Abstract
Cigarette smoking is an established risk factor for lung cancer. However, the magnitude of the relative risk (RR) on lung cancer mortality in relation to cigarette smoking is reported to be lower in Japan than in Western countries. We investigated whether this discrepancy could be explained by differences in the exposure to cigarettes smoked, by differences in sensitivity to smoking, or by differences in lung cancer mortality among non‐smokers. We examined the 10‐year follow‐up data on 88 153 participants in a Japanese population‐based prospective study conducted in three prefectures. Data used as a Western counterpart was retrieved from a published report of the US Cancer Prevention Study (CPS)‐II. Although there was a significant increased risk of lung cancer death among current smokers compared with non‐smokers, the observed RR in the Three‐Prefecture Study were much lower than RR reported in the CPS‐II. Lung cancer mortality of our Japanese sample was lower among current smokers and higher among non‐smokers regardless of age and sex. Current smokers in our sample had initiated smoking at an older age and smoked fewer cigarettes per day for shorter durations than those in the CPS‐II sample. The Poisson regression model (controlling for age, number of cigarettes smoked per day and duration of smoking) showed that male current smokers in our sample had a lower risk of lung cancer compared with those in the CPS‐II sample (rate ratio 0.34 [95%CI 0.27–0.43]). These findings might explain why Japanese risks of lung cancer are lower than those observed in Western countries. (Cancer Sci 2005; 96: 120–126)
Numerous epidemiological studies have consistently reported smoking as a risk factor for lung cancer. Three prospective studies 1 , 2 , 3 and several case‐control studies 4 , 5 , 6 in Japan have shown that the magnitude of the relative risk (RR) associated with cigarette smoking is lower than those in Western countries. (2) For example, in the Six‐Prefecture Study (3) and the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC), (1) the RR of lung cancer death among smokers compared to non‐smokers was estimated at 4.5 for men, whereas the RR for men ranged from 11.6 to 23.2 in prospective studies conducted in the USA 7 , 8 , 9 and the UK. (10) For women, the RR were 2.3 in the Six‐Prefecture Study (3) and 3.6 in the JACC study, (1) while corresponding RR ranged from 2.7 to 12.8 in the USA. 7 , 9 The first aim of this study was to verify these figures by evaluating lung cancer death and smoking habits with a new large‐scale, population‐based prospective survey (The Three‐Prefecture Cohort Study), conducted in three prefectures in Japan.
The RR expresses a single summary estimate of the effects of smoking on lung cancer. However, the RR is computed by simply dividing the death rate among smokers by that among non‐smokers. For a better understanding of the reasons for the lower RR of lung cancer among the Japanese, it would be more accurate to compare the death rates by smoking status. Furthermore, exposure levels to smoking might account for differences in the risk of lung cancer between Japanese and Western current smokers. It is well known that lung cancer risk depends on the amount, duration, and initiation age of smoking. Thus, to determine the reason for the lower RR associated with smoking in Japanese subjects, it is also important to compare the exposure levels to smoking as well as the lung cancer death rates between Japanese and Western subjects.
The second aim of this study was to compare death rates by smoking status and smoking exposure levels with published data from a large American prospective sample, the Cancer Prevention Study II (CPS‐II), (9) which began at nearly the same time as the Three‐Prefecture Cohort Study (1982). Finally, we examined whether any discrepancy in the RR of lung cancer between the studies could be explained by the difference in death rates due to smoking status (i.e. non‐smokers vs smokers) and smoking exposure level between the Japanese and the US samples.
Materials and Methods
Study population. The Three‐Prefecture Cohort Study collected data from February 1, 1983 to November 1, 1985, in selected areas of three prefectures in Japan: Miyagi, Aichi, and Osaka. The study areas of each prefecture included six areas of a city and two towns in Miyagi Prefecture, five elementary school districts in one area of a city and two areas of a city in Aichi Prefecture, and three towns in Osaka Prefecture. An additional study cohort was sampled in December 1, 1990, in one city in the Osaka Prefecture. The study population included all persons aged 40 years or older, who resided in the study areas according to each town's residential registry. A self‐administered questionnaire was distributed to 130 839 persons, and 108 774 (50 544 men and 58 230 women) of them responded (83.1%). We then excluded individuals under 40 years (one man and one woman) and over 80 years of age (1 427 men and 2 465 women), any who moved out before the start of the follow up (five men and three women), and any whose information on smoking status at enrollment was incomplete (4 660 men and 12 059 women). After exclusion of these individuals, 44 451 men and 43 702 women remained in the analysis. This study was approved by the institutional review board of the National Cancer Center, Tokyo, Japan.
