Abstract
Attention has recently focused on the critical role of inflammatory responses in the tumor stroma that provide favorable conditions for cancer‐cell growth and invasion/metastasis. In particular, macrophages recruited into the tumor stroma and activated, known as tumor‐associated macrophages, are suggested to promote tumorigenesis. In this study, we examined the effect of a decrease in the number of monocytes/macrophages in peripheral blood and the tumor stroma on the development of bone and muscle metastases by lung cancer cells. Treatment with clodronate encapsulated by liposomes (Cl2MDP‐LIP) has been developed for the depletion of monocytes/macrophages in an animal model. Subcutaneous administration of Cl2MDP‐LIP markedly reduced the number of monocytes in peripheral blood, resulting in efficient suppression of both bone metastasis and muscle metastasis when lung cancer HARA‐B cells were injected into the left cardiac ventricle of mice. Treatment with Cl2MDP‐LIP significantly reduced the number of macrophages in tumors and the number of osteoclasts in bone marrow, as well as peripheral monocytes in mice harboring lung cancer cells. In contrast, treatment with an osteoclast‐targeting antibiotic, reveromycin A, inhibited bone metastasis by lung cancer cells, but not muscle metastasis. The survival of human macrophages in culture was found to be specifically blocked by Cl2MDP‐LIP, but not by reveromycin A. Cl2MDP‐LIP thus exerted antimetastatic effects in both bone and muscle whereas reveromycin A did so only in bone. Liposome‐encapsulated bisphosphonate may modulate metastasis through decreasing the number of monocytes/macrophages in both peripheral blood and the tumor stroma, suggesting that tumor‐associated macrophages might be suitable targets for antimetastatic therapy. (Cancer Sci 2008; 99: 1595–1602)
Abbreviations:
- Ab
antibody
- BSA
bovine serum albumin
- Cl2MDP‐LIP
clodronate encapsulated by liposomes
- DAPI
4′,6‐diamidino‐2‐phenylindole
- FITC
fluorescein‐isothiocyanate
- HRP
horseradish peroxidase
- OCT
optimal cutting temperature
- PBS
phosphate‐buffered saline
- PE
phycoerythrin
- PTHrP
parathyroid hormone‐related protein
- RT
room temperature
- TAMs
tumor‐associated macrophages
- TRAP
tartrate‐resistant acid phosphatase
Metastases of several malignant cancers including those of the breast, lung, prostate, and kidney have high affinity for bone. Bone metastasis is often accompanied by serious complications such as pathological fractures, bone pain, spinal cord compression, and hypercalcemia. Organ metastasis, including that affecting bone, is a multistep process mediated through mutual interaction between cancer cells and the host microenvironment. In bone metastasis, cancer cells reach the bone via hematogenous spread, followed by osteoclastic bone resorption, and finally proliferate in the bone matrix.( 1 , 2 ) Moreover, osteoclast‐stimulating cytokines such as PTHrP have been shown to promote bone metastasis.( 3 )
Inflammatory responses in the tumor stroma play an important role by providing favorable conditions for cancer cell growth, invasion/metastasis, and angiogenesis as well as malignant progression.( 4 , 5 , 6 ) In particular, monocytes/macrophages are recruited into the tumor stroma, and activated macrophages known as TAMs produce potent angiogenic factors, as well as inflammatory cytokines, growth factors, and proteases, resulting in a promotion of angiogenesis and invasion/metastasis.( 7 , 8 , 9 ) Infiltrating TAMs are often closely associated with poor prognosis and tumor angiogenesis in patients with various tumor types.( 9 , 10 , 11 ) A preparation of Cl2MDP‐LIP has been reported to markedly inhibit angiogenesis in corneas in response to inflammatory cytokines through depletion of macrophages.( 12 ) A recent study has demonstrated that administration of clodronate‐liposomes depleted TAMs in mouse models resulting in significant inhibition of tumor growth and tumor angiogenesis, whereas free clodronate alone did not.( 13 ) Clodronate‐liposomes were also found to inhibit both tumor growth and tumor angiogenesis by lung cancer cells in a xenograft model when stimulated by inflammatory stimuli.( 14 ) Angiogenesis in a tumor microenvironment in bone marrow also played a critical role in the induction of an angiogenic response and invasion/metastasis by cancer cells.( 15 ) Furthermore, monocyte/macrophage precursor cells entered the osteoclastic lineage and expressed the osteoclastic marker TRAP under the influence of the RANK/RNAKL signaling pathway.( 16 ) Tumor burden at bone metastatic sites was markedly decreased in preclinical models on treatment with inhibitors of the RANK/RANKL pathway and neutralizing antibodies against PTHrP as well as bisphosphonate, suggesting a central role for osteoclasts in bone metastasis.( 17 , 18 , 19 ) Together, one can expect a decrease in the number of the monocyte/macrophage‐lineage by clodronate‐liposomes to attenuate the bone metastasis and growth by cancer cells.
In the present study, using an animal model of bone metastasis with the human lung cancer cell line HARA‐B, we investigated whether the administration of clodronate‐liposomes was able to modulate bone metastasis by lung cancer cells.( 3 , 20 ) On the basis of our results, we discuss whether liposome‐encapsulated bisphosphonate may be useful for treating not only bone metastasis from lung cancer, but also metastasis in other tissues/organs.
Materials and Methods
Cell culture. HARA‐B cells were established from bone metastasis of human lung cancer in nude mice and cultured in RPMI‐1640 supplemented with 10% FBS and 10‐U/mL penicillin‐streptomycin.( 3 , 20 ) The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. Human macrophage‐like cell line U937 was purchased from the American Type Culture collection (Manassas, VA, USA) and cultured in RPMI supplemented with 10% FBS.
