Abstract
A total of 152 patients with asbestos‐related lung cancer recognized by the criteria of Japanese compensation law for asbestos‐related diseases were examined and compared with 431 patients with non‐asbestos‐related lung cancer. Male comprised 96% of patients. Ages ranged from 50 to 91 years with a median of 72 years. Eighty‐nine percent were smokers or ex‐smokers. Almost all patients had occupational histories of asbestos exposure. The median duration of asbestos exposure was 31 years and the median latency period was 47 years. Thirty‐four percent of patients exhibited asbestosis and 81% exhibited pleural plaques by radiography. Regarding asbestos particles in the lung for 73 operated or autopsied patients, 62% had more than 5,000 particles per gram. On the other hand, 100% of non‐asbestos‐related lung cancer patients had <5000 particles per gram with a median of 554 particles. The number of asbestos bodies in the lung, male gender, absence of symptoms, smoking index, and early stage of cancer were significantly much more than those of non‐asbestos‐related lung cancer. In this study, a diagnosis of asbestos‐related lung cancer was made in 34% of patients by asbestosis, in 62% by presence of both pleural plaques and more than 10 years’ occupational asbestos exposure, and in 4% by more than 5000 asbestos particles per gram of lung tissue. Occupational histories, duration of asbestos exposure, and pleural plaques are common categories for the recognition of asbestos‐related lung cancer in Japan.
(Cancer Sci 2010; 101: 1194–1198)
The disaster of asbestos exposure has been a serious social problem in Japan since 2005,( 1 ) with neighborhood exposure to asbestos inducing mesothelioma in more than 100 patients in the Amagasaki area. Furthermore, the number of patients with mesothelioma and asbestos‐related lung cancer (Fig. 1) has recently increased. In this study, clinical features and occupational histories for asbestos‐related lung cancer patients in Japan were investigated and compared with those of non‐asbestos‐related lung cancer patients.
Figure 1.
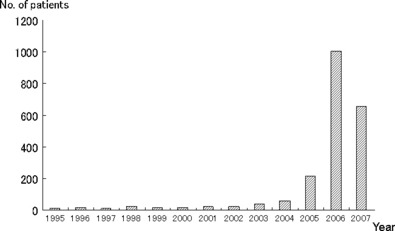
Number of asbestos‐related lung cancer in Japan from 1995 to 2007 was shown in this figure. The number of patients with asbestos‐related lung cancer has drastically increased after the 2005 Kubota Shock. (Data from statistics published by the Ministry of Health, Labor, and Welfare of Japan.)
Materials and Methods
In this study, the definition of asbestos‐related lung cancer was primary lung cancer with the following: (i) asbestosis on chest radiography; (ii) pleural plaques with more than 10 years’ occupational asbestos exposure; (iii) asbestos particles or fibers on the lung tissues with more than 10 years’ occupational asbestos exposure; and (iv) more than 5000 asbestos particles per gram of dry lung tissue with occupational asbestos exposure. These criteria fulfill the Japanese compensation law of asbestos‐related lung cancer.
Retrospective study of asbestos‐related lung cancer patients from 2000 to 2008 treated in 18 Rosai hospitals throughout Japan was performed. Gender, age, diagnostic motive, smoking history, histological type of lung cancer, clinical stage, therapeutic procedures and prognosis, occupational history, and radiological findings of asbestos‐related changes were examined.
Non‐asbestos‐related lung cancer patients treated in Okayama Rosai hospital from 1997 to 2007( 2 ) were also examined for gender, age, smoking history, histological type of lung cancer, clinical stage, and therapeutic procedures and prognosis. Non‐asbestos‐related lung cancer does not fulfill the criteria for the Japanese compensation law of asbestos‐related lung cancer. The findings of asbestos‐related changes such as pleural plaques were examined by chest X‐ray and chest computed tomography (CT) (including high resolution computed tomography (HRCT)) for all patients with asbestos‐related lung cancer and non‐asbestos‐related lung cancer. Prognosis of asbestos‐related lung cancer was calculated by the complication of asbestosis. Prognostic factors in both asbestos‐related and non‐asbestos‐related lung cancers were calculated by multivariate analysis.