Follow up. Information on whether each subject was still alive and living in the same location was obtained from residential registries. If the subject had died, we then searched the population‐based cancer registry in each prefecture and ascertained whether they had died from lung cancer. Sites of any cancers were coded using the International Classification of Disease and Injuries–ninth revision (ICD‐9), except for one city in Osaka where the ICD 10th revision was used. Study subjects were followed for 10 years in each area. Therefore, the end of the study period varied from January 31, 1993 to October 31, 1995 (and February 28, 2000 for the one city in the Osaka Prefecture) according to the dates of enrollment. During the follow up, 8 836 (15.6%) individuals moved out of the study areas.
Smoking Information. At enrollment, study participants completed a self‐administered questionnaire, including demographic information such as sex, date of birth, and smoking habits. The smoking habits questions were the same in each study area, except for one town in the Osaka Prefecture. All participants were asked: ‘Do you smoke?’ Response categories included: (1) yes; (2) smoked but quit; and (3) never smoked. We defined participants who chose response (1) as current smokers; those who chose response (2) as former smokers; and those who chose response (3) as non‐smokers. For one city in the Osaka Prefecture, the response categories were: (1) yes (smoking every day); (2) yes, but occasionally; (3) smoked, but quit; and (4) never smoked. We defined participants who chose response (1) and (2) as current smokers, those who chose response (3) as former smokers, and those who chose response (4) as non‐smokers.
The ages at initiation of smoking and the average number of cigarettes smoked per day for current and former smokers were obtained. The number of years of smoking that current smokers had smoked prior to enrollment was calculated by subtracting the age at initiation of smoking from the age at enrollment. Pack‐years were defined as the number of years of smoking multiplied by the number of packs of cigarettes per day.
Cancer Prevention Study II. The CPS‐II (9) is a prospective cohort study, conducted by the American Cancer Society (ACS). It was selected as the Western counterpart to our Japanese prospective cohort study because it contained detailed data on lung cancer mortality by sex, age group and smoking status, as well as data on smoking patterns of current smokers by sex and age group. The CPS‐II data for the comparison were retrieved from the Smoking and Tobacco Control Monograph no. 8. Study participants were friends, neighbors, and acquaintances of ACS volunteers. Approximately 1.2 million men and women were enrolled in 1982. Enrollment included all household members 30 years of age or older if at least one family member was 45 years of age or older. Study participants completed an initial questionnaire including smoking habits and other lifestyle factors. The vital status of study participants was determined through personal inquiry by the volunteers. The underlying cause of death was obtained through death certificates. During the 6‐year follow up of 711 363 current cigarette smokers and lifelong non‐smokers, 3 229 died of lung cancer.
Statistical Analysis. Person years during the follow‐up were counted from the date of enrollment into the study until the date of death, migration from the study areas, or the end of the study period, whichever came first. The RR was estimated with a Cox proportional hazards model with adjustments for age (continuous variable) and prefecture. Non‐smokers were used as a reference category. A dose–response relationship among current smokers was examined in terms of the number of pack‐years.
Using data from the CPS‐II, we compared the baseline data on smoking patterns among current smokers and the follow‐up data on lung cancer deaths among non‐smokers and current smokers. Follow‐up data were restricted to the first 6 years, the duration of the CPS‐II. The mean number of cigarettes smoked per day and the mean number of years of smoking were calculated within the 5‐year age groups fixed at the baseline. The age‐adjusted number of cigarettes smoked per day and the age‐adjusted number of years of smoking was obtained by directly standardizing to the combined distribution of age groups of the Japanese and US cohorts. Because the mean age at initiation of smoking among the CPS‐II subjects was provided as 10‐year birth cohorts, we calculated mean age of initiation in the Japanese study in the same way.
Sex‐ and age‐specific death rates of lung cancer (per 100 000) were computed for non‐smokers and current smokers. Calculation of the number of person years at risk was based on attained age. To compare the death rates of the Japanese and US cohorts, cumulative death rates between 40 and 84 years were presented. Rate ratios of the Japanese cohort to US cohort were calculated by using a Poisson regression model.
Lung cancer death rates were computed for male current smokers, stratified by the duration of smoking and the number of cigarettes smoked per day. Because of limited CPS‐II data, only subjects who smoked 20 or 40 cigarettes per day were analyzed. To compare the lung cancer risks among male current smokers in Japan to those in the USA, adjusted rate ratios were obtained by Poisson regression analysis. The model included the natural logarithm of the number of lung cancer deaths as a response variable and the natural logarithm of person‐years as an offset. Indicator variables for age group, number of cigarettes per day, and duration of smoking were used as covariates. Statistical computations were carried out using the SAS statistical package (version 8.02; SAS Institute, Cary, NC, USA).
Results
Current and former smokers in the Three‐Prefecture Cohort Study showed a significantly increased risk of lung cancer death for both men and women compared with non‐smokers (Table 1). A statistically significant dose–response trend of RR was observed for men and women current smokers (Table 2).
Table 1.
