Abstract
We recently developed a novel drug delivery system (DDS) using oligomannose‐coated liposomes (OMLs), which are effectively taken up by mouse peritoneal macrophages to carry anticancer drugs to omental milky spots known as initial metastatic sites in the peritoneal cavity in mice. However, the feasibility of the clinical application of this DDS to gastric cancer patients remains essentially unknown. In the present study, we investigated whether human peripheral blood monocytes (PBMs) and human peritoneal macrophages (PEMs) could successfully uptake OMLs and deliver them to the micrometastatic foci in the mouse omentum and resected omentum from cancer patients ex vivo. When OMLs were incubated with the PBMs from four healthy volunteers in vitro, an average 88% of CD14‐positive PBMs, most of which also express CD206, took up OMLs, and this uptake was significantly inhibited by α‐methylmannoside. In the experiment using PEMs obtained from peritoneal washes of five gastric cancer patients, the average uptake rate (63%) of OML by CD14‐positive PEMs was somewhat lower than that of PBMs, but in three advanced gastric cancer patients the uptake rate of OMLs was 76% which was comparable to that of mouse PEMs. Oligomannose‐coated liposome (OML)‐incorporated PBMs and PEMs were successfully accumulated at the micrometastatic foci at the omentum formed after intraperitoneal injection of GFP‐tagged gastric cancer cells into mice. Furthermore, OML‐incorporated PBMs substantially accumulated to tumor foci in the surgically resected human omentum ex vivo. These results suggest that OMLs using human monocytes/macrophages as a cellular vehicle have the potential to target peritoneal micrometastasis in the omentum of gastric cancer patients. (Cancer Sci 2010)
Gastric cancer is the second most common cause of cancer death in Japan and peritoneal metastasis is the most frequent pattern of the recurrence after curative surgery in gastric cancer patients in Japan and Western countries.( 1 ) It causes not only cancer death, but also intestinal obstruction and malignant ascites formation, which severely reduce the quality of life (QOL) of the affected patients. Despite advances in therapeutic modalities for peritoneal metastases such as combination chemotherapy and chemohyperthermia,( 2 , 3 ) conventional chemotherapy has a limited anti‐metastatic effect because advanced deposits are refractory to various chemotherapeutic agents.( 4 ) We previously demonstrated that peritoneal micrometastases at an early stage are much more sensitive to the intraperitoneal chemotherapy with paclitaxel than advanced metastasis.( 5 ) Furthermore, we developed a genetic diagnosis method for the peritoneal lavages which is capable of detecting peritoneal micrometastasis more sensitively than conventional cytology, leading to the selection of patients with high risk for peritoneal relapse after curative surgery.( 6 , 7 ) Based on these findings, it is proposed that intraperitoneal chemotherapy for selected gastric cancer patients with a high risk for relapse is a promising therapeutic strategy to prevent peritoneal recurrence.( 8 ) However, it has been reported that conventional intraperitoneal chemotherapy with paclitaxel sometimes generates severe toxic side effects for patients with ovarian cancer.( 9 ) Therefore, to reduce toxic side effects, development of intraperitoneal chemotherapy capable of specifically targeting peritoneal micrometastasis with low‐dose drug administration is warranted.
The omentum seems to be the central site where many types of disseminated tumor cells develop into solid tumors in the peritoneal cavity.( 10 ) It is well known that the disseminated gastric and ovarian cancer cells preferentially develop overt metastatic foci in the omentum.( 11 , 12 ) There are many small lymphoid tissues, so‐called milky spots, in the omentum and mesentery, and these specific sites are known to provide a good scaffold for cancer cells to facilitate adhesion and proliferation, indicating that a milky spot in the omentum acts as an initial dissemination site for malignant cells in the peritoneal cavity.( 13 , 14 ) Another unique feature of milky spots is the homing sites for peritoneal macrophages in the peritoneal cavity.( 15 ) Based on these characteristics, peritoneal macrophages have been proposed as a cellular vehicle to deliver drugs to the peritoneal micrometastasis at the omental milky spots if the drugs are efficiently and specifically incorporated into the macrophages.( 16 )
We recently developed a novel platform for the specific delivery of anticancer drug to these milky spots in the omentum using peritoneal macrophages (PEMs) as cellular vehicles using oligomannose‐coated liposome (OML). This method consisting of active delivery and controlled release of anticancer drugs effectively suppressed growth of micrometastasis in the omentum in a mouse peritoneal metastasis model,( 17 ) suggesting that the new drug delivery system (DDS) could be exploited therapeutically. To date, however, it remains entirely unknown whether this DDS is applicable to gastric cancer patients in a clinical setting. In the present study, as a first step to translate this DDS into a successful clinical therapy, we investigated uptake of OMLs by human peripheral blood monocytes (PBMs) and PEMs and subsequent delivery of OMLs to micrometastasis in the omentum using both the mouse peritoneal metastasis model in vivo and human omental tissues ex vivo.
