Abstract
Regenerating islet‐derived family, member 4 (REG4, which encodes Reg IV) is a candidate marker for cancer and inflammatory bowel disease. We investigated the potential prognostic role of Reg IV immunostaining in clinically localized prostate cancer (PCa) after radical prostatectomy. Immunohistochemical staining of Reg IV was performed in 98 clinically localized PCa tumors obtained during curative radical prostatectomy. Intestinal and neuroendocrine differentiation was investigated by MUC2 and chromogranin A immunostaining, respectively. The prognostic significance of immunohistochemical staining for these factors on prostate‐specific antigen (PSA)‐associated recurrence was assessed by Kaplan–Meier analysis and a Cox regression model. Phosphorylation of the epidermal growth factor receptor (EGFR) by Reg IV was analyzed by Western blot. In total, 14 (14%) of the 98 PCa cases were positive for Reg IV staining. Reg IV positivity was observed frequently in association with MUC2 (P = 0.0182) and chromogranin A positivity (P = 0.0012). Univariate analysis revealed that Reg IV staining (P = 0.0004), chromogranin A staining (P = 0.0494), Gleason score (P < 0.0001) and preoperative PSA concentration (P = 0.0167) were significant prognostic factors for relapse‐free survival. Multivariate analysis indicated that Reg IV staining (P = 0.0312), Gleason score (P = 0.0014) and preoperative PSA concentration (P = 0.0357) were independent predictors of relapse‐free survival. In the LNCaP cell line, EGFR phosphorylation was induced by the addition of Reg IV‐conditioned medium. These results suggest that Reg IV expression is an independent prognostic indicator of relapse after radical prostatectomy. (Cancer Sci 2008; 99: 1570–1577)
Prostate cancer (PCa) is one of the most common cancers and the second leading cause of cancer death in men in the USA.( 1 ) PCa screening by assessing serum prostate‐specific antigen (PSA) level has led to increased detection of early stage PCa that can be cured by radical prostatectomy or radiation therapy. Nonetheless, a substantial fraction of patients with clinically localized PCa who undergo curative radical prostatectomy will eventually recur with metastatic disease. These patients would benefit most from the discovery of a prognostic factor that can identify individuals for whom adjuvant therapy would be advantageous. Treatment decisions are based mainly on known prognostic factors. High risk of relapse is defined according to preoperative PSA level (>20 ng/mL), biopsy Gleason score (≥8), and the 1992 American Joint Committee on Cancer (AJCC) clinical T stage (≥T2c).( 2 ) These factors are helpful but far from perfect due to significant clinical heterogeneity. Clearly, new biological markers are needed to accurately predict the risk of relapse.
We previously performed serial analysis of gene expression of four primary gastric cancers,( 3 ) and identified several gastric cancer‐specific genes.( 4 ) Of these genes, regenerating islet‐derived family, member 4 (REG4, which encodes Reg IV) is a candidate gene for cancer‐specific expression, at least in patients with gastric cancer. Although various normal tissues express REG4, the levels of expression are much lower in normal tissues than in cancerous tissues.( 3 ) Our previous immunohistochemical analysis revealed that Reg IV was expressed in 30% of gastric cancers and was associated with both intestinal mucin phenotype and neuroendocrine differentiation.( 5 ) Reg IV is a secreted protein, and we also showed that serum Reg IV represents a novel biomarker for gastric cancer.( 6 )
Understanding of the genetic and epigenetic pathways involved in the pathogenesis of PCa is essential for the development of improved diagnostic and treatment modalities. A variety of genetic and epigenetic alterations is associated with PCa.( 7 , 8 ) In addition to gastric, colorectal,( 9 ) and pancreatic( 10 ) cancers, overexpression of REG4 mRNA in PCa has been reported by in situ hybridization.( 11 ) The majority of localized PCa tumors expressed a low level of REG4 mRNA, whereas the majority of metastatic PCa tumors expressed a high level of REG4 mRNA. Although the biological function of Reg IV is poorly understood, it has been reported that Reg IV is a potent activator of the epidermal growth factor receptor (EGFR)/Akt/activator protein‐1 (AP‐1) signaling pathway in colon cancer cells and increases expression of Bcl‐2, Bcl‐xl and surviving, proteins associated with the inhibition of apoptosis.( 12 ) We have also reported that forced expression of Reg IV induces phosphorylation of the EGFR and inhibits 5‐fluorouracil‐induced apoptosis in gastric cancer cells.( 6 ) Taken together, these findings suggest that Reg IV may also participate in tumor cell growth in PCa and may be a new prognostic marker for relapse in patients with PCa. However, the expression and distribution of Reg IV protein and the biological significance of Reg IV in PCa has not been investigated.
In the present retrospective study, we examined the expression and distribution of Reg IV in 98 clinically localized PCa tumors by immunohistochemistry. The relation between staining for Reg IV and clinicopathological characteristics was also examined. We have reported two Reg IV staining patterns (mucin‐like staining and perinuclear staining).( 5 ) Mucin‐like staining, observed in goblet cells and goblet cell‐like vesicles of tumor cells, is associated with MUC2 (a marker of goblet cells) positivity. Perinuclear staining is detected in cells with neuroendocrine differentiation and that show chromogranin A (a marker of neuroendocrine cells) positivity. Therefore, we examined staining for Reg IV and chromogranin A or MUC2 by double‐immunofluorescence. Because Reg IV activates the EGFR, we also performed an immunohistochemical analysis of Reg IV and EGFR expression.