The number of asbestos particles was counted for the operated or autopsied patients (73 patients with asbestos‐related and 23 with non‐asbestos‐related lung cancers). The number of asbestos particles in the lung was counted by the method of Kohyama.( 3 ) One to 2g of lung tissue without cancer invasion was dissolved in sodium hypochlolite and 5% potassium hydroxide (KOH) for 12 h, and complete dissolution of the lung tissue was confirmed. The supernatant was discarded, the sediment was dissolved with 10 mL of chloroform and 50% ethanol, and the solution was centrifuged at 18G for 5 min. The supernatant was discarded, the sediment was dissolved with 95% ethanol, the solution was passed through a Millipore filter, and the asbestos particles on the filter were counted under phase‐contrasted microscope at ×200 magnification. The number of asbestos particles per gram of dry lung tissue was then calculated. The data were statistically analyzed, using Student’s t‐test and P < 0.05 was regarded as statistically significant.
Results
A total of 152 patients with asbestos‐related lung cancer were examined in this study. Regarding gender, 146 were male and six were female. Ages ranged from 50 to 91 years with a median of 72 years. Sixty‐four patients were diagnosed by the chief complaints of dyspnea, cough, etc. Fifty‐nine patients were diagnosed by regular health check‐up and another 20 patients were accidentally diagnosed during the following of other diseases. For nine patients there was no information. Only 15 patients (10%) were non‐smokers and another 134 were smokers or ex‐smokers. The smoking index for 149 patients ranged from 0 to 2550 with a median of 900. The smoking index for 71 patients exceeded 1000. The smoking history of three patients was unknown (Table 1).
Table 1.
Characteristics of asbestos‐related and non‐asbestos‐related lung cancers
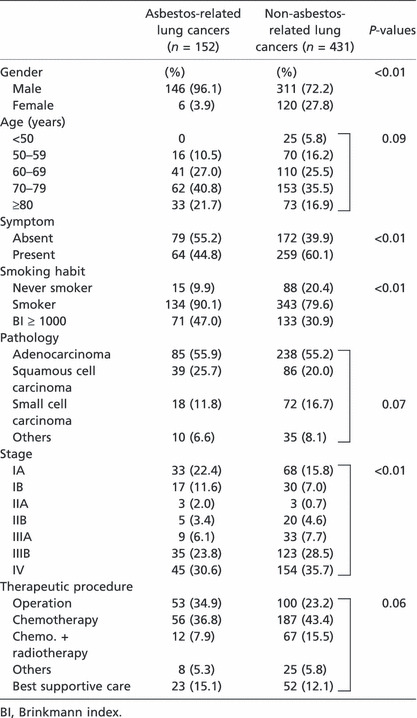
Regarding the histology of 143 patients with asbestos‐related lung cancer, 85 exhibited adenocarcinoma, 39 had squamous‐cell type, 18 had small‐cell type, and one had large‐cell type. The histological types of nine patients were not determined. The features of non‐asbestos‐related lung cancer are described in Table 1. Rates of male gender, being symptom free, smoking, and early stage disease for asbestos‐related lung cancer are significantly (P > 0.01) higher than those of non‐asbestos‐related lung cancer.
The survival term was overall 17.4 months with 57.0% having 1 year‐survival and 25% having 5 year‐survival. On the other hand, the survival term for 431 patients with non‐asbestos‐related lung cancer was 19.2 months with 70.1% having 1 year‐survival and 24.5% of having year‐survival. The difference in survival term between the two groups was not statistically significant (Fig. 2). Three patients with asbestos‐related lung cancer died within 3 months of surgery and another four patients died from respiratory failure due to advanced asbestosis. Two of three patients with asbestosis were did not have good survival after surgery because of acute exacerbation of asbestosis. However, the survival of 51 patients with asbestosis was 17.2 months and that of 101 patients without asbestosis was 18.1 months; there was no statistical significance in either group. Prognostic factors calculated by multivariate analysis in both groups, included age, gender, and stages, but not asbestos exposure or pathology (Table 2).
Figure 2.
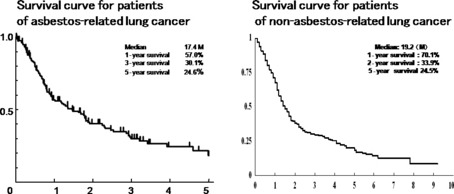
Survival curves for asbestos‐related lung cancer and non‐asbestos‐related lung cancer show almost the same pattern which indicates almost the same rates of survival between these two types of lung cancer.
Table 2.