Relative risk of lung cancer death associated with cigarette smoking, Three‐Prefecture Cohort Study, Japan
| Smoking status | No. subjects | Person‐years | No. lung cancer deaths | Crude mortality rates | Relative risk † (95%CI) |
|---|---|---|---|---|---|
| Men | |||||
| Non‐smokers | 7 590 | 64 645 | 23 | 35.6 | 1.00 |
| Former smokers | 11 164 | 91 792 | 102 | 110.9 | 2.60 (1.65–4.10) |
| Current smokers | 25 697 | 215 139 | 341 | 158.5 | 5.10 (3.34–7.79) |
| Women | |||||
| Non‐smokers | 36 884 | 321 170 | 79 | 24.6 | 1.00 |
| Former smokers | 1 630 | 13 258 | 13 | 98.1 | 2.94 (1.63–5.31) |
| Current smokers | 5 188 | 42 931 | 40 | 93.2 | 3.66 (2.50–5.35) |
Adjusted for age and prefecture.
Table 2.
Relative risk of lung cancer death by pack‐years among current smokers, Three‐Prefecture Cohort Study, Japan
| Pack‐years of smoking | No. subjects | Person‐years | No. lung cancer deaths | Crude death rate | Relative risk † (95%CI) |
|---|---|---|---|---|---|
| Men ‡ | |||||
| <20 | 3 982 | 33 592 | 19 | 56.6 | 1.16 (0.72–1.88) |
| 20–39 | 12 066 | 101 910 | 113 | 110.9 | 2.10 (1.62–2.71) |
| 40–59 | 6 574 | 54 374 | 129 | 237.2 | 2.86 (2.23–3.65) |
| 60 + | 2 765 | 22 770 | 78 | 342.6 | 4.44 (3.34–5.89) |
| P for trend | <0.0001 | ||||
| Women § | |||||
| <20 | 3 136 | 26 212 | 12 | 45.8 | 1.75 (0.96–3.19) |
| 20–39 | 1 545 | 12 642 | 15 | 118.7 | 3.92 (2.27–6.76) |
| 40 + | 397 | 3 157 | 10 | 316.8 | 7.22 (3.75–13.9) |
| P for trend | <0.0001 | ||||
Adjusted for age and prefecture. Reference category was non‐smokers.
‡ 310 men were excluded because of missing data.
§ 110 women were excluded because of missing data.
In the first 6 years of follow up, the Three‐Prefecture Cohort Study had 341 deaths due to lung cancer (260 men and 81 women). Adjusted RR for current smokers versus non‐smokers were 3.16 (95%CI 1.29–3.64) for men and 2.68 (95%CI 1.58–4.53) for women. Corresponding reported RR in the CPS‐II study were 23.2 (95%CI 19.3–27.9) for men and 12.8 (95%CI 11.3–14.7) for women.
Death rates among current smokers and non‐smokers were calculated, based on attained age (Fig. 1). Compared with the CPS‐II, death rates among Japanese current smokers were lower in all age groups, with the exception of the youngest and oldest female age groups. In contrast, death rates among Japanese non‐smokers were higher than those in the USA, for both men and women regardless of age. Cumulative death rates between 40 and 84 years and rate ratios are presented in Table 3. Compared with US non‐smokers, Japanese non‐smokers had a higher cumulative mortality of lung cancer with an approximately threefold increased risk for men and a twofold increased risk for women. However, Japanese current smokers were at a significantly 60% lower risk of lung cancer compared to those in the USA.
Figure 1.
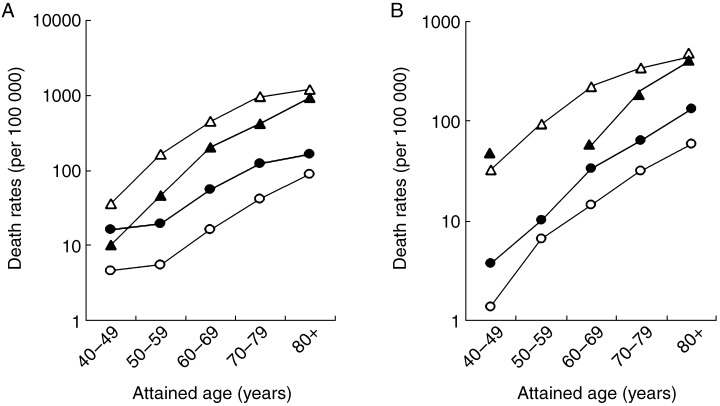
Age‐specific death rates due to lung cancer by attained age among current smokers and non‐smokers in the Three‐Prefecture cohort in Japan and Cancer Prevention Study II (CPS‐II) in the USA. (a), Death rates of men; (b), death rates of women. (▴), Three‐Prefecture cohort current smokers; (•), Three‐Prefecture cohort nonsmokers; (▵), CPS‐II current smokers; (○), CPS‐II non‐smokers.