Reagents. FITC‐conjugated anti‐F4/80 mAb and PE‐conjugated anti‐CD11b mAb were obtained from CALTAG Laboratories (Burlingame, CA, USA). Rat antimouse F4/80 Ab (MAC497R) was obtained from Serotec (Raleigh, NC, USA). Rat antimouse Gr‐1 was purchased from R&D Systems (Minneapolis, MN, USA). Phosphatidylcholine, cholesterol, and clodronate (dichloromethylene diphosphate; Cl2MDP) were from Sigma‐Aldrich (St. Louis, MO, USA). Reveromycin A was a gift from Riken (Saitama, Japan).
Preparation of Cl2MDP‐LIP. Cl2MDP‐LIP was prepared as described previously.( 12 , 21 ) A total of 11 mg of cholesterol and 75 mg of phosphatidylcholine were combined with 10 mL of 0.7‐M Cl2MDP solution and sonicated gently. The resulting liposomes were washed three times to eliminate any free drug. Empty liposomes were prepared as a control under the same conditions using PBS instead of Cl2MDP.
Animals. Female 5‐week‐old BALB/C nude mice were obtained from Clea Japan (Tokyo, Japan) and maintained in a specific pathogen‐free environment throughout the experiment.
Flow cytometry. Blood samples were obtained from the left cardiac ventricle in mice under anesthesia at day 0, 1, and 2 after stimulation with Cl2MDP‐LIP. A total of 50 mL of each sample was stained for 15 min in a dark doom with FITC‐anti‐F4/80 mAb (1:50) to label macrophages, and with PE‐anti‐CD11b mAb (1:50) to label macrophages and neutrophils. Positive cells were measured using a FACScan (Becton Dickinson, USA).( 12 )
Bone and muscle metastasis by cancer cells, and antimetastatic therapeutic protocol. HARA‐B cells (2 × 105/100 µL) were injected into the left cardiac ventricle of mice on day 0 under anesthesia with pentobarbital (0.05 mg/g body weight; Dainippon Pharmaceutical, Osaka, Japan).( 3 ) To assess the inhibitory effect of Cl2MDP‐LIP on the formation of bone and muscle metastasis, Cl2MDP‐LIP at 200 µL or 400 µL was subcutaneously (s.c.) administered into the base of the tail once every 3 days for 6 weeks after the inoculation of HARA‐B cells. A subcutaneous administration of reveromycin A (10 mg/kg) was also performed every day for 6 weeks after the inoculation of HARA‐B cells. Bone metastases were determined on X‐ray photographs at 4 or 6 weeks. Osteolytic bone metastasis on X‐ray photographs was evaluated independently. Mice were sacrificed under anesthesia with pentobarbital (0.5 mg/g body weight) at 5 or 6 weeks after inoculation. The extremities and spine were harvested and fixed in 10% formalin. The bone specimens were decalcified in 10% EDTA solution for 1 week and then embedded in paraffin. Tumor metastases were histologically evaluated by the number of colonies and the tumor area in bone and muscle after hematoxylin–eosin staining.( 22 )
Immunohistochemical and immunofluorescence analysis. Macrophages in bone marrow and tumors were determined using immunohistochemistry for F4/80. Slides were deparaffinized and hydrated, and then rinsed twice with PBS. After 1 h of blocking with 2% goat serum, the sections were incubated overnight with rat antimouse F4/80 (1:200) at 4°C in 1% BSA in PBS. They were then rinsed three times with PBS and treated with HRP‐conjugated goat antirat IgG (DakoCytomation, CA, USA) and stained using the DakoCytomation LSAB2 SYSTEM HRP kit, according to the instructions. The sections were counterstained with diluted hematoxylin according to the manufacturer's directions.
For the detection of osteoclasts, TRAP staining was done using a Sigma Diagnostics Acid Phosphatase kit. The number of TRAP‐positive cells in bone marrow was counted by microscopy in five random fields in each of three sections at ×200 magnification.
To determine the macrophages and neutrophils in HARA‐B tumors, immunofluorescence staining was performed. The tumor samples from bone or muscle were excised and immersed in OCT compound, and immediately frozen in liquid nitrogen. Frozen sections 5‐µm thick were prepared. The sections were rinsed with PBS and briefly fixed in 4% paraformaldehyde/PBS for 20 min at RT, followed by two further rinses in PBS. After 1 h of blocking with 2% goat serum, the sections were incubated overnight with rat antimouse F 4/80 (1:200) or rat antimouse Gr‐1 (1:200) at 4°C in 1% BSA in PBS. They were then rinsed three times with PBS and incubated with goat antirat IgG; 1‐mg/mL Alexa Fluor 488 for F4/80 (Molecular Probes, Eugene, OR, USA) in 1% BSA in PBS for 60 min at RT. Nuclear staining was carried out using DAPI (1:1000; Dojindo, Japan). Coverslips were mounted on sections using gel mount and viewed using an Olympus BX51 florescence microscope (Olympus, Tokyo, Japan) fitted with an Olympus DP‐70 digital camera (Olympus). For quantification, the number of stained cells was counted in five random fields in each of three tumors at ×200 magnification.
Cell survival assay. Cell survival assay was carried out using a Cell Counting Kit (Wako Pure Chemical Industries, Osaka, Japan). In brief, HARA‐B cells and human monocytes were plated in triplicate in 96‐well plates at a density of 5000 cells/well in basal medium. Following overnight culture, PBS‐LIP, Cl2MDP‐LIP, or reveromycin A was added to each concentration, and the cells were incubated for 48 h. After 48 h, WST‐1 was added and the cells were incubated for a further 1 h. The plates were read at a wavelength of 450 nm using a microplate reader (Model 3550; Bio‐Rad, Richmond, CA, USA). Results are presented as the mean ± SD.