Univariate and multivariate analysis for the prognosis of asbestos and non‐asbestos‐related lung cancer
| Univariate analysis | ||||
|---|---|---|---|---|
| Factors | n | MST (95% CI) | Log‐rank test | |
| Asbestos | Related | 152 | 16.2 months (8.3–24.1) | P = 0.673 |
| Non‐related | 431 | 17.2 months (15.1–19.3) | ||
| Age (years) | ≤70 | 278 | 20.8 months (15.9–25.7) | P < 0.001 |
| 71+ | 305 | 14.1 months (11.7–16.5) | ||
| Gender | Male | 457 | 15.4 months (13.7–17.1) | P < 0.001 |
| Female | 126 | 24.5 months (17.8–31.2) | ||
| Pathology | NSCLC | 493 | 18.1 months (15.4–20.8) | P = 0.001 |
| SCLC | 90 | 13.4 months (11.1–15.7) | ||
| Stage | I–II | 179 | 21.5 months (17.7–25.3) | P < 0.001 |
| III–IV | 399 | 13.4 months (11.1–15.7) | ||
| Multivariate analysis | |||
|---|---|---|---|
| Factors | Exp (β) | 95% CI | P‐values |
| Asbestos | 1.051 | 0.816–1.353 | 0.699 |
| Age | 1.625 | 1.312–2.013 | <0.001 |
| Gender | 1.686 | 1.255–2.273 | 0.001 |
| Pathology | 1.290 | 0.973–1.710 | 0.077 |
| Stage | 1.945 | 1.548–2.443 | <0.001 |
CI, confidence interval; MST, median survival term; NSCLC, non‐small‐cell lung cancer; SCLC, small‐cell lung cancer.
Regarding therapy, 53 patients underwent surgery; 56 received chemotherapy, with nine receiving a combination of surgery and chemotherapy; 16 received radiation, with 12 receiving a combination of chemotherapy and radiation; and 23 received the best available supportive care. These numbers resemble those for non‐asbestos‐related lung cancer. Survival for patients who received surgery was 55.1 months with 45% having 5‐year survival; and for radiation, chemotherapy, and best supportive care, survival was 9.3 months, 10.3 months, and 7.0 months, respectively.
One hundred and fifty (98%) of 152 patients whose occupational histories were ascertained had occupational exposure to asbestos. For another two patients with more than 5000 asbestos particles in the lung, occupational histories were not confirmed. Thirty‐four patients had occupational histories of shipyard work, 29 had construction work, 15 had exposure due to making asbestos products, 15 had piping works, and 14 had insulation work, with the remainder also having been employed in asbestos‐related work (Table 3).
Table 3.
Occupational histories of asbestos‐related lung cancer patients
| Area of occupation | n |
|---|---|
| Shipbuilding | 34 |
| Construction | 29 |
| Asbestos product making | 15 |
| Piping | 15 |
| Insulation | 14 |
| Electrician | 8 |
| Chemicals | 6 |
| Arc welding | 5 |
| Transportation | 4 |
| Steel company | 4 |
| Asbestos spraying | 3 |
| Fire bricklaying | 3 |
| Automobile making | 3 |
| Metal making | 2 |
| Furnace making | 2 |
| Warehousing | 1 |
| Casting | 1 |
| Other | 1 |
Age at exposure to asbestos for the first time ranged from 14 to 50 years with a median of 21 years. The duration of asbestos exposure for 146 patients ranged from 1 to 60 years with a median of 31 years, and the latency period from first exposure to the appearance of lung cancer ranged from 5 to 71 years with a median of 47 years.
Regarding the radiographical findings of asbestos‐related changes, only 51 patients (34%) exhibited asbestosis; 122 (81%) exhibited pleural plaques and 100 (66%) showed calcified plaques. Seven patients exhibited rounded atelectasis and four diffuse pleural thickening. Only 33 patients (22%) had complicated pleural effusion (Table 4). Thirty‐two patients with asbestosis were exposed to asbestos due to work in asbestos product making, insulation, and asbestos spraying. And other 19 patients were exposed to asbestos due to work in shipyards and construction work and work with piping. Ninety‐four patients (62%) with asbestos‐related lung cancer were diagnosed by the presence of pleural plaques and had more than 10 years’ occupational asbestos exposure (Fig 4). On the other hand, 10 patients with non‐asbestos‐related lung cancer showed pleural plaques, but no other findings such as asbestosis or diffuse pleural thickening.
Table 4.