Table 3.
Cumulative mortality and rate ratios for lung cancer among non‐smokers and current smokers, Three‐Prefecture Cohort Study in Japan compared to Cancer Prevention Study II in the USA
| Non‐smokers | Current smokers | |||
|---|---|---|---|---|
| Three‐Prefecture | CPS‐II | Three‐Prefecture | CPS‐II | |
| Men | ||||
| Cumulative mortality rate (%) † | 3.0 | 1.1 | 11.6 | 27.5 |
| Rate ratio ‡ (95%CI) | 2.95 (1.79–4.87) | 1.00 | 0.38 (0.32–0.41) | 1.00 |
| Women | ||||
| Cumulative mortality rate (%) † | 1.9 | 0.8 | 5.3 | 11.6 |
| Rate ratio ‡ (95%CI) | 2.10 (1.56–2.82) | 1.00 | 0.42 (0.27–0.67) | 1.00 |
Analysis restricted to first 6 years of follow‐up to enhance comparability to Cancer Prevention Study II (CPS‐II) data. †Cumulative mortality rates between 40 and 84 years. ‡Estimated based on Poisson regression model.
The mean number of cigarettes smoked per day (Fig. 2a) decreased with age for men and women in both Japan and the USA. However, current smokers in Japan had a lower daily cigarette consumption for all age groups and for both men and women than current smokers in the USA. The differences ranged from 0.8 (aged 40–44 years) to 4.4 (aged 55–59 years) for men. Daily consumption of cigarettes in the youngest male age group showed the least difference. Japanese women constantly used approximately five fewer cigarettes per day in all age groups. The age‐adjusted number of cigarettes per day for the Japanese and US cohorts were 21.5 and 24.8 for men, respectively, and 14.1 and 19.4 for women, respectively.
Figure 2.
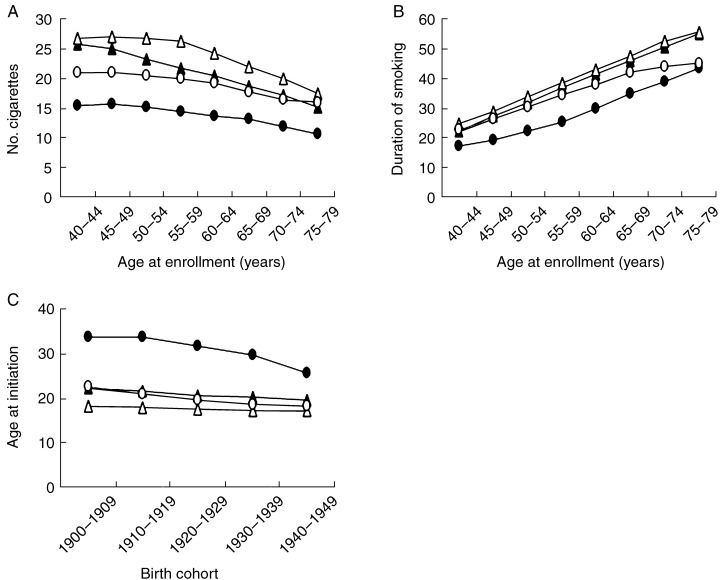
Comparison of smoking patterns of current smokers at baseline between Three‐Prefecture study in Japan and Cancer Prevention Study II (CPS‐II) in the US. (a), Mean number of cigarettes smoked per day by age at enrolment; (b), mean duration of smoking by age at enrolment; (C), mean age of initiation of smoking by birth cohort. (▴), Three‐Prefecture cohort men; (•), Three‐Prefecture cohort women; (▵), CPS‐II men; (○), CPS‐II women.
The mean number of years of smoking was slightly lower among Japanese men in all age groups than those in the USA (range 0.8–2.1) (Fig. 2b). Except for the youngest and oldest age groups, Japanese women had smoked for a much shorter time than comparable women in the USA. The range of differences was from 1.7 (aged 75–79 years) to 8.9 (aged 55–59 years). The age‐adjusted years of smoking for the Japanese and US smokers were 37.1 and 38.6 years for men, respectively, and 26.8 and 34.2 years for women, respectively.
Japanese smokers in all age groups started smoking later than their counterparts in the USA, and this was especially true for women (Fig. 2c). While the age at initiation of smoking for Japanese women gradually became younger in recent birth cohorts, they still began smoking much later than US women. The mean age at initiation of smoking among Japanese men in all birth cohorts was slightly older than those in the USA.