Statistical analysis. The significance of tumor incidence was determined by χ2‐test. The significance of differences in the number of metastases was determined by the Mann–Whitney U‐test. The significance of differences in the numbers of TRAP‐positive cells and tumor area was estimated using the unpaired Student's t‐test. P‐values of <0.05 were considered statistically significant.
Results
Decrease in the number of monocytes/macrophages by Cl2MDP‐LIP in vitro and in vivo. Blood samples were harvested from the left cardiac ventricles of mice under anesthesia as a control. Subsequently, Cl2MDP‐LIP at 200 µL and 400 µL was s.c. administered into the base of the tail, and a 100‐µL blood sample was obtained after 24 and 48 h. Each sample was stained with FITC‐anti‐F4/80 mAb and PE‐anti‐CD11b mAb to label macrophages, and double‐positive cells were measured using a FACScan. The rate of double‐positive staining was about 5–7% before stimulation with Cl2MDP‐LIP. The number of double‐positive cells in peripheral blood was decreased significantly at 24 h after stimulation with 400 µL of Cl2MDP‐LIP but had recovered slightly after 48 h. Although stimulation with 200 µL of Cl2MDP‐LIP also suppressed the percentage of monocytes at 24 h, the effect was less marked than that of 400 µL of Cl2MDP‐LIP (Fig. 1a,b). PBS‐LIP and reveromycin A did not decrease the number of monocytes in peripheral blood (Fig. 1c). We also compared the effects of Cl2MDP‐LIP and reveromycin A on the survival of lung cancer HARA‐B cells and macrophage U937 cells in culture. Survival of cancer cells was specifically inhibited by reveromycin A at both 5 and 10 µg/mL, but Cl2MDP‐LIP had no effect up to 200 µM (Table 1). By contrast, survival of macrophages was blocked only by 20–200 µM Cl2MDP‐LIP, but not by reveromycin A at up to 10 µg/mL (Table 1). Thus, Cl2MDP‐LIP had a more specific effect on macrophage survival than reveromycin A in both in vitro and in vivo.
Figure 1.
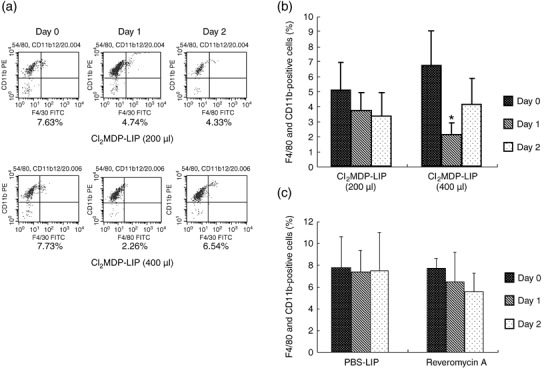
The decrease in the number of monocytes in peripheral blood by treatment with clodronate encapsulated by liposomes (Cl2MDP‐LIP). (a) FACS analysis of macrophages on days 0, 1, and 2 in peripheral blood of nude mice untreated or treated with Cl2MDP‐LIP. Each blood sample (100 µL) was harvested from the left cardiac ventricle under anesthesia before stimulation. Subsequently, blood samples were taken on days 1 and 2 after the subcutaneous administration of Cl2MDP‐LIP at 200 µL or 400 µL/mouse. Cells were stained with fluorescein‐isothiocyanate (FITC)–anti‐F4/80 monoclonal antibody (mAb) (1:50) and phycoerythrin (PE)–anti‐CD11b mAb (1:50). F4/80‐ and CD11b‐positive cells were measured by FACScan. Upper lane, Cl2MDP‐LIP 200 µL; lower lane, Cl2MDP‐LIP 400 µL. Quantification of the number of monocytes in peripheral blood of nude mice treated with (b) Cl2MDP‐LIP at 200 µL or 400 µL, and (c) PBS‐LIP (400 µL) or reveromycin A (10 mg/kg). Each value represents the mean number of monocytes/macrophages ± SD (n = 4). *P < 0.05.
Table 1.
Effect of clodronate, Cl2MDP‐LIP, and reveromycin A on the survival of macrophages and lung cancer cells
| Drug | Dose | Lung cancer cells † | Macrophages+ |
|---|---|---|---|
| PBS‐LIP | 0 | 100.0 ± 4.0 | 100.0 ± 5.9 |
| Clodronate | 2 | 93.3 ± 5.6 | 98.3 ± 6.6 |
| 20 | 93.8 ± 7.1 | 99.9 ± 11.5 | |
| 200 (µM) | 99.1 ± 1.8 | 92.7 ± 8.9 | |
| Cl2MDP‐LIP | 2 | 100.0 ± 2.6 | 94.0 ± 9.1 |
| 20 | 100.0 ± 0.4 | 74.7 ± 2.4 | |
| 200 (µM) | 100.0 ± 5.7 | 26.4 ± 3.7 | |
| Reveromycin A | 1 | 100.0 ± 5.6 | 101.2 ± 1.6 |
| 5 | 33.1 ± 2.5 | 100.7 ± 2.4 | |
| 10 (µg/mL) | 12.2 ± 0.4 (%) | 105.3 ± 1.5 (%) |
HARA‐B cells (5 × 103/well) and macrophages (5 × 103/well) were incubated for 2 days in the absence or presence of various doses of drugs, and surviving fractions were determined.