Radiological findings in asbestos‐related diseases
| Findings | n (%) |
|---|---|
| Asbestosis | 51 (34.0) |
| Pleural plaques | 122 (81.3) |
| Calcified PQ | 81 (73.6) |
| Rounded atelectasis | 7 (4.7) |
| Diffuse pleural thickening | 4 (2.7) |
| Pleural effusion | 33 (22.3) |
PQ, pleural plaque.
Figure 4.
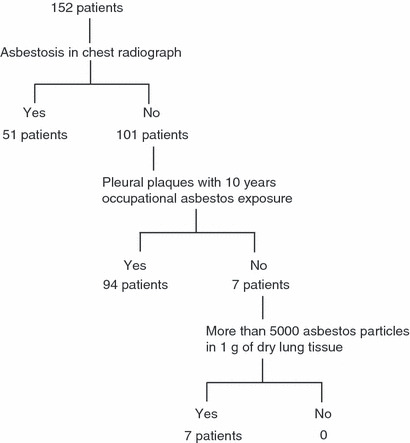
Flow chart of asbestos‐related lung cancer diagnoses.
As for the number of asbestos particles in the lung in 73 patients, 45 (62%) had more than 5000 asbestos bodies per gram of dry lung tissue which meant they had an occupational history of asbestos exposure. Furthermore, 21 (29%) exceeded 50 000 particles which meant heavy exposure. Seven (4%) were diagnosed with more than 5000 asbestos particles per gram of lung tissue (Fig. 4). However, 14 (19%) had <1000 asbestos particles which indicated the non‐exposed citizen level. These 14 patients had pleural plaques with more than 10 years’ occupational asbestos exposure and were diagnosed with asbestos‐related lung cancer.
Among 18 asbestosis patients, 10 (56%) exceeded 50 000 particles, but two patients had <5000 particles. On the other hand, for 55 patients without asbestosis, 14 had <1000 particles and 11 (20%) exceeded 50 000 particles (Fig. 3). Twenty‐three patients with non‐asbestos‐related lung cancer had 0 to 3751 asbestos particles per gram of lung tissue with a median of 554 particles.
Figure 3.
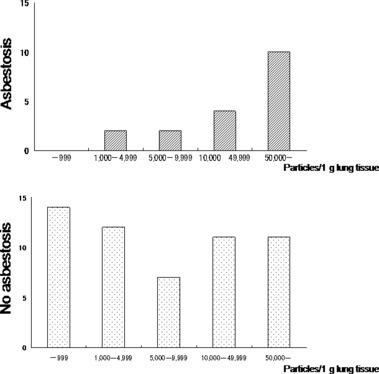
Number of asbestos particles per gram of lung tissue for asbestosis and non‐asbestosis by chest X‐ray. For asbestosis, two patients (11.1%) had <5000 asbestos particles; 14 (77.8%) had more than 10 000 particles; while 10 had more than 50 000 particles (55.6%) which means heavy asbestos exposure. On the other hand, for non‐asbestosis, 31 patients had more than 5000 particles and 14 had <1000 particles. For the patients with non‐asbestosis, no particular pattern in number of asbestos particles could be observed.
Discussion
Asbestos is known to be carcinogenic for malignant mesothelioma and lung cancer. It has been established that exposure to asbestos can induce malignant mesothelioma. Regarding the onset of primary lung cancer, the involvement of smoking has been emphasized.( 4 ) Asbestos enhances the mutagenicity of tobacco carcinogen and it acts independently to tissue damage responsible for fibrosis, that is asbestosis.( 5 ) High incidences of lung cancer among individuals exposed to asbestos have been demonstrated by various cohort studies.( 3 ) The present study was undertaken to characterize primary lung cancer observed in asbestos‐exposed individuals in Japan and to examine these patients for the presence or absence of concomitant lung lesions such as asbestosis and pleural plaques. While no definition of asbestos‐related lung cancer has been established, Helsinki criteria( 6 ) indicates that having 25 asbestos fiber‐years doubles the risk of lung cancer.
The present study adopted the criteria of asbestos‐related lung cancer defined by the Japanese compensation law of asbestos‐related diseases in 2006. A total of 152 patients with asbestos‐related lung cancer were examined and the median age was 72 years which was 7 years older than that of a group with Japanese malignant pleural mesothelioma described in 2004.( 7 ) Ninety‐six percent of them were males who had occupational histories of asbestos exposure, and the median duration of asbestos exposure was 31 years. Forty‐two percent were diagnosed by subjective complaints, but another 68% were diagnosed during regular check‐ups or accidentally diagnosed due to abnormal chest shadows without subjective complaints. This data seems to be due to the Japanese system of having regular check‐ups for lung cancer, because 60% of patients with non‐asbestos‐related lung cancer were diagnosed by subjective complaints.