Materials and Methods
Tissue samples. Ninety‐eight primary tumors were collected from patients diagnosed with PCa who underwent surgery during 2000–2002 at the Department of Urology, Hiroshima University Hospital (Hiroshima, Japan). All 98 patients were treated by radical prostatectomy and bilateral lymphadenectomy for clinically localized PCa and were confirmed to be node negative by pathological examination. None of the patients were treated preoperatively with hormonal or radiation therapy, and none had secondary cancer. All 98 specimens were archival, formalin‐fixed, paraffin‐embedded tissues. Tumor staging was performed according to the AJCC classification system. After prostatectomy, the serum PSA level was measured by E‐test Tosoh II Assay (Tosoh, Tokyo, Japan). Patients were followed up by PSA measurement monthly during the first 6 months after prostatectomy and then every 3 months thereafter. Biochemical relapse was defined as a PSA level of 0.2 ng/mL or greater. Because written informed consent was not obtained, for strict privacy protection, identifying information for all samples was removed before analysis. This procedure was in accordance with the Ethical Guidelines for Human Genome/Gene Research of the Japanese Government.
Immunohistochemistry. Formalin‐fixed, paraffin‐embedded samples were sectioned, deparaffinized and stained with hematoxylin–eosin to ensure that the sectioned block contained tumor cells. Adjacent sections were then stained immunohistochemically. Antigen retrieval was performed by microwave heating in citrate buffer (pH 6.0) for 30 min for Reg IV, MUC2, chromogranin A, EGFR, phospho‐EGFR (Tyr1068) and transforming growth factor (TGF)‐α. Peroxidase activity was blocked with 3% H2O2‐methanol for 10 min, and sections were then incubated with normal goat serum (Dako Cytomation, Carpinteria, CA, USA) for 20 min to block nonspecific antibody binding. Sections were incubated with primary antibody against Reg IV (rabbit polyclonal antibody, diluted 1:50; anti‐Reg IV antibody was raised in our laboratory),( 5 ) MUC2 (1:50; Novocastra, Newcastle, UK), chromogranin A (1:50; Novocastra), EGFR (1:50; Dako Cytomation), phospho‐EGFR (Tyr1068) (1:50; Cell Signaling Technology, Beverly, MA, USA) or TGF‐α (Calbiochem, San Diego, CA, USA) for 1 h at room temperature, followed by incubation with peroxidase‐labeled antirabbit or antimouse IgG for 1 h. Staining was completed with a 10‐min incubation in substrate–chromogen solution. The sections were counterstained with 0.1% hematoxylin. The specificity of the Reg IV antibody has been characterized in detail elsewhere.( 5 )
Double‐immunofluorescence staining was performed as described previously.( 5 ) Alexa Fluor 546‐conjugated antirabbit immunoglobulin (Ig)G (Molecular Probes, Eugene, OR, USA) and Alexa Fluor 488‐conjugated antimouse IgG (Molecular Probes) were used as secondary antibodies.
The specificity of immunohistochemical detection for Reg IV was verified by triple‐immunofluorescence staining with different antibodies against Reg IV (goat polyclonal, rabbit polyclonal and mouse monoclonal). Goat polyclonal and mouse monoclonal anti‐Reg IV antibodies were purchased from R&D Systems (Abingdon, UK). Alexa Fluor 405‐conjugated antigoat IgG (Molecular Probes), Alexa Fluor 488‐conjugated antimouse IgG (Molecular Probes) and Alexa Fluor 546‐conjugated antirabbit IgG (Molecular Probes) were used as secondary antibodies.
Cell culture and conditioned medium production. Colon cancer cell line, colo320, and human prostate cell line, LNCaP, were maintained in RPMI‐1640 medium (Nissui Pharmaceutical, Tokyo, Japan) containing 10% fetal bovine serum (FBS) (Whittaker, Walkersville, MD, USA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Reg IV‐conditioned medium (Reg IV‐CM) and colo320 control medium were prepared as follows. Colo320 cells stably transfected with a Reg IV cDNA were grown to 80–90% confluence in RPMI‐1640 medium containing 10% FBS. The medium was removed and the cells were washed twice with phosphate‐buffered saline (PBS). Cells were incubated for 48 h in 20 mL serum‐free RPMI‐1640 medium. After 48 h, the medium was collected and filtered (0.22‐µm pores; Becton Dickinson Labware, Bedford, MA, USA). Control medium from colo320 cells stably transfected with the pcDNA3.1 vector alone was prepared under the same conditions. Conditioned medium was then normalized for DNA content between samples by adding RPMI‐1640 medium. Levels of EGF and TGF‐α in Reg IV‐CM and control medium were assessed by sandwich‐type enzyme‐linked immunosorbent assay (ELISA) (R&D Systems).
Western blot analysis. Western blot analysis was performed as described previously.( 13 ) To examine whether Reg IV activates phosphorylation of the EGFR, cells were serum starved for 24 h and treated with Reg IV‐CM or control medium for 3 min. To examine whether EGF or TGF‐α induce Reg IV expression, cells were serum starved for 24 h and treated with EGF (100 nM, Sigma, Saint Louis, MO, USA) or TGF‐α (10 nM, Sigma) for 1, 2 and 3 days. Cells were scraped in PBS supplemented with 1 mM Na3VO4, centrifuged, and lysed in ice‐cold RIPA buffer (20 mM Tris‐HCl [pH 7.5], 0.15 M NaCl, 1% Triton X‐100, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 1 mM ethyleneglycotetraacetic acid [EGTA], 1 µg/mL leupeptin, 2 µg/mL aprotinin and 10 µg/mL pepstatin). Supernatant protein concentration was measured with a the Bio‐Rad Protein Assay kit (Bio‐Rad, Hercules, CA, USA). Protein (20 µg/lane) was electrophoresed on sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS‐PAGE) gels and transferred to nitrocellulose filters. Filters were incubated for 1 h at room temperature with anti‐Reg IV antibody (rabbit polyclonal antibody raised in our laboratory),( 5 ) anti‐EGFR antibody (Cell Signaling Technology), antiphospho‐EGFR (Tyr992) antibody (Cell Signaling Technology) or antiphospho‐EGFR (Tyr1068) antibody (Cell Signaling Technology).