Finally, we calculated lung cancer death rates by years of smoking among current male smokers who had consumed 20 cigarettes per day (Table 4). Similar calculations for men who had smoked 40 cigarettes per day are not presented because there were too few of these men. We were unable to calculate lung cancer death rates in strata where no deaths occurred. For strata where calculations could be made, death rates of current Japanese smokers were lower than those in the USA. Rate ratios in all strata were less than 0.6. After controlling for age, duration of smoking and number of cigarettes smoked per day by the Poisson regression analysis, rate ratios of male Japanese current smokers relative to those in the USA was 0.34 (95%CI 0.27–0.43).
Table 4.
Death rates by duration of smoking among current male smokers of 20 cigarettes per day, Three‐Prefecture Study in Japan compared to the Cancer Prevention Study II in the USA
| Attained age (years) | Three‐Prefecture Duration* | CPS‐II Duration † | Rate ratio Duration † | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30–39 | 40–49 | 50 + | 30–39 | 40–49 | 50 + | 30–39 | 40–49 | 50 + | |
| 50–59 | 42.0 | — | — | 143.1 | 267.3 | 483.1 | 0.29 | — | — |
| 60–69 | 119.0 | 170.1 | — | 215.7 | 452.3 | 848.5 | 0.55 | 0.38 | — |
| 70–79 | 180.5 | 142.1 | 590.6 | 455.9 | 702.1 | 1149.0 | 0.40 | 0.20 | 0.51 |
Duration of smoking was fixed at enrollment. ––, no lung cancer deaths observed (Three‐Prefecture cohort study), or no data available because of five or fewer deaths observed (Cancer Prevention Study II).
Discussion
The present large‐scale, population‐based prospective study confirmed an increased lung cancer risk among smokers, as compared with non‐smokers, in Japan. The RR observed for Japanese smokers was lower than that observed in the USA. This finding is consistent with other studies conducted in Japan. 1 , 2 , 3 , 4 , 5 , 6 Comparison of death rates and exposure levels of current smokers in the two samples revealed one reason for the lower RR in Japan, namely, higher death rates among non‐smokers combined with lower death rates among smokers. A lower exposure level to smoking was responsible for the lower death rates among current smokers. However, even after adjustment for age, duration of smoking and daily cigarette consumption, male Japanese current smokers had a lower risk of lung cancer compared to those in the USA.
Death rates for non‐smokers in all Japanese age groups were higher than those for non‐smokers in the USA. The CPS‐II used more detailed questions regarding smoking habits. For example, the CPS‐II questionnaire clearly asked whether or not participants had smoked at least one cigarette per day for 1 year. (9) However, the questionnaire in our study did not specify the number of cigarettes or the duration of smoking. Therefore, the definition of non‐smokers in the CPS‐II was more strictly limited in terms of lifelong non‐smokers, while non‐smokers in our study might have included former smokers who had quit and not smoked for a long time. Such a difference in classification of non‐smokers might have led to overestimation of death rates among Japanese non‐smokers. Second‐hand smoking might also have contributed to the difference. The prevalence of current smokers among Japanese subjects was higher than in the CPS‐II. Among Japanese men, the prevalence was 58% for current smokers and 83% for ever smokers (ever smokers = current + former smokers); somewhat higher than the prevalence reported in the CPS‐II (24% for white, male current smokers, 75% for white, male ever smokers, 36% for black, male current smokers, and 73% for black, male ever smokers). (9) Therefore, Japanese non‐smokers might have had more opportunity to be exposed to environmental tobacco smoke (ETS). Furthermore, it was only in 2003 that Japanese law promoted the separation of smoking and non‐smoking areas at the workplace and in public places. As well, since Japanese residences are small, Japanese non‐smokers who had lived with parents or a spouse who smoked would have been exposed to concentrated tobacco carcinogens. Some, but not all, Japanese studies showed higher RR associated with spousal ETS, (11) and a pooled RR calculated from Japanese studies (1.41) was higher than the pooled RR calculated from US studies (1.19). (11) Therefore, until recently, Japanese non‐smokers would have had a much higher cumulative exposure to ETS at home and in the workplace than their US counterparts.
Other risk factors, such as air pollution, radon and asbestos, do not offer a clear explanation for the observed differences. Several observational studies have shown an association between air pollution levels and lung cancer. 12 , 13 Even if a difference in air pollution levels exists between the two countries, it is unlikely that this small difference could account for the large difference in the risk of lung cancer among non‐smokers given the only moderate association between air pollution and lung cancer. (14) The level of indoor radon in Japan, a known risk factor for lung cancer in Western countries (15) is much lower than in the USA. (16) Although asbestos consumption per capita was higher in Japan than in the USA during the mid‐1970s, (17) it remains unknown whether low environmental exposure to asbestos (in contrast to heavy occupational exposure) causes lung cancer. (18)
In contrast to non‐smokers, death rates among current smokers in our sample were lower than those observed in the CPS‐II sample, regardless of age and sex. Because lung cancer risk and exposure level to smoking are clearly dose‐related, the discrepancy in exposure levels among current smokers is probably a major factor explaining the difference in death rates among current smokers. However, considering lower exposure as a reason for the lower death rates among current smokers assumes that individuals with similar exposure levels have the same risk of lung cancer. However, the risk of lung cancer among male Japanese current smokers was lower than those in the USA, even after adjustment for age, duration of smoking and number of cigarettes smoked per day.
