastasis, and thus were especially well suited for EMR. As a result, 44% of the cases detected by the PG test alone underwent EMR, and this percentage was higher than in the other two groups: EMR was used to treat 22% of gastric cancers in the single DR‐positive group and 12% in the two test‐positive group.
Table 3 shows the costs for the cancer screening program using the two methods. Taking into account both the primary and secondary screening costs, the total cost for the present screening approach was ¥198 955 000, which included ¥35 294 000 for the initial PG test screening, ¥88 235 000 for the DR, and ¥75 426 000 for endoscopy. Using the present screening approach, the cost to find a single cancer case was ¥4 060 306. Using the PG test alone, the cost to find a single cancer case was ¥2 275 387, while the calculated cost to find a single cancer case by using the DR method alone was ¥3 872 588.
Table 3.
Cost‐effectiveness analysis of gastric cancer screening according to the screening methods used
| Method | Two screening methods (PG plus DR) | PG | DR |
|---|---|---|---|
| Total number of subjects screened | 17 647 | ||
| Total number of subjects undergoing further tests | 5 802 | 2 711 | 3 341 |
| Number of cancer cases detected | 49 | 31 | 34 |
| Total cost (¥) | 198 955 000 | 70 537 000 | 131 668 000 |
| Cost for detecting a single cancer (¥) | 4 060 306 | 2 275 387 | 3 872 588 |
PG, pepsinogen test; DR, barium X‐ray with digital radiography. Cost for PG test: ¥2000/subject; cost for barium X‐ray with DR: ¥5000/subject; cost for endoscopy: ¥13 000/subject.
Figure 1 shows examples of gastric cancer cases detected by the PG test alone. Case (a) had a 10‐mm type IIa lesion located in the anterior wall of the gastric angle, and case (b) had an 8‐mm type IIa lesion located in the lesser curvature of the proximal antrum. These cases had an intestinal type mucosal cancer and were successfully treated by EMR. Case (c) had a 5‐mm IIc + III lesion located in the anterior wall of the lower gastric body. Partial gastrectomy was performed, and pathological examination of the resected stomach revealed a minute, diffuse type mucosal cancer. It is noteworthy that these three cases had been essentially symptom‐free and that the DR did not document any abnormalities that would indicate the existence of these lesions. In these three cases no recurrence has been observed to date. These cases clearly indicate that the PG test is especially useful in detecting minute lesions that escape diagnosis by barium X‐ray.
Figure 1.
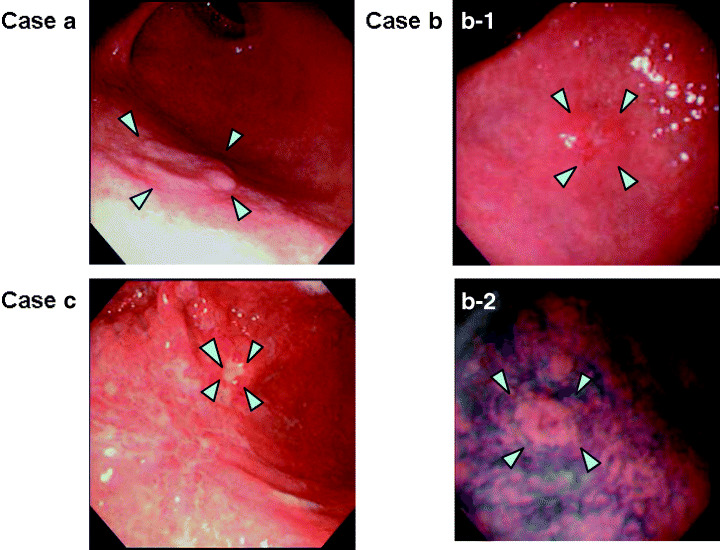
Representative gastric cancer cases in which only the pepsinogen (PG) test was positive. The PG test efficiently detected small early cancers, especially those with an elevated type morphology and intestinal type histology. (a) A 10‐mm type IIa lesion located in the anterior wall of the gastric angle (arrow heads). The serum PG levels were: PGI 27.2 µg/L, PGII 22.6 µg/L, and the PGI/PGII ratio was 1.2. (b) An 8‐mm type IIa lesion located in the lesser curvature of the proximal antrum as indicated by the arrow heads (b‐1). The indigo carmine dye‐spraying method revealed the existence of the tiny elevated lesion more