Statistical methods. Association between clinicopathological variables and Reg IV expression was analyzed by Fisher's exact test. Kaplan–Meier survival curves were constructed for Reg IV‐positive and Reg IV‐negative patients. Survival rates were compared between Reg IV‐positive and Reg IV‐negative groups. Differences in survival between groups were tested for statistical significance by log–rank test.( 14 ) The Cox proportional hazards multivariate model was used to examine the association of clinical and pathological factors and Reg IV and chromogranin A staining with survival. P < 0.05 was considered statistically significant.
Results
Immunohistochemical analysis of Reg IV in PCa tissues. We performed an immunohistochemical analysis of Reg IV expression in 98 clinically localized PCa cases. In adjacent non‐neoplastic prostate tissue, focal Reg IV staining was found in five (5%) of 98 cases. Periodic luminal epithelial cells stained for Reg IV, but stromal cells showed no staining for Reg IV (Fig. 1a). We confirmed that Reg IV‐positive cells were not stained by 34βE12 (a marker of basal cells) (Fig. 1b). Although the specificity of the Reg IV antibody has been characterized in detail,( 5 ) the specificity of immunohistochemical detection for Reg IV was further verified by triple‐immunofluorescence staining with different antibodies against Reg IV (goat polyclonal, rabbit polyclonal and mouse monoclonal). Cells stained with anti‐Reg IV goat polyclonal antibody also stained with anti‐Reg IV rabbit polyclonal antibody and anti‐Reg IV mouse monoclonal antibody, indicating that these anti‐Reg IV antibodies specifically recognize Reg IV protein (Fig. 1c). In PCa tissues, Reg IV staining was observed in 14 (14%) of 98 PCa cases. In all 14 PCa cases, few tumor cells (1–10%) showed Reg IV staining. Stromal cells showed no Reg IV staining. Reg IV staining was considered positive if any tumor cells were stained. In each Reg IV‐positive case, both mucin‐like staining (Fig. 1d) and perinuclear staining (Fig. 1e) were observed. We analyzed relations between Reg IV staining and clinicopathological characteristics. Reg IV staining was not correlated with pT stage, Gleason score or preoperative PSA concentration (Table 1).
Figure 1.
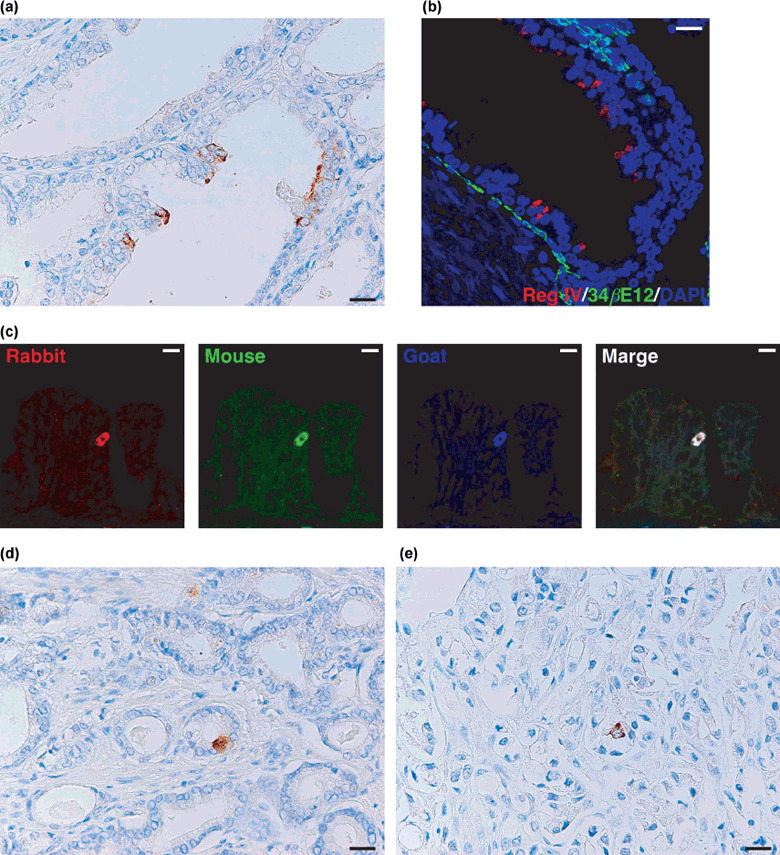
Immunohistochemical analysis of Reg IV expression in clinically localized prostate cancer. (a) Immunostaining of Reg IV in adjacent non‐neoplastic prostate tissue. Several luminal epithelial cells show Reg IV staining. Scale line, 25 µm. (b) Double‐immunostaining of Reg IV (red) and 34βE12 (a marker for basal cells; green). Nuclei are stained with 4′,6‐diamidino‐2‐phenylindole (DAPI; blue). Scale line, 25 µm. (c) Triple‐immunostaining of rabbit polyclonal anti‐Reg IV (red), mouse monoclonal anti‐Reg IV (green), and goat polyclonal anti‐Reg IV (blue). Scale line, 13 µm. (d) Immunostaining of Reg IV in prostate cancer (PCa). Mucin‐like staining of Reg IV is present in goblet cell‐like vesicles of tumor cells. Scale line, 25 µm. (e) Immunostaining of Reg IV in PCa. Perinuclear staining of Reg IV is present in tumor cells. Scale line, 25 µm.
Table 1.