Materials and Methods
Mice. Male athymic nude mice of the KSN strain, 7–10 weeks old, were obtained from the Shizuoka Laboratory Animal Center (Hamamatsu, Japan). KSN athymic mice were maintained under specific pathogen‐free conditions. All animal experiments were performed according to the experimental protocol approved by the Ethics Review Committee for Animal Experimentation of the Aichi Cancer Center Research Institute and met the standard as defined by the UK co‐ordinating Committee on Cancer Research (UKCCR) guidelines.
Reagents and cell lines. For flow cytometry, mouse monoclonal antibody to human CD14 (BD Bioscience, CA, USA) and rabbit polyclonal antibody to human CD206 (Santa Cruz Biotechnology, CA, USA) were used as first antibodies for staining human monocytes/macrophages. Alexa Fluor 488‐conjugated goat polyclonal antibody to mouse IgG (H + L) and Alexa Fluor 488‐ and 568‐conjugated goat polyclonal antibody to rabbit IgG (H + L) were used as secondary antibodies (Invitrogen, OR, USA).
For liposome preparation, dipalmitoylphosphatidylcholine (DPPC), cholesterol, and dipalmitoylphosphatidylethanolamine (DPPE) were purchased from Sigma‐Aldrich (St Louis, MO, USA). Rhodamine‐DPPE (lissamin Rhodamine B) was purchased from Avanti Polar Lipids (Alabaster, AL, USA) and Mannotriose (Man3: Manα1‐6 [Manα1‐3] Man) was from Funakoshi (Tokyo, Japan). α‐Methylmannoside was obtained from Sigma‐Aldrich (St Louis, MI, USA). Calcein‐AM for vital staining of macrophages was obtained from Dojin Kagaku (Kumamoto, Japan). Activated carbon particles (CH40) was kindly provided by Dr Hagiwara (Kyoto Prefectural University of Medicine, Japan). Green fluorescent protein (GFP)‐tagged gastric cancer cell lines such as GCIY‐EGFP and MKN28‐EGFP were established and maintained as described previously.( 18 )
Peripheral blood, peritoneal wash, and omentum tissues. Peri‐pheral blood samples (20 mL each) were collected into sterilized tubes in the presence of EDTA as an anticoagulant from the popliteal vein of four healthy volunteers immediately before the experiment. As for peritoneal washes, aliquots of 100–200 mL of saline were introduced into the Douglas cavity and left subphrenic space of gastric cancer patients with or without peritoneal metastasis at the beginning of each operation and aspirated soon after gentle stirring. One‐half of each wash was sent to routine cytopathology with conventional Papanicolaou staining and the other half of the wash was used for the present experiment to examine uptake of OMLs by macrophages of human origin and its delivery to omental milky spots. Small pieces of surgically resected omental tissue were obtained from two advanced ovarian cancer patients with omentectomy. Tumors were staged according to the TNM classification (6th edition, 2003)( 19 ) and the categories were determined from pathological findings based on surgically resected specimens. This study was approved by the institutional review board of Aichi Cancer Center and the written informed consent of the patients was obtained.