Each value was the average of triplicate dishes, and presented as a relative percentage, the cell number in the absence of any drug being taken as 100%. †Mean ± SD.
Inhibition of bone and muscle metastases by Cl2MDP‐LIP. Bone and muscle metastases in mice were followed using X‐ray photographs at 4 or 6 weeks after inoculation of 2 × 105 cancer cells (Fig. 2a). Colonies of abundant proliferating cancer cells were observed in both bone and muscle when both regions were histologically examined at 6 weeks after cancer cell inoculation (Fig. 2b). We first examined the inhibitory effect of Cl2MDP‐LIP on bone and muscle metastases of cancer cells in comparison with PBS‐LIP (control). Based on the effects of Cl2MDP‐LIP (Fig. 1), we determined the protocols shown in Fig. 3a. Cl2MDP‐LIP was administered s.c. at 200 µL and 400 µL once every 3 days for 6 weeks just after the inoculation of cancer cells (Fig. 3a). All of the mice treated with PBS‐LIP showed destructive bone changes in X‐ray photographs or paralysis in the hind limbs at 6 weeks. By contrast, treatment with Cl2MDP‐LIP at 200 µL and 400 µL inhibited the development of bone metastases by cancer cells (Fig. 3b). Fig. 3b also shows the therapeutic effects of reveromycin A on bone metastasis when administered s.c. at 10 mg/kg daily after cancer cell inoculation. Treatment with reveromycin A markedly inhibited bone metastasis by lung cancer cells. Quantitative analysis showed that both the incidence of bone metastasis and the number of metastatic foci were significantly decreased by treatment with Cl2MDP‐LIP at 200 µL and 400 µL, and reveromycin A (Table 2). The inhibitory effect of Cl2MDP‐LIP at 400 µL was strongest among those agents (Table 2).
Figure 2.
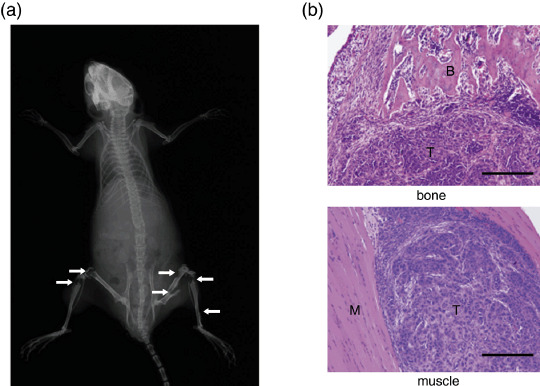
Radiographic and histological analysis of untreated mice. The human lung cancer cell line HARA‐B (2 × 105 cells per mouse) was injected into the left cardiac ventricle of nude mice. (a) Bone metastases were determined by radiography on the indicated days after inoculation. Arrows indicate osteolytic bone metastases. (b) The mice were sacrificed at 6 weeks, and bone and muscle metastases were examined histologically. Bar, 200 µm; T, tumor; B, bone; M, muscle.
Figure 3.
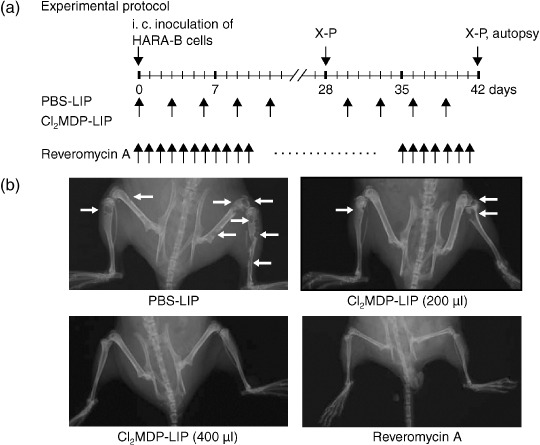
Clodronate‐liposomes and reveromycin A decreased the frequency of bone metastasis by lung cancer cells. (a) Experimental protocol of the treatment with clodronate encapsulated by liposomes (Cl2MDP‐LIP) and reveromycin A on bone or muscle metastases of HARA‐B cells. (b) Radiographs of the hind limbs of nude mice treated with PBS‐LIP (400 µL), Cl2MDP‐LIP (200 µL), Cl2MDP‐LIP (400 µL), and reveromycin A (10 mg/kg). HARA‐B cells (2 × 105 per mouse) were injected into the left cardiac ventricle. At 6 weeks after the subcutaneous administration of PBS‐LIP, Cl2MDP‐LIP (200 µL and 400 µL/mouse once every 3 days), and reveromycin A (every day) the extent of bone and muscle metastases was determined by radiography and autopsy. Arrows indicate osteolytic bone metastases.
Table 2.
Radiological analysis of inhibitory effects of clodronate‐liposomes and reveromycin A on bone metastasis
| Treatment | Incidence of bone metastasis | No. of metastatic foci + |
|---|---|---|
| PBS‐LIP | 8/9 | 6.6 ± 4.2 |
| Cl2MDP‐LIP (200 µl) | 3/7 † | 0.9 ± 1.2 ‡ |
| Cl2MDP‐LIP (400 µl) | 1/9 ‡ | 0.1 ± 0.3 ‡ |
| Reveromycin A | 1/9 ‡ | 0.3 ± 1.0 ‡ |
HARA‐B cells (2 × 105 per mouse) were injected into the left cardiac ventricle of nude mice on day 0. The mice were s.c. administered PBS‐LIP, Cl2MDP‐LIP (200 and 400 µl/mouse once every three days), or reveromycin A (10 mg/kg daily) from day 0 to 6 weeks. Bone metastases were determined by radiographs at 4 and 6 weeks after inoculation.