Association between Reg IV immunostaining and clinicopathological variables
| Reg IV | |||
|---|---|---|---|
| Positive | Negative | P‐value | |
| pT stage | |||
| ≤2b | 6 (22%) | 21 | 0.2001 |
| ≥2c | 8 (11%) | 63 | |
| Gleason score | |||
| ≤7 | 8 (11%) | 66 | 0.1001 |
| ≥8 | 6 (25%) | 18 | |
| Preoperative PSA concentration | |||
| <20 | 12 (13%) | 77 | 0.6122 |
| ≥20 | 2 (22%) | 7 | |
| MUC2 | |||
| Positive | 14 (19%) | 58 | 0.0182 |
| Negative | 0 (0%) | 26 | |
| Chromogranin A | |||
| Positive | 10 (32%) | 21 | 0.0012 |
| Negative | 4 (6%) | 63 | |
| EGFR | |||
| Positive | 8 (32%) | 17 | 0.0067 |
| Negative | 6 (8%) | 67 | |
| Phospho‐EGFR (Tyr1068) (n = 25) | |||
| Positive | 6 (67%) | 3 | 0.0099 |
| Negative | 2 (13%) | 14 | |
EGFR, epidermal growth factor receptor; PSA, prostate‐specific antigen.
We also performed an immunohistochemical analysis of MUC2 expression because Reg IV is associated with intestinal differentiation in gastric cancer.( 5 ) In PCa tissues, MUC2 staining was observed in goblet cell‐like vesicles (Fig. 2a) and perinuclear regions (Fig. 2b) of tumor cells. Of the 98 PCa cases, MUC2 staining was observed in 72 (73%). MUC2‐positive PCa cells comprised 1–30% of tumor cells. MUC2 staining was considered positive if any tumor cells were stained. We analyzed the relation between MUC2 staining and clinicopathological characteristics. MUC2 positivity was found more frequently in PCa showing a pT stage of 2c or more (59/71, 83%) than in PCa showing a pT stage of 2b or less (13/27, 48%, P = 0.0182, Fisher's exact test). MUC2 staining was not correlated with Gleason score or preoperative PSA concentration. Association between Reg IV and MUC2 staining was also analyzed. Reg IV positivity was found more frequently in MUC2‐positive cases (14/72, 19%) than in MUC2‐negative cases (0/26, 0%, P = 0.0182, Fisher's exact test) (Table 1). We confirmed that almost all tumor cells showing mucin‐like staining of Reg IV were positive for MUC2 by double‐immunofluorescence staining (Fig. 2c). Some PCa cells showing perinuclear Reg IV staining also showed MUC2 staining (Fig. 2d). These results indicated that Reg IV is associated with intestinal differentiation of PCa.
Figure 2.
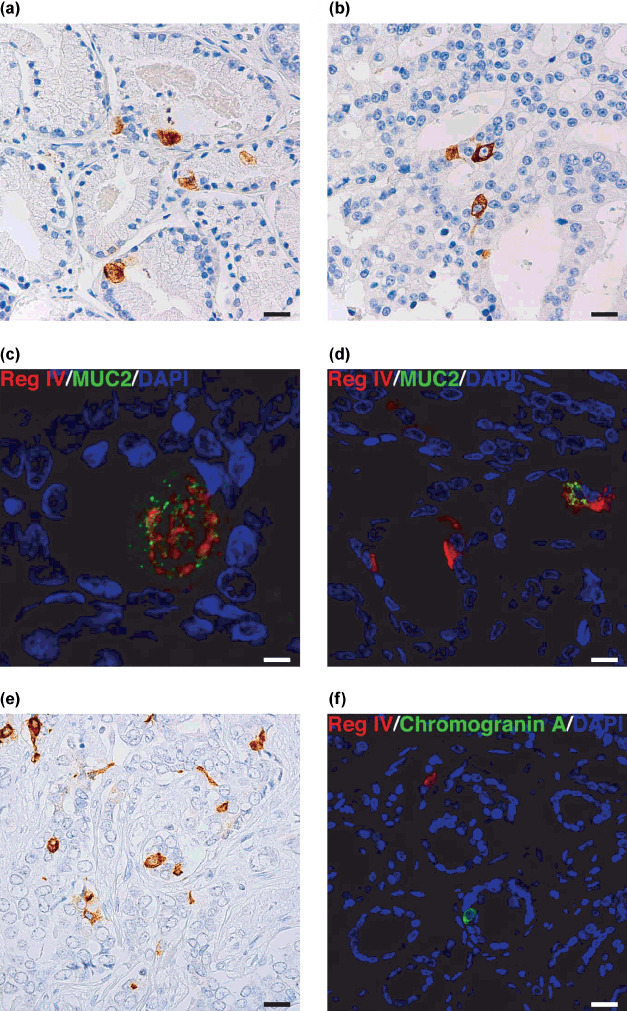
Immunohistochemical analysis of MUC2 and chromogranin A expression in clinically localized prostate cancer (PCa). (a) Immunostaining of MUC2 in PCa. Mucin‐like staining of MUC2 is present in goblet cell‐like vesicles of a tumor cell. Scale line, 25 µm. (b) Immunostaining of MUC2 in PCa. Perinuclear staining of MUC2 is present in a tumor cell. Scale line, 25 µm. (c) Double‐immunostaining of Reg IV (red) and MUC2 (green). Nuclei are stained with 4′,6‐diamidino‐2‐phenylindole (DAPI; blue). Reg IV staining is present with MUC2 in goblet cell‐like vesicles of a tumor cell. Scale line, 13 µm. (d) Double‐immunostaining of Reg IV (red) and MUC2 (green). Nuclei are stained with DAPI (blue). A tumor cell showing perinuclear staining of Reg IV also shows MUC2 staining. Scale line, 25 µm. (e) Immunostaining of chromogranin A in PCa. Scale line, 25 µm. (f) Double‐immunostaining of Reg IV (red) and chromogranin A (green). Nuclei are stained with DAPI (blue). Scale line, 25 µm.