Although the difference in smoking patterns between the Japanese and US samples was greater among women than among men, the rate ratio for the current smokers was not very different between men and women. We have no clear explanation for this. However, the unit change in the lung cancer risk between Japanese female smokers and US female smokers with low levels of smoking exposure might not have the same magnitude as the unit change seen between Japanese male smokers and US male smokers with high levels of smoking exposure. Furthermore, Japanese women might under‐report their smoking history. A single inquiry about smoking at baseline might not reflect the whole smoking history of individuals in either the Japanese or US samples.
Caution is advised when exposure levels to smoking are assessed, based on self‐reported smoking history collected from a single questionnaire at the point of enrollment. Cigarette consumption per capita was much lower in Japan than in the USA from 1920 to 1970, (19) when the participants in these two cohorts were in adolescence to young adulthood. Furthermore, Japanese smokers experienced an extreme tobacco shortage during and immediately after World War II. It was not until the late 1970s that Japanese cigarette consumption per capita caught up with US consumption levels. Japanese participants classified in the same strata by smoking exposure undoubtedly experienced periods of cigarette shortage, and this bias toward overestimation of exposure may have produced spurious lower lung cancer death rates in our sample. Similarly, possible bias in the CPS‐II sample may have included smokers who underreported usage of cigarettes due to strong social prohibitions to smoking in the USA.
Changes in tar content and the prevalence of filter‐tipped cigarettes were also influential. The sales‐weighted average yields of tar in the 1980s, and the reduction in tar levels during the 1960s and 1970s were similar in Japan and the USA. 20 , 21 Filter‐tipped cigarettes were first marketed in the 1950s and their market share grew to more than 80% in the 1970s, reaching over 90% in both countries. However, as Stellmen et al. have noted, American manufactured cigarettes contain higher tobacco‐specific nitrosamines than Japanese cigarettes. (22) Furthermore, charcoal filters, which remove certain compounds that inhibit lung clearance, are more widely used in Japanese cigarettes than American cigarettes.
Causes of death, other than lung cancer, might be influential in the estimation of lung cancer death rates among current smokers. Coronary heart disease (CHD) was the second leading cause of death among CPS‐II smokers. (9) Premature death from CHD among CPS‐II smokers might have led to somewhat lower lung cancer death rates in the USA. An increase in the discrepancy of lung cancer death rates among current smokers might have occurred, because death rates from CHD in Japan are not as high as in the USA. (23)
Other confounding factors, such as lifestyle or genetic factors, might also lower lung cancer death rates among Japanese smokers. The traditional Japanese diet, which is low in fat and high in several phytochemicals, might help decrease the risk of death due to lung cancer. 24 , 25 , 26 , 27 Deletion‐type polymorphism CYP2A6, the principal enzyme in the metabolic activation of tobacco‐specific nitrosamines, was found to be inversely associated with lung cancer among Japanese male smokers. (28) It has been demonstrated that the frequency of occurrence of this variant is higher amongst Japanese than among Caucasians. (29) However, caution is required, because diet and the odds of having CYP2A6 can be assumed to be constants (i.e. would be equally likely to affect non‐smokers) and non‐smokers presented the opposite pattern to current smokers.
Another potential explanation is different histological distribution of lung cancer between American and Japanese populations. (30) Adenocarcinoma, which is less strongly related to smoking than squamous cell carcinoma, (2) contributes to a larger proportion of Japanese lung cancer than US lung cancers. The relatively lower incidence of squamous cell carcinoma among Japanese smokers would reduce the overall Japanese lung cancer incidence for the same level of exposure to smoking as in the US cohort.
Generally, in Western countries non‐smokers have a higher socioeconomic status than smokers. People with a high socioeconomic status tend to have more health conscious lifestyles, such as a higher intake of fruits and vegetables, as well as lower occupational exposures to other factors, such as asbestos. In the USA, the socioeconomic gap between smokers and non‐smokers is much larger due to a strong societal antismoking campaign. This larger disparity of background risk factors resulted in a larger difference of lung cancer mortality between US non‐smokers and smokers, as compared with Japanese non‐smokers and smokers.