Preparation of oligomannose‐coated liposomes. Oligomannose‐coated liposomes (OMLs) using neoglycolipid Man3‐DPPE, which was prepared by conjugation of the mannotriose with DPPE by reductive amination, were obtained from BioMedCore (Yokohama, Japan). Briefly, a chloroform‐methanol (2:1, v/v) solution containing 1.5 μmol of DPPC and 1.5 μmol of cholesterol was placed in a conical flask and dried by rotary evaporation. Subsequently, 2 mL ethanol containing 0.15 μmol of Man3‐DPPE was added to the flask and evaporated to prepare a lipid film containing Man3‐DPPE. The multilamellar vesicles were generated with PBS in the dried lipid film by intense vortex dispersion, and these vesicles were then extruded 10 times through polycarbonate membranes of 1‐μm pore (Nucleopore, Pleasanton, CA, USA) to produce oligomannose (mannotriose)‐coated liposomes. For fluorescence (Rhodamine B) labeling of the lipid bilayer in the OMLs, a lipid film containing 0.15 μmol Rhodamine B–DPPE was used instead of DPPE.( 17 )
Uptake of OMLs by human PBMs and PEMs in vitro. Leukocytes fraction of peripheral blood from healthy volunteers were isolated using Histopaque‐1077 (Sigma‐Aldrich) and were cultured in RPMI‐1640 (Sigma‐Aldrich) medium in the presence of 10% human serum in a plastic culture dish (Falcon; BD Labware, Franklin Lakes, NJ, USA) in a humidified 5% CO2 incubator at 37°C. One day after culture, cells adhering to the culture dish were harvested by 0.125% trypsin/0.25 mM EDTA/PBS and used as PBMs in the experiments. Isolated PBMs were preincubated with Rhodamine B‐labeled OMLs or Rhodamine B‐labeled bare liposomes (BLs) without mannotriose‐coating at 37°C for 1 h in vitro in the RPMI‐1640 in the presence of 10% human serum. To investigate inhibition of OML uptake by carbohydrates, PBMs were incubated with OMLs at 37°C for 5 min in the presence of 250 mM α‐methylmannoside, α‐methylgalactoside, and α‐methylglucoside. After washing with PBS, uptake of liposomes by human PBMs and PEMs was measured by flow cytometry and with an in vivo fluorescence imaging system (Ivis lumina II; Xenogen, CA, USA). In the latter, fluorescence images were captured on Windows PC and analyzed with Living Image software. With peritoneal wash samples from gastric cancer patients, peritoneal cells including mesothelial cells were incubated with OMLs at 37°C for 1 h and uptake of OMLs by PEMs was measured by flow cytometry.
Flow cytometry and confocal laser scanning microscopy. First, PBMs and PEMs were gated based on CD14‐positive expression and then incorporation of OMLs were evaluated by flow cytometry with dual‐color fluorescence emission (FACSCalibur; BD Science, San Diego, CA, USA). Rhodamine B‐labeled OML‐ or BL‐incorporated macrophages were further stained with antibodies to CD14 or CD206 for 15 min on ice, reacted by Alexa Fluor 488‐ or 568‐conjugated secondary antibodies, followed by nuclear staining with Hoechst 33324. The stained cells were also mounted on slide glass using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) to reduce photo‐bleaching and then viewed with a ×40 objective lens using a LSM510META confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
Delivery of OMLs to the omental milky spots of mice. Oligomannose‐coated lipsome (OML)‐incorporated PBMs were stained with 1 μg/mL Calcein‐AM at 37°C for 15 min, followed by washing twice with PBS. Thereafter, PBMs (3 × 106 in 0.4 mL) were injected into the peritoneal cavity of mice. After 24 h, mice were autopsied and the localization of Rhodamine B‐labeled OMLs, Calcein‐AM stained PBMs, and GFP‐tagged gastric cancer cells in the peritoneal cavity was observed using a macro fluorescence in vivo imaging system. This system consists of Macro lens (J6x11; Macro, Tokyo, Japan) and a 150 W high brightness halogen Lamp (PICL‐NEX; NPI, Tokyo, Japan) with dual flexible guides at up to ×6.7 magnification as reported previously.( 20 ) Fluorescence images were recorded on a cooled coupled‐charged device (CCD) camera (Penguin 600CL; Pixera, CA, USA), and images of 2000 × 1312 pixels were captured on a Windows PC with In Studio software.
Delivery of OMLs to the milky spots of the human omentum ex vivo. Fresh omental tissues were denoted from advanced ovarian cancer patients with omentectomy. Noncancerous omental tissues were harvested, cut into small pieces <1 mm in diameter and co‐cultured with OML‐incorporated and Calcein‐AM pre‐stained PBMs in 3 mL of RPMI‐1640 medium in the presence of 10% human serum for 12 h in a humidified 5% CO2 incubator at 37°C with mild shaking. In two cases, GCIY‐EGFP cells were preincubated with small pieces of omental tissues before co‐culture with PBMs. Rhodamine B‐labeled OMLs incorporated PBMs and GFP‐tagged tumor cells in the omental tissues were observed with an in vivo imaging system and with a confocal laser scanning microscope with ×20 and ×60 objective lenses.