Immunostaining of chromogranin A was also performed. Representative results of chromogranin A immunostaining in PCa are shown in Fig. 2(e). Of the 98 PCa cases, chromogranin A staining was observed in 31 (32%). Chromogranin A positivity was observed in 1–40% of PCa cells. Chromogranin A staining was considered positive if any tumor cells were stained. We analyzed the relation between chromogranin A staining and clinicopathological characteristics. Chromogranin A positivity were found more frequently in PCa showing a Gleason score of 8 or more (12/24, 50%) than in PCa showing a Gleason score of 7 or less (19/74, 26%, P = 0.0418, Fisher's exact test). Chromogranin A staining was not correlated with pT stage or preoperative PSA concentration. Association between Reg IV and chromogranin A staining was also analyzed. Reg IV positivity was found more frequently in chromogranin A‐positive cases (10/31, 32%) than in chromogranin A‐negative cases (4/67, 6%, P = 0.0012, Fisher's exact test) (Table 1). These results indicated that Reg IV is associated with neuroendocrine differentiation. However, double‐immunofluorescence staining revealed that Reg IV‐positive cells did not show chromogranin A staining (Fig. 2f). Therefore, a paracrine effect of secreted Reg IV may be involved in neuroendocrine differentiation in PCa.
Relation between Reg IV immunostaining and relapse‐free survival of PCa patients. We next examined the relation between Reg IV immunostaining and relapse‐free survival in PCa. Univariate analysis revealed that Reg IV staining (P = 0.0004, log–rank test), chromogranin A staining (P = 0.0494), Gleason score (P < 0.0001) and preoperative PSA concentration (P = 0.0167) (Fig. 3a–d) were significant prognostic factors for relapse‐free survival in patients with PCa, whereas MUC2 staining and pT stage did not correlate with relapse‐free survival. We then used the Cox proportional hazards multivariate model to examine the association of clinicopathological factors and Reg IV and chromogranin A staining with relapse‐free survival. Multivariate analysis indicated that Reg IV staining, Gleason score and preoperative PSA concentration were independent predictors of relapse‐free survival in patients with PCa (Table 2). These results suggested that Reg IV expression directly contributes to the malignant potential of PCa.
Figure 3.
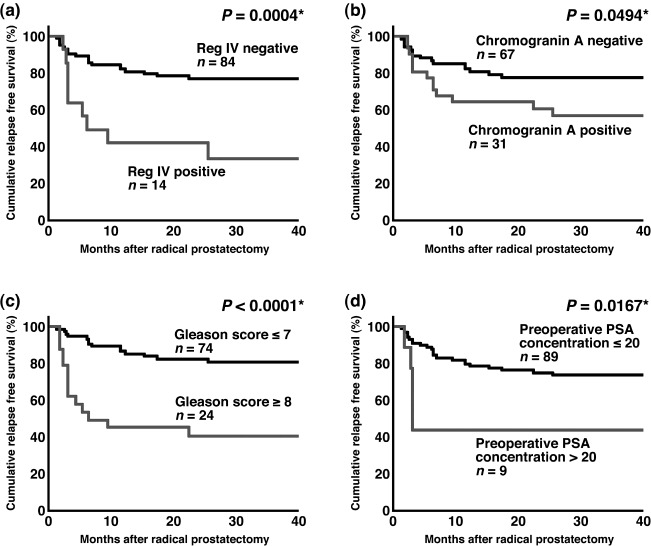
Relapse‐free survival of patients with prostate cancer (PCa). (a) Kaplan–Meier curves of patients with Reg IV‐negative or Reg IV‐positive PCa. (b) Kaplan–Meier curves of patients with chromogranin A‐negative or chromogranin A‐positive PCa. (c) Kaplan–Meier curves of patients with low Gleason score (≤7) or high Gleason score (≥8) PCa. (d) Kaplan–Meier curves of patients with low preoperative prostate specific antigen (PSA) concentration (≤20) or high preoperative PSA concentration (>20) PCa. *Log–rank test.
Table 2.
Multivariate analysis of factors influencing relapse‐free survival
| Hazard ratio | (95% CI) | χ2‐test | P‐value | |
|---|---|---|---|---|
| Reg IV staining | ||||
| Negative | 1 | (Reference) | 4.640 | 0.0312 |
| Positive | 2.848 | (1.099–7.381) | ||
| Chromogranin A staining | ||||
| Negative | 1 | (Reference) | 0.005 | 0.9431 |
| Positive | 1.034 | (0.414–2.582) | ||
| Gleason score | ||||
| ≤7 | 1 | (Reference) | 10.235 | 0.0014 |
| ≥8 | 3.747 | (1.668–8.416) | ||
| Preoperative PSA concentration | ||||
| <20 | 1 | (Reference) | 4.410 | 0.0357 |
| ≥20 | 2.938 | (1.074–8.036) | ||
CI, confidence interval; PSA, prostate‐specific antigen.