clearly as shown by the arrow heads (b‐2). The serum PG levels were: PGI 39.5 µg/L, PGII 39.5 µg/L, and the PGI/PGII ratio was 1. (c) An 8‐mm type IIc + III lesion located in the anterior wall of the lower gastric body (arrow heads). The serum PG levels were: PGI 48.5 µg/L, PGII 30.3 µg/L, and the PGI/PGII ratio was 1.6. All three cases had been essentially symptom‐free at detection. (a) and (b) were intestinal type mucosal cancers and were successfully treated by endoscopic mucosal resection (EMR). (c) was treated with a partial gastrectomy, and pathological examination revealed a diffuse type mucosal cancer. In all these cases, the background mucosa revealed extensive atrophy, as would be expected with a positive PG test.
Figure 2 shows examples of gastric cancer cases detected only by DR. Case (a) had a 10‐mm type IIc + III lesion located in the lesser curvature of the gastric angle. A partial gastrectomy was performed, and pathology revealed a diffuse type mucosal cancer. Currently, no recurrence has been observed. Case (b) had a 30‐mm type IIc + III lesion located in the greater curvature of the gastric angle. A partial gastrectomy was performed, and the resected stomach revealed a diffuse type submucosal cancer. Case (c) had a 45‐mm Borrmann 3 lesion located in the gastric cardia. Total gastrectomy was performed, and pathology revealed a large, ulcerated, advanced intestinal type cancer invading the subserosa. There have been no signs of recurrence in any of these three cases to date. In these three cases, although they were not symptom‐free at screening, the lesion could have been missed if the primary screening was conducted using the PG test alone, because the background mucosa was not atrophic, and thus, the serum pepsinogen level was high. These cases clearly show that gastric cancers, irrespective of stage, can easily escape detection by the PG test alone; for such cancers, barium X‐ray is the preferred screening strategy.
Figure 2.
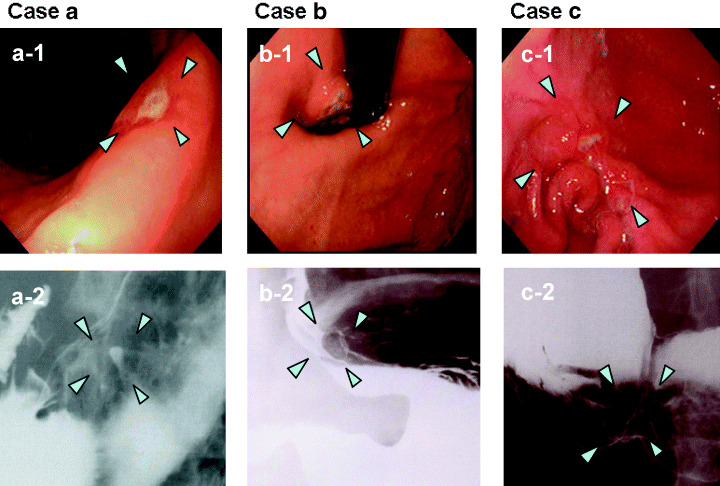
Representative gastric cancer cases in which only barium digital radiography (DR) was positive. The DR efficiently detected cancers with depressed or ulcerated type morphology and diffuse type histology. Images marked 1 show endoscopic views of each case, and images marked 2 show the DR images of each case. (a) A 10‐mm type IIc + III lesion located in the lesser curvature of the gastric angle. Serum PG levels were: PGI 44.6 µg/L, PGII 13.5 µg/L, and the PGI/PGII ratio was 3.3. (b) A 45‐mm Borrmann 3 lesion located in the gastric cardia. Serum PG levels were: PGI 43.1 µg/L, PGII 5.9 µg/L, and the PGI/PGII ratio was 7.3. (c) A 30‐mm type IIc + III lesion located in the greater curvature of the gastric angle. Serum PG levels were: PGI 77.9 µg/L, PGII 22.9 µg/L, and the PGI/PGII ratio was 2.6. (a) and (c) were both treated by partial gastrectomy, and both had diffuse type histology. (a) was a mucosal cancer, and (c) was a submucosal cancer. (b) underwent a total gastrectomy, and the pathology revealed a large ulcerated advanced intestinal type cancer invading the subserosa. In all these cases, the background mucosa was not atrophic, which would be expected with a negative PG test.