Finally, the comparability of the Japanese and US samples should be considered. A potential advantage was that both studies were conducted using a prospective design during approximately the same time period. Dates of birth of participants covered approximately the same years. Because cigarette types, such as non‐filtered versus filtered cigarettes changed similarly in both the USA and Japan from the 1950s to the 1970s, (31) different study periods or birth cohorts might have weakened the comparability, especially in terms of exposure. In addition, lung cancer deaths were basically diagnosed by the same ICD‐9 codes. Lung cancer deaths were determined based on death certificates for the US sample, and the Japanese sample lung cancer deaths were determined using the cancer registry, which was based on death certificate data. Death certificates were usually considered complete both in the US and Japan. As well, the cause of death was also considered to have been identified with reasonable accuracy. In 1988, the percentage of deaths with no classifiable diagnosis, including unknown cause of morbidity and mortality (ICD‐9: 780–799) was 3.9% for Japan and 1.4% for USA. Therefore, both studies appeared to be equal in their precision of determining lung cancer deaths. Finally, follow‐up periods were restricted to 6 years in both studies. However, over this relatively short time interval, there were too few deaths among the Japanese cohort to produce stable and informative estimates of death rates, especially at high exposure levels. To solve this problem, further investigation with samples as large as the CPS‐II sample, or the pooling of several studies, are needed.
Acknowledgments
T. M. received a Research Resident Fellowship from the Foundation of Cancer Research (Japan) for the 3rd Term Comprehensive 10‐Year‐Strategy for Cancer Control.
References
- 1. Ando M, Wakai K, Seki N, Tamakoshi A, Suzuki K, Ito Y, Nishino Y, Kondo T, Watanabe Y, Ozasa K, Ohno Y. Attributable and absolute risk of lung cancer death by smoking status: findings from the Japan Collaborative Cohort Study. Int J Cancer 2003; 105: 249–54. [DOI] [PubMed] [Google Scholar]
- 2. Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle‐aged Japanese men and women. JPHC Study Int J Cancer 2002; 99: 245–51. [DOI] [PubMed] [Google Scholar]
- 3. Akiba S, Hirayama T. Cigarette smoking and cancer mortality risk in Japanese men and women − results from reanalysis of the six‐prefecture cohort study data. Environ Health Perspect 1990; 87: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sobue T, Suzuki T, Fujimoto I, Matsuda M, Doi O, Mori T, Furuse K, Fukuoka M, Yasumitsu T, Kuwahara O, Kono K, Taki T, Kuwabara M, Nakahara K, Endo S, Sawamura K, Kurata M, Ichitani M, Hattori S. Case‐control study for lung cancer and cigarette smoking in Osaka, Japan: comparison with the results from Western Europe. Jpn J Cancer Res 1994; 85: 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimizu H, Hisamichi S, Motomiya M, Oizumi K, Konno K, Hashimoto K, Nakada T. Risk of lung cancer by histologic type among smokers in Miyagi Prefecture. Jpn J Clin Oncol 1986; 16: 117–21. [DOI] [PubMed] [Google Scholar]
- 6. Wakai K, Ohno Y, Genka K, Ohmine K, Kawamura T, Tamakoshi A, Aoki R, Kojima M, Lin Y, Aoki K, Fukuma S. Smoking habits, local brand cigarettes and lung cancer risk in Okinawa, Japan. J Epidemiol 1997; 7: 99–105. [DOI] [PubMed] [Google Scholar]
- 7. Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW Jr. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst 1997; 89: 1580–6. [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF Jr. Smoking and cancer mortality among US veterans: a 26‐year follow‐up. Int J Cancer 1995; 60: 190–3. [PubMed] [Google Scholar]
- 9. Thun MJ, Day‐Lally C, Myers DG, Calle EE, Flanders WD, Zhu BP, Namboodiri MM, Heath CW. Trends in tobacco smoking and mortality from cigarette use in Cancer Prevention Studies I (1959 through 1965) and II (1982 through 1988). In: National Cancer Institute, Smoking and Tobacco Control, Monograph 8: Changes in Cigarette‐Related Disease Risks and their Implication for Prevention and Control. NIH Publication no. 97–4213. Washington D.C.: NIH, 1997;. 305–82. [Google Scholar]
- 10. Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994; 309: 901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Environmental Protection Agency. Respiratory health effects of passive smoking: lung cancer and other disorders. EPA/600/6–90/006F. Washington D.C.: EPA, 1992. [Google Scholar]
- 12. Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long‐term exposure to fine particulate air pollution. JAMA 2002; 287: 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr, Speizer FE. An association between air pollution and mortality in six US cities. N Engl J Med 1993; 329: 1753–9. [DOI] [PubMed] [Google Scholar]
- 14. Cohen AJ, Pope CA III. Lung cancer and air pollution. Environ Health Perspect 1995; 103 (Suppl. 8): 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12: 1341–51. [DOI] [PubMed] [Google Scholar]
- 16. Fujimoto K. Locality of indoor radon concentration. In: Radon and Thoron − Opportunities, Properties and Health Effects. Proceedings of the 27th NIRS Seminar on Environmental Research; 2–3 Dec 1999. Chiba: National Institute of Radiological Sciences, 2000, 39–45.