Reg IV activates EGFR in LNCaP cells. Statistical analysis revealed that Reg IV is an independent predictor of relapse‐free survival in patients with clinically localized PCa. However, the underlying mechanism remains unclear. Therefore, Reg IV‐CM was prepared and the function of Reg IV was analyzed. It has been reported that recombinant human Reg IV induces rapid phosphorylation of the EGFR at Tyr992 and Tyr1068 and of Akt at Thr308 and Ser473 resulting in increased AP‐1 transcription factor activity.( 12 ) Western blot analysis of the EGFR showed that colo320 did not express EGFR protein (data not shown). Therefore, we assumed that Reg IV overexpression has little effect in colo320 cells. Thus, Reg IV‐CM was prepared with the colo320 cell line. With a specific antibody, we verified the expression of Reg IV protein in Reg IV‐CM prepared from colo320 cells (Fig. 4a). We also examined whether EGF and TGF‐α could be detected by ELISA in Reg IV‐CM and control medium. EGF or TGF‐α was not detected in Reg IV‐CM or control medium (data not shown). We tested the specificity of the antiphospho‐EGFR (Tyr1068) and antiphospho‐EGFR (Tyr992) antibodies. Western blotting of lysates of LNCaP cell line was performed. The antiphospho‐EGFR (Tyr1068) antibody detected a single band of 170 kDa on Western blots of LNCaP cells treated with TGF‐α or EGF (Fig. 4b). The antiphospho‐EGFR (Tyr992) antibody also detected a single band of 170 kDa on Western blots of LNCaP cells treated with TGF‐α or EGF (data not shown). These data show that these antiphospho‐EGFR (Tyr1068) and antiphospho‐EGFR (Tyr992) antibodies specifically recognize phospho‐EGFR protein. The effect of Reg IV‐CM on EGFR phosphorylation was then investigated in LNCaP cells. EGFR was phosphorylated at Tyr1068 but not Tyr992 in LNCaP cells treated with Reg IV‐CM (Fig. 4c). These findings indicated that Reg IV is involved in EGFR phosphorylation in an EGF‐ and TGF‐α‐independent manner in LNCaP cells. Because it has been reported that growth factors enhance REG4 mRNA expression in colon cancer cells,( 15 ) the effect of EGF and TGF‐α on REG4 expression was also investigated in LNCaP cells. Neither EGF or TGF‐α had any significant effect on Reg IV protein expression in LNCaP cells (Fig. 4d). Similar results were also obtained from the DU145 cell line (data not shown).
Figure 4.
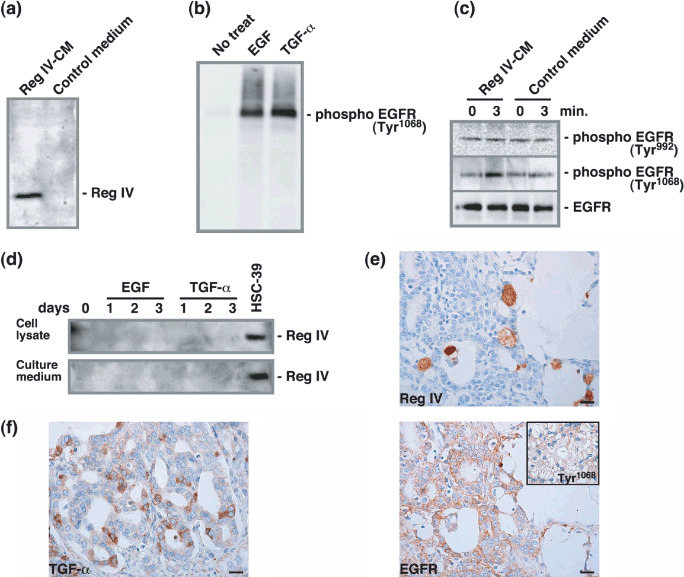
Effect of Reg IV on epidermal growth factor receptor (EGFR) phosphorylation. (a) 10 µL of Reg IV‐conditioned medium and control medium were analyzed by Western blot with a rabbit polyclonal antibody against Reg IV. (b) LNCaP cells were cultured with either EGF (100 nM) or transforming growth factor (TGF)‐α (10 nM) for 3 min. Whole‐cell lysates were prepared and analyzed by Western blot with antiphospho‐EGFR (Tyr1068) antibody. (c) LNCaP cells were cultured with either Reg IV‐CM or control medium for 3 min. Whole‐cell lysates were prepared and analyzed by Western blot with antiphospho‐EGFR (Tyr992) or antiphospho‐EGFR (Tyr1068) antibody. The samples were also probed with anti‐EGFR antibody to verify equal loading. (d) LNCaP cells were cultured with either EGF (100 nM) or TGF‐α (10 nM) for 1, 2 or 3 days. Whole‐cell lysates and culture medium were analyzed by Western blot with anti‐Reg IV antibody. (e) Expression of Reg IV and EGFR was examined by immunohistochemistry in serial sections of prostate cancer. Inset, phospho‐EGFR (Tyr1068) staining of a serial section. Scale line, 25 µm. (f) Expression of TGF‐α was examined by immunohistochemistry. Scale line, 25 µm.