Mean ±SD,
P < 0.05,
P < 0.01.
We also compared the therapeutic effects of various agents on both muscle metastasis and bone metastasis by histological analysis. Treatment with Cl2MDP‐LIP at 400 µL and reveromycin A significantly decreased the number of tumor colonies in bone (Table 3). We also observed a marked decrease of tumor colony numbers in muscle by Cl2MDP‐LIP at 400 µL (P < 0.05) but not by reveromycin A. Furthermore, Cl2MDP‐LIP at 400 µL significantly (P < 0.05) inhibited tumor area in bone as compared with the control, whereas reveromycin A or Cl2MDP‐LIP at 200 µL decreased the tumor area by only 30–50% compared with the control. In contrast, there was no significant difference in the inhibitory effect on tumor area in muscle between the untreated control and treated groups. Of the various treatments against tumor area in muscle, only Cl2MDP‐LIP at 400 µL had an inhibitory effect, although the inhibition was not statistically significant (Table 3).
Table 3.
Histological analysis of inhibitory effects of clodronate‐liposomes and reveromycin A on bone metastasis
| Treatment | Incidence of metastasis | No. of tumor colonies + | Tumor area (mm2) + | ||
|---|---|---|---|---|---|
| bone | muscle | bone | muscle | ||
| PBS‐LIP | 8/9 | 4.7 ± 4.1 | 13.2 ± 14.1 | 8.1 ± 6.9 | 16.4 ± 20.2 |
| Cl2MDP‐LIP (200 µl) | 5/7 | 2.0 ± 3.0 | 7.9 ± 13.1 | 5.6 ± 9.3 | 10.1 ± 11.3 |
| Cl2MDP‐LIP (400 µl) | 5/9 | 0.4 ± 0.7 † | 1.2 ± 1.2 † | 0.4 ± 0.8 † | 2.0 ± 3.3 |
| Reveromycin A | 6/9 | 0.7 ± 1.3 † | 4.3 ± 4.6 | 3.7 ± 9.2 | 14.9 ± 13.9 |
HARA‐B cells (2 × 105 per mouse) were injected into the left cardiac ventricle of nude mice on day 0. The mice were s.c. administered PBS‐LIP, Cl2MDP‐LIP (200 and 400 µl/mouse once every three days), or reveromycin A (10 mg/kg daily) from day 0 to 6 weeks. The mice were sacrificed at 6 weeks and the formation of metastasis in bone or muscle was examined. The tumor area is represented as the sum of the individual tumor areas in bone or muscle of each mouse calculated as πd2/4, where d is the diameter of each tumor in mm. +Mean ± SD, † P < 0.05.
Decrease in the number of both macrophages in tumor and osteoclasts in bone by Cl2MDP‐LIP. We examined whether the number of macrophages was affected by treatment with Cl2MDP‐LIP. The number of macrophages in tumors was also estimated by immunofluorescence analysis with a rat antimouse F4/80 antibody. The immunofluorescence analysis of mice treated with PBS‐LIP revealed numerous macrophages stained with F4/80 in tumors, whereas infiltrating macrophages in tumors were decreased in mice treated with Cl2MDP‐LIP (Fig. 4a). Immunostaining of macrophages also showed a marked decrease in the number of infiltrating macrophages in tumors on treatment with Cl2MDP‐LIP in comparison with the untreated control (Fig. 4b). By contrast, the number of macrophages in bone marrow was not affected by treatment with Cl2MDP‐LIP (data not shown). Quantitative analysis showed a significant decrease of infiltrating macrophages in tumors after treatment with Cl2MDP‐LIP (Fig. 4c). The number of neutrophils in tumors was not affected by treatment with Cl2MDP‐LIP in comparison with the untreated control (Fig. 4d,e).
Figure 4.
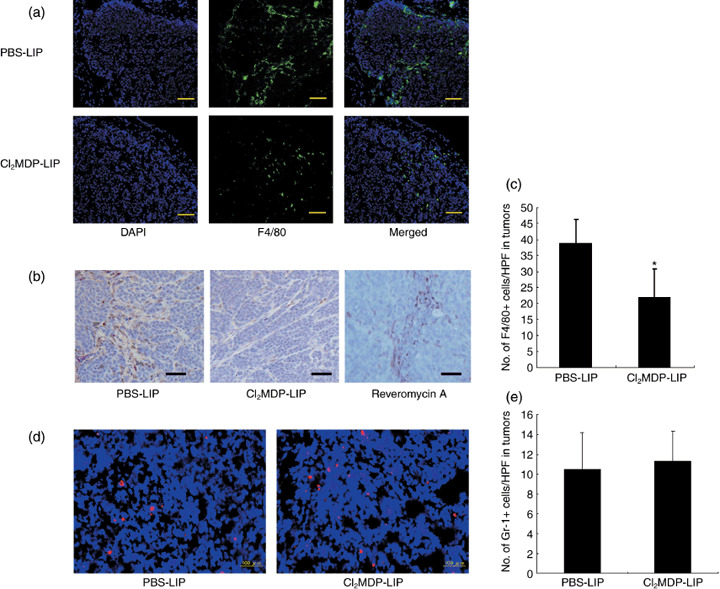
Reduced infiltration of macrophages in tumors by clodronate encapsulated by liposomes (Cl2MDP‐LIP). (a) Immunofluorescence analysis of F4/80‐positive cells in HARA‐B tumors. The tumors derived from mice treated with PBS‐LIP, Cl2MDP‐LIP (400 µL), and reveromycin A (10 mg/kg) were excised at 6 weeks after inoculation as described in the experiment protocol. The samples were stained with rat anti‐F4/80 antibody (1:200), and ant‐rat Alexa‐Fluor‐488 (1:1000) was used as secondary antibody to detect the F4/80‐positive macrophages. Nuclear staining was carried out using 4′,6‐diamidino‐2‐phenylindole (DAPI) (1:1000) to present profiles of tumor masses, and the merged figures were used to localize the F4/80‐positive cells in tumor masses. (b) Some sections were incubated with rat anti‐F4/80 antibody and horseradish peroxidase (HRP)–conjugated goat antirat IgG was used as secondary antibody. Bar, 100 µm. (c) Quantification of macrophages in HARA‐B tumors. The number of stained cells was counted in five random fields for each of three tumors derived from mice treated with PBS‐LIP and Cl2MDP‐LIP at ×200 magnification. Each value represents the mean number of macrophages ± SD. *P < 0.05. (d) Some sections were incubated with rat anti‐Gr‐1 antibody. Bar, 100 µm. (e) Quantification of neutrophiles in HARA‐B tumors. The number of stained cells was counted in five random fields for each of three tumors at ×200 magnification. Each value represents the mean number of neutrophiles ± SD.