Regarding smoking, only 15 patients (10%) were non‐smokers and 134 were smokers or ex‐smokers. Seventy‐one patients (47%) exceeded the smoking index of 1000 which meant they were heavy smokers. Lung cancer can occur in nonsmokers exposed to asbestos; however, the risk is magnified several‐fold by smoking,( 8 ) and increased risk for lung cancer remains up to 20 years after cessation of smoking.( 9 ) Forty‐seven percent of our patients were heavy smokers whose lung cancer was suggested to be related not only to asbestos exposure but also to smoking. Ex‐smokers stopped smoking within 20 years of the appearance of lung cancer.
Histological classification of asbestos‐related lung cancer indicated that 59% had adenocarcinoma and 27% had squamous cell type, which is a similar pattern to that of non‐asbestos‐related lung cancer (control group) in Japan.
Overall survival of 152 patients with asbestos‐related lung cancer was 17.4 months with 25% having 5 year‐survival. This data is similar that of the control group which had an overall survival of 19.2 months with 25% having 5‐year survival. As for therapeutic procedures, the survival for patients who underwent surgery was 55.1 months with a 5‐year survival rate of 45% and that of chemotherapy was 10.3 months. Lung cancer which shows limited small areas of ground glass opacity by CT scanning is typically early stage; therefore, the survival of this type is better after surgery. These data showed that therapeutic procedures for asbestos‐related lung cancer and survival were also similar to controls. Some patients with early stage experienced the exacerbation of asbestosis after surgery. But the presence of asbestosis did not affect the survival of those with asbestos‐related lung cancer. Prognostic factors of survival for asbestos‐related and non‐asbestos‐related lung cancers by multivariate analysis proved not to be asbestos exposure or pathology, but rather age, gender, and stage.
As for occupational history, shipyard workers, asbestos product makers, piping workers, and insulation workers mainly comprised asbestos‐related lung cancer patients, which was similar to that for malignant mesothelioma as described previously). Thirty‐four patients who were shipyard workers with more than 10‐year occupational histories were considered as proof of moderate density of exposure to asbestos. Insulation workers, asbestos product makers, and piping workers were considered to have had heavy exposure to asbestos. The term of exposure to asbestos ranged from 1 to 60 years with a median of 31 years. Workers who were suggested to have had heavy exposure to asbestos through insulation or asbestos spraying tended to have had short‐term exposure, and construction workers thought to have had lower levels of exposure tended to have had longer histories of asbestos exposure. The latency period had a median of 47 years which is longer than that of malignant mesothelioma (37 years)( 6 ) and that of asbestos‐related‐lung cancer patients (43 years) who were treated in several Rosai Hospitals from 1995 to 2000.( 10 )
Regarding the radiological findings for asbestos‐related changes, 34% of patients had complicated asbestosis, but 81% had pleural plaques which occurred even due to low density of asbestos exposure, which was about same percent as Bianchi’s data.( 11 ) Sixty‐two percent of patients were diagnosed as having asbestos‐related lung cancer on the evidence of both pleural plaques and 10 years’ occupational asbestos exposure. Ten patients with non‐asbestos‐related lung cancer also exhibited pleural plaques, but no evidence of enough asbestos particles or occupational asbestos exposure. Therefore, these patients were not diagnosed as having asbestos‐related lung cancer.
Regarding asbestos particles in the lung tissues of asbestos‐related lung cancer patients, 61% exceeded 5000 particles per gram of dry lung tissue, but 19% had <1000 particles which corresponded to the citizen’s level of exposure. These patients with pleural plaques on chest X‐ray were exposed to low‐density asbestos for more than 10 years. More than 5000 asbestos particles per gram of lung tissue corresponds with 25 fiber‐years, and is consistent with a doubling of the lung cancer risk.( 6 , 12 ) Bianchi et al. ( 11 ) reported that 31% of 414 necropsy cases of lung cancer exceeded 5000 particles per gram of dry lung cancer. Our data is double this data which suggests denser exposure to asbestos.