In the workplace in which the screening was done, six employees died from gastric cancer during the period from 1991 to 1994 when cancer screening was conducted by conventional barium X‐ray using photofluorography. With the introduction of the new screening program, there was no significant reduction in the number of gastric cancer deaths during the first 4 years between 1995 and 1998, as seven employees died. The SMR (95% confidence interval) of gastric cancer in the workplace compared with the same aged male population living in the same area during the period was 2.74 (1.20–5.92). However, in the following 3 years (1999–2001), there was a drastic reduction in cancer deaths, with only one employee dying from gastric cancer; the SMR for this period decreased to 0.87 (0.22–2.76).
Discussion
Previous seroepidemiological studies have clearly indicated a significant correlation between gastric cancer mortality and the prevalence of extensive atrophic gastritis as determined by serum PG testing.( 33 , 34 ) Therefore, PG test‐positive subjects are considered to constitute a population at high risk for gastric cancer, and the PG test is a valuable tool for selecting a population that needs further examination by endoscopy. It has also been reported that barium X‐ray with DR is superior to conventional barium X‐ray in image quality and diagnostic accuracy for detecting gastric cancer, especially when a high concentration barium meal is used.( 35 , 36 ) Furthermore, DR has the following advantages over the conventional screen‐film system: (i) digital imaging allows image quality to be optimized and radiation dose to be reduced; (ii) DR provides rapid data acquisition, digital image processing, and instant image display; and (iii) DR is compatible with a picture archiving and imaging network.( 26 , 27 , 28 ) Given these features, DR can be particularly useful for barium X‐rays using the double‐contrast imaging method.( 26 , 27 , 28 ) To improve the efficiency of gastric cancer screening, we introduced the PG test and the barium X‐ray with DR as initial screening tools. The results of the present study clearly indicate that both the PG test and DR effectively detect gastric cancer. According to the 2001 annual report of gastric cancer screening prepared by the Japanese Association of Gastrointestinal Mass Screening, the cancer detection rate of nationwide screening using the conventional barium X‐ray method was 0.10%.( 10 ) Thus the 0.28% cancer detection rate found in the present screening over a 7‐year period was considerably higher than that of the nationwide gastric cancer screening program using conventional barium X‐ray examination. Based on their positive predictive values, the PG test and DR are almost equally efficient, and both of them are superior to conventional barium X‐ray examination. The detection rate is 1.8 times higher with the PG test and 1.9 times higher with DR than with conventional barium X‐ray examination.