We further examined whether treatment with Cl2MDP‐LIP at 400 µL as well as reveromycin A affected the number of osteoclasts in bone marrow. The hind limbs of mice treated with PBS‐LIP, Cl2MDP‐LIP, and reveromycin A were harvested at 6 weeks after inoculation, and osteoclasts were identified by TRAP staining (Fig. 5a). We observed many TRAP‐positive osteoclasts in control mice with bone metastasis. By contrast, the number of osteoclasts was decreased in reveromycin A– and Cl2MDP‐LIP‐treated mice. Quantitative analysis demonstrated that the number of osteoclasts was significantly decreased in bone marrow by Cl2MDP‐LIP at 400 µL and by reveromycin A in comparison with the controls (Fig. 5b).
Figure 5.
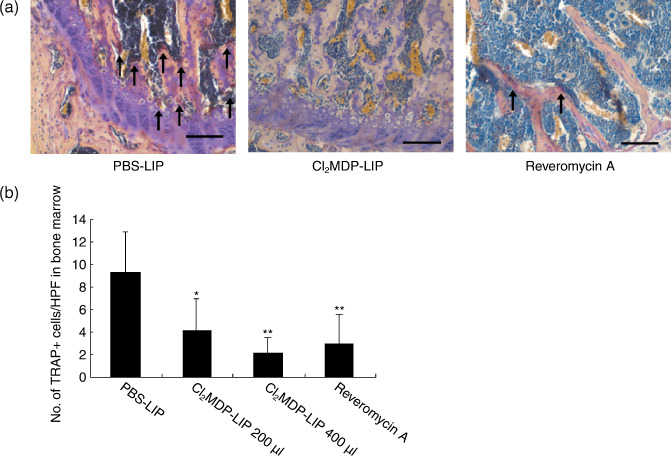
Reduced number of osteoclasts in bone marrow by clodronate encapsulated by liposomes (Cl2MDP‐LIP). (a) Tartrate‐resistant acid phosphatase (TRAP) staining was done using a Sigma Diagnostics Acid Phosphatase kit. The hind limbs of mice treated with PBS‐LIP, Cl2MDP‐LIP (200 µL and 400 µL), and reveromycin A in the experiment protocol were taken and fixed in 10% formalin. The specimens were decalcified in a 10% EDTA solution for 1 week and then embedded in paraffin. Arrows indicate TRAP‐positive osteoclasts in bone marrow. Bar, 100 µm. (b) Quantification of osteoclasts in bone marrow. The number of TRAP‐positive cells in bone marrow was counted under a microscope in five random fields in each of three sections at ×200 magnification. The statistical significance of differences between the controls and other groups was analyzed using the unpaired Student's t‐test. *P < 0.05, **P < 0.01.
Discussion
In the present study, we assessed the effect of a decreasing number of monocytes/macrophages in peripheral blood and the tumor stroma on bone and muscle metastases using an experimental bone metastasis model in nude mice inoculated with human lung cancer cells which showed strong bone metastasis activity.( 3 , 20 ) Clodronate‐liposomes reduced the number of monocytes in peripheral blood as well as the number of osteoclasts in bone marrow, accompanied by marked inhibition of metastasis to both bone and skeletal muscle by lung cancer cells. Clodronate is a bisphosphonate, and bisphosphonates targeting osteoclast‐mediated bone metastasis have been used to treat bone metastasis.( 23 ) The underlying mechanism of their effects is inhibition of a key enzyme in the mevalonate pathway, farnesyl diphosphate synthase, resulting in prevention of protein phenylation and Ras activation, and also producing a unique adenosine triphosphate analog (Apppi), resulting in induction of apoptosis of both osteoclasts and cancer cells.( 23 ) Clodronate encapsulated by liposomes has been developed and successfully applied in several studies for depletion of macrophages.( 12 , 13 , 21 ) Although free clodronate is not ingested by macrophages and is rapidly removed from circulation,( 24 ) the liposome‐encapsulated form is phagocytosed, and intracellular release of clodronate promotes apoptosis.( 21 )
Treatment with the osteoclast‐targeting agent reveromycin A also significantly decreased bone metastasis by lung cancer cells. Reveromycin A, a novel antibiotic, inhibits bone resorption by inducing the specific apoptosis of activated osteoclasts, possibly because reveromycin A is specifically transported into osteoclasts at acidic pH.( 25 , 26 , 27 ) In this study, histological analyses showed that reveromycin A markedly decreased the number of osteoclasts in bone lesions, suggesting that reveromycin A specifically inhibits osteolytic bone metastasis by targeting osteoclasts in bone lesions. Reveromycin A only slightly inhibited the number of muscle metastases, perhaps through its direct inhibition of cancer cell survival. By contrast, clodronate‐liposomes inhibited the survival of macrophages in culture, whereas reveromycin A did not. These findings suggest that the clodronate‐liposome‐induced inhibition of both bone and muscle metastasis may be due to a reduction the number of not only osteoclasts, but also macrophages infiltrating the metastatic lesions in both bone and muscle.