We next examined whether expression of Reg IV activates phosphorylation of the EGFR at Tyr1068 in PCa tissue samples. Staining of the EGFR was found in 25 (26%) of the 98 PCa cases. All 25 PCa cases were considered EGFR‐positive. Of 25 PCa cases positive for EGFR, eight (32%) were positive for Reg IV. In eight PCa cases positive for both Reg IV and EGFR, Reg IV‐positive tumor cells were found near EGFR‐positive tumor cells (Fig. 4e). Because Reg IV‐CM phosphorylated the EGFR at Tyr1068 in LNCaP cells, we further investigated phosphorylation of the EGFR at Tyr1068 in 25 PCa cases positive for EGFR staining. Phosphorylation of the EGFR at Tyr1068 was found in nine (36%) PCa cases (Fig. 4e, inset). Reg IV positivity was found more frequently in Tyr1068‐positive EGFR cases than in Tyr1068‐negative EGFR cases (P = 0.0099, Fisher's exact test) (Table 1). Because it is possible that EGFR phosphorylation is due to the stimulation by some growth factors rather than Reg IV protein in the human PCa tissues, we performed immunohistochemical analysis of TGF‐α. As reported previously,( 16 ) in most cases, TGF‐α expression was present in the stroma; however, co‐expression of EGFR and TGF‐α in tumor cells was observed in several PCa cases. Tumor cell staining of the TGF‐α was found in 17 (17%) of the 98 PCa cases (Fig. 4f). We regarded these cases with TGF‐α‐positive tumor cells as TGF‐α‐positive. Of 25 PCa cases positive for EGFR, nine (36%) were positive for TGF‐α. TGF‐α positivity was found more frequently in Tyr1068‐positive EGFR cases (7/9, 78%) than in Tyr1068‐negative EGFR cases (2/16, 13%, P = 0.0022, Fisher's exact test). Because it has been reported that growth factors enhance REG4 mRNA expression in colon cancer cells,( 15 ) association between Reg IV and TGF‐α staining was analyzed. Reg IV positivity was found more frequently in TGF‐α‐positive cases (8/17, 47%) than in TGF‐α‐negative cases (6/81, 7%, P = 0.0003, Fisher's exact test).
Discussion
Overexpression of REG4 mRNA has been reported in PCa by in situ hybridization.( 11 ) A majority of metastatic PCa tumors express high levels of REG4 mRNA. In addition, REG4 expression is significantly more intense in high‐grade PCa (Gleason score, 7–10) than in low‐grade PCa (Gleason score, 5–6). In the present study of clinically localized PCa, Reg IV‐positive cases showed unfavorable prognosis with respect to relapse‐free survival. Several autocrine and paracrine signaling pathways involving the EGFR and its ligands, EGF and TGF‐α, are postulated to stimulate tumor cell proliferation independent of androgen activity.( 17 ) These pathways become much more essential to PCa growth once androgen insensitivity occurs. EGFR signaling pathways are also involved in PCa invasion and angiogenesis, both of which are crucial for progression and metastasis.( 18 , 19 ) In the present study, we showed that Reg IV‐CM phosphorylated the EGFR at Tyr1068. These findings suggest that Reg IV activates phosphorylation of the EGFR in human PCa tissues. Although the precise mechanism of EGFR phosphorylation by Reg IV remains unclear, it is not likely that secretion of the EGFR ligands, such as EGF and TGF‐α, is involved because no EGF or TGF‐α was detected in Reg IV‐CM by ELISA. Taken together, these results suggest that Reg IV plays important roles in tumor progression and unfavorable prognosis. However, TGF‐α positivity was also found more frequently in Tyr1068‐positive EGFR cases than in Tyr1068‐negative EGFR cases. Because frequency of Reg IV positivity in Tyr1068‐positive EGFR cases (67%) was lower than that of TGF‐α positivity in Tyr1068‐positive EGFR cases (78%), EGFR phosphorylation may be due to the stimulation by TGF‐α rather than Reg IV protein in PCa tissues.
Androgen‐deprivation therapy has been used for decades in the treatment of prostate cancer.( 20 ) Although this treatment is initially very effective in hormone‐dependent cancers, they invariably become hormone‐refractory and metastasize, resulting in death.( 21 ) Novel therapies that target androgen‐independent proliferation, as well as invasion and angiogenesis, may have enormous potential for improving the care of patients with advanced PCa. Several possible mechanisms by which PCa can escape the effects of androgen‐deprivation therapy have been reported.( 21 ) Among them, EGFR expression has been reported to be associated with hormone‐refractory status.( 22 ) Therefore, phosphorylation of the EGFR by Reg IV may participate in the acquisition of hormone‐refractory status. In the present study, few tumor cells (1–10%) in clinically localized PCa showed Reg IV staining, although residual hormone‐refractory PCa has been reported to express high levels of REG4 mRNA.( 11 ) These results suggest that Reg IV‐positive tumor cells may escape the effects of androgen‐deprivation therapy, resulting in an increased number of Reg IV‐positive tumor cells in residual hormone‐refractory PCa.
It has been reported that growth factors enhance REG4 mRNA expression in colon cancer cells.( 15 ) In fact, in PCa tissues, Reg IV positivity was found more frequently in TGF‐α‐positive cases than in TGF‐α‐negative cases. In contrast, neither EGF or TGF‐α had any significant effect on Reg IV protein expression in LNCaP and DU145 cell lines, suggesting that, in addition to TGF‐α, other factors are needed to express Reg IV in PCa cells.
In the present study, Reg IV‐positive cases were frequently found in association with MUC2 positivity. It has been suggested that the acquisition by tumor cells of a mucinous phenotype, such as MUC2 expression, is involved in hormonal escape in PCa.( 23 ) Reg IV‐positive cases were frequently found in association with chromogranin A positivity. Neuroendocrine differentiation has been also reported to be correlated with tumor aggressiveness, short survival and poor response to androgen‐deprivation therapy.( 24 , 25 ) Therefore, Reg IV may be a key factor mediating the hormone‐refractory phenotype in MUC2‐positive or chromogranin A‐positive PCa. We observed two Reg IV staining patterns, mucin‐like staining and perinuclear staining. In gastric cancer, mucin‐like Reg IV staining is associated with MUC2 positivity.( 5 ) Perinuclear Reg IV staining is detected in cells with neuroendocrine differentiation and that show chromogranin A positivity. In PCa tissue, although almost all tumor cells showing mucin‐like staining of Reg IV were positive for MUC2, some tumor cells showing perinuclear Reg IV staining also showed MUC2 staining. The significance of the difference between mucin‐like Reg IV staining and perinuclear Reg IV staining is unclear; however, there were several PCa cases in which both staining patterns were observed and we presume that these staining patterns are not independent.