In the workplace where this study was done, cancer screening by using the conventional barium X‐ray technique with photofluorography had been done until the present screening system was introduced. In the initial year, the cancer detection rates of the PG test and DR were at the same high level (0.38%). The mean detection rates of the two methods for the first 3 years and the next 4 years were: PG test, 0.28% first 3 years, 0.14% next 4 years; DR, 0.29% first 3 years, 0.17% next 4 years. Because the PG test was a new type of screening test applied for the first time in that workplace, the test may have detected prevalent cancers in the initial year. Thus, it can be expected that by repeating the PG test screening during subsequent years, the detection rate of the serum test would decrease until it finally detected only the annual incident cancers. In contrast, since DR screening was essentially the same type of screening that had already been done annually, it was quite unexpected that the detection rate would be high in the first year of screening, and that it would then decrease in subsequent years. It is therefore likely that, in the first year, DR detected cancers missed by conventional screening, and in the following years, the DR detection rate, although it did decrease, remained higher than that of the conventional barium X‐ray screening approach. This clearly indicates the higher sensitivity of DR in comparison with conventional barium X‐ray. Thus, the sensitivity of the two screening methods is almost equivalent, both in the initial phase of screening and on subsequent screening, indicating that the combined approach is much better than conventional screening using photofluorography.
In our screening, the overlap between the cancers detected by the PG test and by DR was relatively small, accounting for only 32.7% of the total cancer cases detected. The PG test is a sensitive and specific indicator of extensive mucosal atrophy, while DR is a method that directly visualizes mucosal abnormalities. As could be expected from the difference in the diagnostic mechanisms of the two screening methods, the PG test efficiently detected small early cancers with elevated type morphology and intestinal type histology that tended to be derived from atrophic gastric mucosa. In contrast, DR was efficient at detecting cancers with a depressed or ulcerated type morphology and a diffuse type histology. PG test‐positive cancers are usually asymptomatic, which is a reflection of reduced acid secretion due to extensive atrophic gastritis. However, DR‐positive cancers, of which approximately a half are PG test‐negative and in whom acid secretion is not impaired, are by no means asymptomatic. In our study, cases detected only by the PG test were all asymptomatic early gastric cancers, 83% of which were intestinal type. Macroscopically, 22% of these cases had elevated morphology, and 89% were limited to the mucosa. These small intestinal type mucosal cancers, especially the elevated type, are particularly well suited for EMR. Thus, the PG test is a screening method that can contribute greatly to the patients’ quality of life (QOL) by detecting cancer in its early stages. In other words, the PG test is especially good at screening asymptomatic subjects, whereas symptomatic subjects or PG test‐negative subjects should be screened by barium X‐ray examination.
In 2002, the Japanese Association of Gastroenterological Mass Survey reported that 5 843 904 subjects underwent mass screening for gastric cancer nationwide, and that 379 965 underwent further examination.( 10 ) Given that initial screening with conventional barium X‐ray cost ¥3500 per subject, and endoscopy cost ¥13 000 per subject, the total cost for the screening program was estimated as ¥25 393 209 000 per year. During screening in 2002, 5410 subjects were identified as having gastric cancer. Based on these data, the cost required to find a single case of gastric cancer can be estimated as ¥4 408 543, simply by dividing the total cost by the number of detected cancers. In contrast, using the screening system presented in this paper it would cost ¥4 060 306 to find a single gastric cancer case. Using the PG test alone, the cost to find a single cancer case was ¥2 275 387, whereas it cost ¥3 872 588 to find a single gastric cancer case using DR alone. Both of these screening methods were found to be more cost‐effective than conventional barium X‐ray. Furthermore, with the introduction of DR, there has been a reduction in costs for films, reagents for development, and waste disposal.