Monocytes in peripheral blood are versatile precursors with the potential to differentiate into the various types of specialized macrophages.( 28 ) Macrophages in the tumor environment are activated by inflammatory responses during the acquisition of malignant characteristics in both the primary tumor and bone metastases.( 6 ) Infiltrating macrophages under conditions of inflammation are derived mainly from peripheral blood monocytes, and create conditions in the tumor stroma and bone metastases that favor metastasis/invasion and angiogenesis through the production of various chemokines, cytokines, growth factors, proteases, and hypoxia.( 7 , 8 , 9 ) Clodronate‐liposomes have been shown to significantly reduce the number of monocytes in peripheral blood in vivo ( 12 ) (see also Fig. 1), and the survival of macrophages in vitro. Treatment with clodronate‐liposomes also markedly inhibits inflammatory cytokine‐induced angiogenesis and infiltration of monocytes/macrophages in the cornea,( 12 ) and also tumor growth by lung cancer cells.( 14 ) Thus, a decrease in the number of macrophages by clodronate‐liposomes might also block the metastasis of cancer cells to bone.
Tumor angiogenesis is often closely associated with bone metastasis by cancer cells,( 15 , 29 ) possibly through angiogenesis in the tumor stroma and also in the metastases themselves. Activation of the VEGF, IL‐8, bFGF, and cyclooxygenase‐2 genes in both tumor cells and macrophages in the tumor stroma by inflammatory cytokines induces angiogenesis.( 12 , 30 ) It has been reported that synergistic interaction between macrophages and tumor cells is required for tumor cell migration through a paracrine loop involving reciprocal signaling of EGF and colony‐stimulating factor‐1.( 31 ) Inflammatory cytokines produced by macrophages affect tumor invasion and angiogenesis, suggesting that the recruitment of macrophages into the tumor stroma is prerequisite for the acquisition of malignant characteristics.( 4 , 5 , 6 , 9 ) Previous studies have demonstrated the apparent involvement of macrophages in inflammatory cytokine‐induced angiogenesis( 12 ) and also in tumor‐induced angiogenesis.( 13 ) The blocking of bone metastasis from lung cancer might be due in part to decreased macrophage‐induced angiogenesis. However it still remains to be clarified how bone metastasis is linked to angiogenesis in metastatic lesions.
Treatment with clodronate‐liposomes markedly inhibited the metastasis of cancer cells to muscle as well as bone. However, whether a decrease in the number of macrophages by this drug is directly involved in its inhibitory effect on the metastasis to muscle needs to be further studied. Jones et al. have recently reported that bone metastasis after the intracardiac injection of melanoma cells is dependent on RANK/RANKL signaling.( 32 ) However, in our present study, the role of RANK/RANKL signaling was not examined. Regarding the pleiotropic mechanisms of bone metastasis by cancer cells, we consider it likely that macrophage lineages provide a microenvironment that is favorable for metastasis and tumor growth not only in bone but also in muscle.( 33 ) The inhibition of metastasis in both bone and muscle by clodronate‐liposomes might be due to depletion of osteoclasts precursors as well as tumor‐associated macrophages.
In conclusion, osteoclasts are well known to play pivotal roles in bone metastasis by cancer cells, and osteoclasts are derived from monocytes/macrophages. Bisphosphonates are most frequently used to treat bone metastasis in cancer patients, and act by possibly targeting osteoclasts and cancer cells.( 34 ) The present study demonstrated that treatment with a bisphosphonate encapsulated by liposomes markedly decreased both bone and muscle metastases by lung cancer cells. In contrast, treatment with the osteoclast‐targeting drug, reveromycin A, specifically inhibited bone metastasis, but not muscle metastasis, by lung cancer cells. These findings suggest that bisphosphonates encapsulated by liposomes may be a novel and potent therapeutic agent against not only bone metastasis but also other organ metastases of lung cancer cells in humans.
Acknowledgments
We thank Kazuhiro Yoshida, Hiromi Kuboyama, and Hitomi Wakita for technical assistance. This study was partly supported by the 21st Century COE Program for Medical Science; a grant‐in‐aid for the 3rd term comprehensive 10‐year strategy for cancer control from the Ministry of Health, Welfare (M.K.), and Labor of Japan; and the Innovation Center for Medical Redox Navigation, Kyushu University (M.O., M.K.).