- 17. Takahashi KHM, Tossavanen A, Higashi T, Okubo T, Rantanen J. Ecological relationship between mesothelima incidence/mortality and asbestos consumption in ten Western countries and Japan. J Occup Health 1999; 41: 8–11. [Google Scholar]
- 18. Camus M, Siemiatycki J, Meek B. Non‐occupational exposure to chrysotile asbestos and the risk of lung cancer. N Engl J Med 1998; 338: 1565–71. [DOI] [PubMed] [Google Scholar]
- 19. Forey B HJ, Lee P. International Smoking Statistcs. A Collection of Histrogical Data from 30 Economically Developed Countries, 2nd edn. London: Oxford University Press, 2002. [Google Scholar]
- 20. Hoffmann D, Hoffmann I. The changing cigarette, 1950–95. J Toxicol Environ Health 1997; 50: 307–64. [DOI] [PubMed] [Google Scholar]
- 21. Sato H, Araki S. Yields and daily consumption of cigarettes in Japan in 1969–96. J Epidemiol 2000; 10: 7–15. [DOI] [PubMed] [Google Scholar]
- 22. Stellman SD, Takezaki T, Wang L, Chen Y, Citron ML, Djordjevic MV, Harlap S, Muscat JE, Neugut AI, Wynder EL, Ogawa H, Tajima K, Aoki K. Smoking and lung cancer risk in American and Japanese men: an international case‐control study. Cancer Epidemiol Biomarkers Prev 2001; 10: 1193–9. [PubMed] [Google Scholar]
- 23. Sekikawa A, Satoh T, Hayakawa T, Ueshima H, Kuller LH. Coronary heart disease mortality among men aged 35–44 years by prefecture in Japan in 1995–99 compared with that among white men aged 35–44 by state in the United States in 1995–98: vital statistics data in recent birth cohort. Jpn Circ J 2001; 65: 887–92. [DOI] [PubMed] [Google Scholar]
- 24. Ozasa K, Watanabe Y, Ito Y, Suzuki K, Tamakoshi A, Seki N, Nishino Y, Kondo T, Wakai K, Ando M, Ohno Y. Dietary habits and risk of lung cancer death in a large‐scale cohort study (JACC Study) in Japan by sex and smoking habit. Jpn J Cancer Res 2001; 92: 1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takezaki T, Hirose K, Inoue M, Hamajima N, Yatabe Y, Mitsudomi T, Sugiura T, Kuroishi T, Tajima K. Dietary factors and lung cancer risk in Japanese. with special reference to fish consumption and adenocarcinomas. Br J Cancer 2001; 84: 1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wakai K, Ohno Y, Genka K, Ohmine K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Aoki K, Fukuma S. Risk modification in lung cancer by a dietary intake of preserved foods and soyfoods: findings from a case‐control study in Okinawa. Japan Lung Cancer 1999; 25: 147–59. [DOI] [PubMed] [Google Scholar]
- 27. Takezaki T, Inoue M, Kataoka H, Ikeda S, Yoshida M, Ohashi Y, Tajima K, Tominaga S. Diet and lung cancer risk from a 14‐year population‐based prospective study in Japan: with special reference to fish consumption. Nutr Cancer 2003; 45: 160–7. [DOI] [PubMed] [Google Scholar]
- 28. Ariyoshi N, Miyamoto M, Umetsu Y, Kunitoh H, Dosaka‐Akita H, Sawamura Y, Yokota J, Nemoto N, Sato K, Kamataki T. Genetic polymorphism of CYP2A6 gene and tobacco‐induced lung cancer risk in male smokers. Cancer Epidemiol Biomarkers Prev 2002; 11: 890–4. [PubMed] [Google Scholar]
- 29. Miyamoto M, Umetsu Y, Dosaka‐Akita H, Sawamura Y, Yokota J, Kunitoh H, Nemoto N, Sato K, Ariyoshi N, Kamataki T. CYP2A6 gene deletion reduces susceptibility to lung cancer. Biochem Biophys Res Commun 1999; 261: 658–60. [DOI] [PubMed] [Google Scholar]
- 30. Yoshimi I, Ohshima A, Ajiki W, Tsukuma H, Sobue T. A comparison of trends in the incidence rate of lung cancer by histological type in the Osaka Cancer Registry, Japan and in the Surveillance, Epidemiology and End Results Program, USA. Jpn J Clin Oncol 2003; 33: 98–104. [DOI] [PubMed] [Google Scholar]
- 31. Wynder EL, Fujita Y, Harris RE, Hirayama T, Hiyama T. Comparative epidemiology of cancer between the United States and Japan. A second look. Cancer 1991; 67: 746–63. [DOI] [PubMed] [Google Scholar]