( 26 , 27 , 28 ) The total cost over 7 years for cancer screening using barium X‐ray was ¥88 235 000 (¥5000/subject), which includes the depreciation cost for the DR system (¥4 000 000/year), the cost for the optical disk (¥250 000/year), hard copy paper (¥220 000/year), the cost for barium meals, effervescent granules, anticholinergics (¥1714 280/year, ¥680/subject) and other costs, including personnel expenses. Since the cost of DR can be decreased considerably, the total cost of DR could be reduced to less than ¥3500/subject, in which case the cost to detect a single case of cancer using a DR screening program would be reduced to less than ¥3 094 044. Furthermore, the PG test cost could be reduced to at least ¥1000/subject, so that the cost would be ¥1 706 129 per cancer using the PG test alone. Thus, it would cost ¥3 159 949 per cancer for a screening program using both the PG test and DR. These figures are comparable to the cost of doing a surgical resection of a stomach cancer in Japan, for which the costs have been calculated as ¥3 417 750 ± 19 982 per patient in patients with preoperative disorders or complications and ¥1 534 070 ± 280 560 per patient in patients without any preoperative disorders or complications.( 37 ) In order to establish a stable and efficient cancer screening system, it is important to optimize the economic aspect of the screening test program.( 38 ) However, neither the PG test nor DR can be recommended as a single screening method for the general population, because, as has been shown, more than 30% of the cancers cannot be detected when these screening methods are used separately. Asymptomatic early cancers, a considerable number of which are particularly suitable for minor invasive endoscopic mucosal resection, escape diagnosis with DR screening, while the PG test cannot detect non‐gastritis based cancers, especially those with a depressed type morphology and diffuse type histology, including those in the advanced stage, which can be easily detected by barium X‐ray and which have a higher malignant potential than PG test‐positive cancers. Because each of the two screening methods detect a distinct subgroup of gastric cancer with distinct clinicopathological characteristics, the combination of the two methods has greatly improved screening sensitivity and is more cost effective than conventional screening.
With the introduction of the new two test screening system, the number of gastric cancer deaths has decreased markedly, and there has been a steady decrease over time in the SMR (using the same aged male population living in the same area as the standard). As yet, the reduction in cancer mortality has not been significant, although it is noteworthy that there have been only two cancer deaths since 1999 in this workplace: one cancer death occurred in a patient who skipped the screening, and the other underwent gastrectomy in 1990 and was thereafter excluded from the screening program and followed separately. Thus, there have been no cancer deaths in the population screened for the last 5 years, and it is thus highly probable that our cancer screening system has successfully reduced gastric cancer mortality.
The next improvement of the present screening system will depend on target setting. Recent studies have clearly indicated that H. pylori infection and the resulting severe atrophic gastritis together with intestinal metaplasia are involved in the development of gastric cancer. By following approximately 5000 male subjects of cancer‐susceptible age for more than 7 years, we have found that it is quite rare for gastric cancer to develop in H. pylori‐free stomachs.( 39 ) In Japan today, 20–30% of the middle‐aged population (40–60 years of age) is H. pylori free, and it is highly probable that subjects from this group, if asymptomatic, could be excluded from screening. In contrast, PG test‐positive subjects are at high risk of developing cancer, and according to our data the cancer incidence in this group is 255/100 000 person‐years.( 39 ) This incidence increases in a stepwise manner with the progression of atrophic gastritis and reaches 871/100 000 person‐years in the group with severe atrophic gastritis and extensive intestinal metaplasia (metaplastic gastritis), which can be easily identified because they are PG test‐positive and H. pylori antibody‐negative.( 39 ) Such cancer high‐risk groups, especially the metaplastic gastritis group, should be the target of regular screening by endoscopy. However, in target setting we should pay special attention to the PG test‐negative, H. pylori antibody‐positive group: the cancer incidence in this group is by no means low (107/100 000 person‐years), and approximately half of the diffuse cancers that are clinically and biologically more malignant occur in this group. Therefore, this group should also be included in the screening program, and DR should be used to screen this group. Thus, initial screening requires the combination of the PG test and barium DR.
In conclusion, the present results strongly indicate that the establishment of a cancer screening system with a high efficacy is possible by using a combination of the PG test and barium DR. The best way to combine the two methods needs further investigation, and this may vary significantly depending upon the target population. It is probable that such screening would be beneficial not only in Japan but also in other high risk areas outside Japan, such as East Asia, Central and South America, and Eastern Europe.
Acknowledgment
This work was supported by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor, and Welfare of Japan.