References
- 1. Fidler IJ, Radinsky R. Genetic control of cancer metastasis. J Natl Cancer Inst 1990; 82: 166–8. [DOI] [PubMed] [Google Scholar]
- 2. Guise TA. Molecular mechanism of osteolytic bone metastasis. Cancer 2000; 88: 2892–8. [DOI] [PubMed] [Google Scholar]
- 3. Iguch H, Tanaka S, Ozawa Y et al . An experimental model of bone metastasis by human lung cancer cells: the role of parathyroid hormone‐related protein in bone metastasis. Cancer Res 1996; 56: 4040–3. [PubMed] [Google Scholar]
- 4. Balkwill F, Mantovani A. Inflammation and cancer: back to Vichow? Lancet 2001; 357: 539–45. [DOI] [PubMed] [Google Scholar]
- 5. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollard JW. Tumor‐educated macrophages promote tumor progression and metastasis. Nat Rev Cancer 2004; 4: 71–8. [DOI] [PubMed] [Google Scholar]
- 7. Polverini PJ, Cotran PS, Gimbrone MA Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature 1977; 269: 804–6. [DOI] [PubMed] [Google Scholar]
- 8. Mantovani A, Allavena P, Sica A. Tumor‐associated macrphages as a prototypic type II plarised phagocytic population: role in tumor progression. Eur J Cancer 2004; 40: 1660–7. [DOI] [PubMed] [Google Scholar]
- 9. Kuwano M, Basaki Y, Kuwano T et al . The critical role of inflammatory cell infiltration in tumor angiogenesis – a target for antitumor drug development? In: New Angiogenesis Research. New York: Nova Science Publishers, Inc, 2005; 157–70. [Google Scholar]
- 10. Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol 1994; 55: 410–22. [DOI] [PubMed] [Google Scholar]
- 11. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56: 4625–9. [PubMed] [Google Scholar]
- 12. Nakao S, Kuwano T, Tsutsumi‐Miyahara C et al . Infiltration of COX2‐expressiong macrophages is prerequisite for IL‐1b‐induced neovascularization and tumor growth. J Clinic Invest 2005; 115: 2979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeisberger SM, Odermatt B, Marty C, Zehnder‐Fjallman AHM, Ballmer‐Hofer K, Schwendener RA. Clodronate‐liposome‐mediated depletion of tumor‐associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 2006; 95: 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimura Y, Watari K, Fotovati A et al . Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci 2007; 98: 2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribatti D, Nico B, Vacca A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene 2006; 25: 4257–66. [DOI] [PubMed] [Google Scholar]
- 16. Gardner CR. Morphological analysis of osteoclastogenesis induced by RANKL in mouse bone marrow cell cultures. Cell Biol Int 2007; 31: 672–82. [DOI] [PubMed] [Google Scholar]
- 17. Gallwitz WE, Guise TA, Mundy GR. Guanosine nucleotides inhibit different syndromes of PTHrP excess caused by human cancers in vivo . J Clin Invest 2002; 110: 1559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guise TA, Yin JJ, Taylor SD et al . Evidence for a causal role of parathyroid hormone‐related protein in the pathogenesis of human breast cancer‐mediated osteolysis. J Clin Invest 1996; 98: 1544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–93. [DOI] [PubMed] [Google Scholar]
- 20. Iguchi H, Ono M, Matsushima K, Kuwano M. Overproduction of IL‐8 results in suppression of bone metastasis by lung cancer cells in vivo . Int J Oncol 2000; 17: 329–33. [DOI] [PubMed] [Google Scholar]
- 21. Van Rooijen N. The liposome‐mediated macrophage ‘suicide’ technique. J Immunol Meth 1989; 124: 1–6. [DOI] [PubMed] [Google Scholar]
- 22. Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer 2005; 116: 458–63. [DOI] [PubMed] [Google Scholar]
- 23. Green JR. Bisphosphonates: preclinical review. Oncologist 2004; 9 (Suppl 4): 3–13. [DOI] [PubMed] [Google Scholar]
- 24. Fleisch H. Bisphosphonates: a new class of drugs in disease of bone and calcium metabolism. Handbook Exp Pharmacol 1988; 83: 441. [DOI] [PubMed] [Google Scholar]
- 25. Osada H, Koshino H, Isono K, Takahashi H, Kawanishi G. Reveromycin A, a new antibiotic which inhibits the mitogenic activity of epidermal growth factor. J Antibiot 1991; 44: 259–61. [DOI] [PubMed] [Google Scholar]
- 26. Muguruma H, Yano S, Kakiuchi S et al . Reveromycin A inhibits osteolytic bone metastasis of small‐cell lung cancer cells, SBC‐5, through an antiosteoclastic activity. Clin Cancer Res 2005; 11: 8822–8. [DOI] [PubMed] [Google Scholar]
- 27. Woo JT, Kawatani M, Kato M et al . Reveromycin A, an agent for osteoporosis, inhibits bone resorption by inducing apoptosis specifically in osteoclasts. Proc Natl Acad Sci USA 2006; 103: 4729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo Y, Zhou H, Krueger J et al . Targeting tumor‐associated macrophages as a novel strategy against breast cancer. J Clin Invest 2006; 116: 2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muguruma H, Matsumori Y et al . Antitumor vascular strategy for controlling experimental metastatic spread of human small‐cell lung cancer cells with ZD6474 in natural killer cell‐depleted severe combined immunodeficient mice. Clin Cancer Res 2005; 11: 8789–98. [DOI] [PubMed] [Google Scholar]
- 30. Torisu H, Ono M, Kiryu H et al . Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNF alpha and IL‐1 alpha. Int J Cancer 2000; 85: 182–8. [PubMed] [Google Scholar]
- 31. Wyckoff J, Wang W, Lin EY et al . A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004; 64: 7022–9. [DOI] [PubMed] [Google Scholar]
- 32. Jones DH, Nakashima T, Sanchez OH et al . Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006; 440: 692–6. [DOI] [PubMed] [Google Scholar]
- 33. Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 2008; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Green JR, Clezardin P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am J Clin Oncol 2002; 25: S3–9. [DOI] [PubMed] [Google Scholar]