However, 11 patients without asbestosis had more than 50 000 particles and two who were construction workers with asbestosis had <5000 particles. Construction workers who ordinarily had treated chrysotile asbestos tended to have less asbestos particles in the lung than insulation or piping workers. We should examine asbestos fibers for these two patients by electron microscopy. On the other hand, the carcinogenicity and fibrogenicity of asbestos has been described as not always being correlated. Fischer suggested that 42% of asbestosis on chest radiograph had fewer asbestos particles than 25 fiber‐year occupational histories.( 13 ) Our result of asbestos particles in the lung and radiographic asbestosis corresponds with his data. Lung cancer risk was elevated in the presence of radiographic asbestosis, but occurred as a result of asbestos exposure in the absence of asbestosis. The incidence of non‐small‐cell lung cancer for patients with asbestosis increased, compared with asbestos‐exposed patients without asbestosis.( 14 ) Lung cancer risk increased almost linearly with cumulative dose of asbestos.( 15 ) It is still controversial that lung cancer in the absence of asbestosis can be attributed to asbestos exposure. We should extend the number of the patients for asbestos‐related lung cancer and clarify the criteria for the diagnosis of asbestos‐related lung cancer without asbestosis.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by research and development and dissemination projects related to the 13 fields of occupational injuries and illnesses by the Japan Labor Health and Welfare Organization.
References
- 1. Kurumatani N, Kumagai S. Mapping risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med 2008; 178: 624–9. [DOI] [PubMed] [Google Scholar]
- 2. Nishi H, Washio K, Fujimoto N et al. Evaluation of asbestos‐related lung cancer. JJLC 2009; 49: 167–73. (In Japanese). [Google Scholar]
- 3. Kohyama N. Asbestos bodies and fibers in medical findings for asbestos exposure. In: Morinaga K, ed. Asbestos Exposure and Asbestos‐related Diseases. Tokyo: Sanshintosho, 2008; 69–87. (In Japanese). [Google Scholar]
- 4. Hammond EC, Selikoff IJ, Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci 1979; 330: 473–90. [DOI] [PubMed] [Google Scholar]
- 5. Nelson HH, Kersey KT. The molecular epidemiology of asbestos and tobacco in lung cancer. Oncogene 2002; 21: 7284–8. [DOI] [PubMed] [Google Scholar]
- 6. Consensus Report . Asbestos, asbestosis and cancer. The Helsinki criteria for diagnosis and attribution. Scand J Work Environ Health 1997; 23: 311–6. [PubMed] [Google Scholar]
- 7. Kishimoto T, Ozaki S, Kato K et al. Malignant pleural mesothelioma in parts of Japan to relationship to asbestos exposure. Ind Health 2004; 42: 435–9. [DOI] [PubMed] [Google Scholar]
- 8. Berry G, Liddleli FK. The interaction of asbestos and smoking in lung cancer: a modified measure of effect. Ann Occup Hyg 2004; 48: 459–62. [DOI] [PubMed] [Google Scholar]
- 9. Reid A, De Klerk NH, Ambrosini GL et al. The risk of lung cancer with increasing time since ceasing exposure to asbestos and quitting smoking. Occup Environ Med 2006; 63: 509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kishimoto T, Ohnishi K, Saito Y. Clinical study of asbestos‐related lung cancer. Ind Health 2003; 41: 94–100. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi C, Brollo A, Ramani L, Zuch C. Asbestos exposure in lung carcinoma: a necropsy‐based study of 414 cases. Am J Ind Med 1999; 36: 360–4. [DOI] [PubMed] [Google Scholar]
- 12. Pahlabeln H, Wild P, Schill W et al. Asbestos fibre‐years and lung cancer: a two phase case‐control study with expert exposure assessment. Occup Environ Med 2002; 59: 410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisher M, Gunther S, Muller KM. Fibre‐years, pulmonary asbestos burden and asbestosis. Int J Hyg Environ Health 2002; 205: 245–8. [DOI] [PubMed] [Google Scholar]
- 14. Van Loon AJ, Kant IJ, Swaen GM et al. Occupational exposure to carcinogen and risk of lung cancer: result from The Netherlands cohort study. Occup Environ Med 1997; 54: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustavsson P, Nyberg F, Pershagen G et al. Low‐dose exposure to asbestos and lung cancer: dose‐response relations and interaction with smoking in a population‐based case‐referent study in Stockholm, Sweden. Am J Epidemiol 2002; 155: 1016–22. [DOI] [PubMed] [Google Scholar]