References
- 1. Correa P. The epidemiology of gastric cancer. World J Surg 1991; 15: 228–34. [DOI] [PubMed] [Google Scholar]
- 2. Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol 2002; 12: 111–27. [DOI] [PubMed] [Google Scholar]
- 3. Research Group for Population‐Based Cancer Registration in Japan. Cancer incidence rates in Japan in 1997: estimates based on data from 12 population‐based cancer registries. Jpn J Clin Oncol 2002; 32: 318–22. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med 1995; 333: 32–41. [DOI] [PubMed] [Google Scholar]
- 5. Ministry of Health, Labour and Welfare . Vital Statistics of Japan, Statistics and Information, vol. 1. Tokyo: Minister's Secretariat, Ministry of Health, Labour and Welfare, 2002. [Google Scholar]
- 6. Fukao A, Tsubono Y, Tsuji I, Hisamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population‐based case‐control study. Int J Cancer 1995; 60: 45–8. [DOI] [PubMed] [Google Scholar]
- 7. Murakami R, Tsukuma H, Ubukata T et al. Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer 1990; 65: 1255–60. [DOI] [PubMed] [Google Scholar]
- 8. Hisamichi S. Screening for gastric cancer. World J Surg 1989; 13: 31–7. [DOI] [PubMed] [Google Scholar]
- 9. Kawai K. Screening for gastric cancer in Japan. Clin Gastroenterol 1978; 7: 605–22. [PubMed] [Google Scholar]
- 10. Koga M, Miyakawa K, Ikeda S. Annual Report of Gastroenterological Mass Survey in Japan, 2002. Tokyo: Japanese Society of Gastroenterology Mass Survey, 2004. [Google Scholar]
- 11. Nishizawa M. Present status and prospects for cancer screening. J Gastroenterol Mass Survey 1993; 78: 100–3. (In Japanese.) [Google Scholar]
- 12. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–40. [PubMed] [Google Scholar]
- 13. Correa P. A human model of gastric carcinogenesis. Cancer Res 1988; 48: 3554–60. [PubMed] [Google Scholar]
- 14. Miki K, Ichinose M, Shimizu A et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987; 22: 133–41. [DOI] [PubMed] [Google Scholar]
- 15. Miki K, Ichinose M, Kawamura N et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res 1989; 80: 111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ichinose M, Yahagi N, Oka M, Ikeda H, Miki K, Omata M. Screening for gastric cancer in Japan. In: Wu GY, Aziz K, eds. Cancer Screening. Totowa, New Jersey: Humana Press, 2001; 255–68. [Google Scholar]
- 17. Miki K, Morita M, Sasajima M, Hoshina R, Kanda E, Urita Y. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am J Gastroenterol 2003; 98: 735–9. [DOI] [PubMed] [Google Scholar]
- 18. Hattori Y, Tashiro H, Kawamoto T, Kodama Y. Sensitivity and specificity of mass screening for gastric cancer using the measurement of serum pepsinogens. Jpn J Cancer Res 1995; 86: 1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshihara M, Sumii K, Haruma K et al. The usefulness of gastric mass screening using serum pepsinogen levels compared with photofluorography. Hiroshima J Med Sci 1997; 46: 81–6. [PubMed] [Google Scholar]
- 20. Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut 1999; 44: 693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ngayo T. Precursors of human gastric cancer. Their frequencies and histological characteristics. In: Farber E, ed. Pathophysiology of Carcinogenesis in the Digestive Organs. Baltimore: University Park Press, 1977; 151–60. [Google Scholar]
- 22. Correa P. Chronic gastritis and gastric cancer. In: Ming SC, ed. Precursors of Gastric Cancer. New York: Praeger Publishers, 1984; 105–16. [Google Scholar]