In conclusion, we showed that Reg IV immunostaining is an independent predictor of relapse‐free survival in patients with clinically localized PCa. To clarify whether Reg IV immunostaining is useful for the identification of patients most likely to benefit from adjuvant treatment, the association between Reg IV staining and response to adjuvant therapies should be investigated. We also showed that Reg IV‐CM induces EGFR phosphorylation. Because Reg IV expression is narrowly restricted in non‐cancerous tissues, Reg IV may be a good therapeutic target for PCa.
Acknowledgments
This work was supported, in part, by Grants‐in‐Aid for Cancer Research from the Ministry of Education, Culture, Science, Sports and Technology of Japan; and from the Ministry of Health, Labor and Welfare of Japan. We thank Ms Emiko Hisamoto for excellent technical assistance and advice. This work was carried out with the kind cooperation of the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University. We thank the Analysis Center of Life Science, Hiroshima University, for the use of their facilities.
References
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43–66. [DOI] [PubMed] [Google Scholar]
- 2. D’Amico AV, Whittington R, Malkowicz SB et al . Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol 2000; 18: 1164–72. [DOI] [PubMed] [Google Scholar]
- 3. Oue N, Hamai Y, Mitani Y et al . Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res 2004; 64: 2397–405. [DOI] [PubMed] [Google Scholar]
- 4. Aung PP, Oue N, Mitani Y et al . Systematic search for gastric cancer‐specific genes based on SAGE data: melanoma inhibitory activity and matrix metalloproteinase‐10 are novel prognostic factors in patients with gastric cancer. Oncogene 2006; 25: 2546–57. [DOI] [PubMed] [Google Scholar]
- 5. Oue N, Mitani Y, Aung PP et al . Expression and localization of Reg IV in human neoplastic and non‐neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol 2005; 207: 185–98. [DOI] [PubMed] [Google Scholar]
- 6. Mitani Y, Oue N, Matsumura S et al . Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5‐fluorouracil‐based chemotherapy. Oncogene 2007; 26: 4383–93. [DOI] [PubMed] [Google Scholar]
- 7. Isaacs W, De Marzo A, Nelson WG. Focus on prostate cancer. Cancer Cell 2002; 2: 113–16. [DOI] [PubMed] [Google Scholar]
- 8. Hasegawa Y, Matsubara A, Teishima J et al . DNA methylation of the RIZ1 gene is associated with nuclear accumulation of p53 in prostate cancer. Cancer Sci 2007; 98: 32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oue N, Kuniyasu H, Noguchi T et al . Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology, 2007; 72: 371–80. [DOI] [PubMed] [Google Scholar]
- 10. Takehara A, Eguchi H, Ohigashi H et al . Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci 2006; 97: 1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu Z, Rubin MA, Yang Y et al . Reg IV: a promising marker of hormone refractory metastatic prostate cancer. Clin Cancer Res 2005; 11: 2237–43. [DOI] [PubMed] [Google Scholar]
- 12. Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP‐1 signaling pathway in colon adenocarcinomas. Gastroenterology 2006; 130: 137–49. [DOI] [PubMed] [Google Scholar]
- 13. Yasui W, Ayhan A, Kitadai Y et al . Increased expression of p34cdc2 and its kinase activity in human gastric and colonic carcinomas. Int J Cancer 1993; 53: 36–41. [DOI] [PubMed] [Google Scholar]
- 14. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–70. [PubMed] [Google Scholar]
- 15. Nanakin A, Fukui H, Fujii S et al . Expression of the REG IV gene in ulcerative colitis. Lab Invest 2007; 87: 304–14. [DOI] [PubMed] [Google Scholar]
- 16. Cohen DW, Simak R, Fair WR, Melamed J, Scher HI, Cordon‐Cardo C. Expression of transforming growth factor‐alpha and the epidermal growth factor receptor in human prostate tissues. J Urol 1994; 152: 2120–4. [DOI] [PubMed] [Google Scholar]
- 17. Culig Z, Hobisch A, Cronauer MV et al . Regulation of prostatic growth and function by peptide growth factors. Prostate 1996; 28: 392–405. [DOI] [PubMed] [Google Scholar]
- 18. Connolly JM, Rose DP. Angiogenesis in two human prostate cancer cell lines with differing metastatic potential when growing as solid tumors in nude mice. J Urol 1998; 160: 932–6. [DOI] [PubMed] [Google Scholar]
- 19. Bonaccorsi L, Carloni V, Muratori M et al . EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen‐sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR). Int J Cancer 2004; 112: 78–86. [DOI] [PubMed] [Google Scholar]
- 20. Huggins C. Endocrine‐induced regression of cancers. Science 1967; 156: 1050–4. [DOI] [PubMed] [Google Scholar]
- 21. Feldman BJ, Feldman D. The development of androgen‐independent prostate cancer. Nat Rev Cancer 2001; 1: 34–45. [DOI] [PubMed] [Google Scholar]
- 22. Shah RB, Ghosh D, Elder JT. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate 2006; 66: 1437–44. [DOI] [PubMed] [Google Scholar]
- 23. Legrier ME, De Pinieux G, Boye K et al . Mucinous differentiation features associated with hormonal escape in a human prostate cancer xenograft. Br J Cancer 2004; 90: 720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McWilliam LJ, Manson C, George NJ. Neuroendocrine differentiation and prognosis in prostatic adenocarcinoma. Br J Urol 1997; 80: 287–90. [DOI] [PubMed] [Google Scholar]
- 25. Di Sant’Agnese PA, Cockett AT. Neuroendocrine differentiation in prostatic malignancy. Cancer 1996; 78: 357–61. [DOI] [PubMed] [Google Scholar]