- 23. Miki K, Ichinose M. Serum pepsinogen I level in patients with stomach cancer: its value and limitation in clinical use. Gan Kagaku Ryoho 1989; 16: 1122–8. (In Japanese.) [PubMed] [Google Scholar]
- 24. Yahagi N, Shimizu Y, Ichinose M, Miki K, Omata M. Corroborative evidence on mass screening method using serum pepsinogen test for long term follow up of gastric cancer patients. Clin Gastroenterol 2002; 17: 1577–83. (In Japanese with English abstract.) [Google Scholar]
- 25. Matsumoto J, Arai T, Yahagi K, Hashimoto Y, Masuda J. A study of serum pepsinogen test method negative gastric cancer. J Gastroenterol Mass Surv 2002; 40: 20–7. (In Japanese.) [Google Scholar]
- 26. Iinuma G, Ushio K, Ishikawa T, Nawano S, Sekiguchi R, Satake M. Diagnosis of gastric cancers: comparison of conventional radiography and digital radiography with a 4 million‐pixel charge‐coupled device. Radiology 2000; 214: 497–502. [DOI] [PubMed] [Google Scholar]
- 27. Sugino Y, Imai Y, Fujisawa H, Hiramatsu K, Amoh H, Kumakura K. Clinical usefulness of digital radiography in the gastrointestinal tract: efficacy of magnification method. J Digit Imaging 1995; 8: 84–8. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi M, Ueno S, Yoshimatsu S et al. Gastrointestinal examinations with digital radiography. Radiographics 1992; 12: 969–78. [DOI] [PubMed] [Google Scholar]
- 29. Ichinose M, Miki K, Furihata C et al. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta 1982; 126: 183–91. [DOI] [PubMed] [Google Scholar]
- 30. Miki K, Ichinose M. Chronic atrophic gastritis and serum pepsinogen levels. Jpn J Cancer Clin 1992; 38: 221–9. (In Japanese with English abstract.) [Google Scholar]
- 31. Miki K, Ichinose M, Ishikawa KB et al. Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn J Cancer Res 1993; 84: 1086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishi M, Ishihara S, Nakajima T, Ohta K, Ohyama S, Ohta H. Chronological changes of characteristics of early gastric cancer and therapy: experience in the Cancer Institute Hospital of Tokyo, 1950–94. J Cancer Res Clin Oncol 1995; 121: 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukao A, Hisamichi S, Osato N. Correlation between the prevalence of gastritis and gastric cancer in Japan. Cancer Causes Control 1993; 4: 17–20. [DOI] [PubMed] [Google Scholar]
- 34. Kabuto M, Imai H, Tsugane S. Correlation between atrophic gastritis prevalence and gastric cancer mortality among middle aged‐man in 5 areas in Japan. J Epidemiol 1993; 3: 35–9. [Google Scholar]
- 35. Asazaki M, Hirukawa K, Chiyasu S et al. Optimal system of the digital radiography on mass survey (computed radiography vs digital radiography). J Gastroenterol Mass Surv 2000; 38: 107–17. (In Japanese.) [Google Scholar]
- 36. Tsukeshiba I, Iinuma M, Niwa Y, Goto H, Segawa K. Assessment of high‐density barium for X‐ray examination of the upper gastrointestinal tract using digital radiography. J Gastroenterol Mass Surv 2000; 40: 155–9. (In Japanese.) [Google Scholar]
- 37. Kiyama T, Tajiri T, Yoshiyuki T et al. An economic evaluation of gastrectomy. Jpn J Cancer Clin 2004; 50: 187–90. (In Japanese.) [Google Scholar]
- 38. Tsuji I, Fukao A, Sugawara N, Shoji T, Kuwajima I, Hisamichi S. Cost‐effectiveness analysis of screening for gastric cancer in Japan. Tohoku J Exp Med 1991; 164: 279–84. [DOI] [PubMed] [Google Scholar]
- 39. Ohata H, Kitauchi S, Yoshimura N et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004; 109: 138–43. [DOI] [PubMed] [Google Scholar]