Results
Uptake of OMLs by human PBMs. Flow cytometric analysis demonstrated that an average 86% (range, 83–90%) of peripheral blood leukocytes showing adhesion to culture dish were CD14‐positive, confirming the majority of adherent cells of peripheral blood leukocytes to be of monocyte/macrophage lineage (data not shown). Approximately 85% of CD14‐positive PBMs took up OMLs, whereas 8.6% of CD14‐positive PBMs took up bare liposomes (BLs), indicating that OML uptake by human PBMs is much more efficient than that of BLs (Fig. 1a, Table 1). Confocal fluorescence microscopy confirmed the abundant rhodamine B‐OMLs were detected in the cytoplasm of CD14 membrane‐positive PBMs, while the incidence and abundance of BL uptake were much lower than in OMLs (Fig. 1b). The fraction (%) of OMLs taken up by PBMs was measured with the Ivis fluorescence imaging system. The results demonstrated that 67% of RhoB‐OMLs injected are incorporated into the PBM fraction, while <1% of RhoB‐BLs are transfered to the cell fraction (Fig. 1c).
Figure 1.
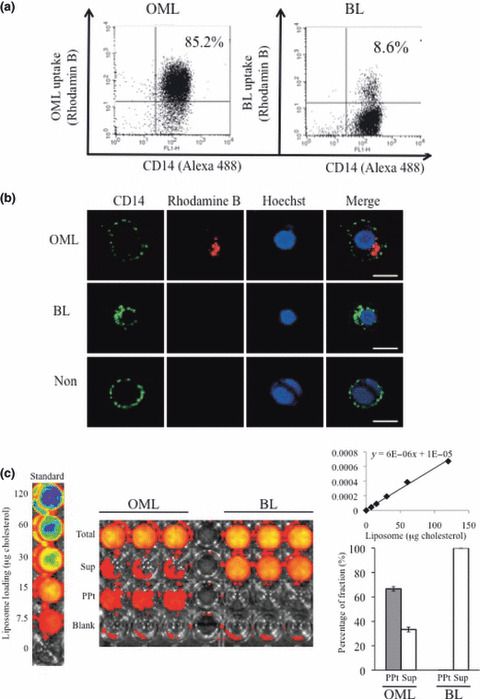
Uptake of oligomannose‐coated liposomes (OMLs) by human peripheral blood monocyte (PBM) from healthy volunteers. (a) Flow cytometric analysis of OML uptake by PBMs. Rhodamine B‐labeled OMLs (left panel) and bare liposomes (BLs) (right panel) were incubated with the human PBMs for 1 h at 37°C. After washing with PBS, macrophages were stained with anti‐CD14 antibody and measured by flow cytometer. (b) Confocal laser scanning microscopic analysis of OML uptake by PBMs. Efficient uptake of Rhodamine B‐labeled OML (red) by CD14‐positive PBMs (green) is seen. Nucleus was stained with Hoechst 33342 (blue). Bars = 10 μm. (c) Analysis of the fraction (%) of OMLs uptaken by PBMs. Rhodamine B‐labeled OMLs or BLs were incubated with the human PBMs for 1 h at 37°C. After centrifugation, cell pellet (ppt) and supernatant fluids (sup) were harvested and measured with the Ivis in vivo imaging system.
Table 1.
Patient characteristics and OML uptake by human PBMs and PEMs according to CD14 status
| Cases | Sample | Stage | Cytology | FACS analysis (%) | |||
|---|---|---|---|---|---|---|---|
| CD14 (+) OML (+) | CD14 (−) OML (+) | CD14 (+) OML (−) | CD14 (−) OML (−) | ||||
| Patient 1† | Peritoneal wash | III | − (I) | 60.54 | 8.17 | 15.19 | 16.11 |
| Patient 2 | Peritoneal wash | IV | + (V) | 33.13 | 11.20 | 15.46 | 40.20 |
| Patient 3 | Peritoneal wash | I | − (I) | 28.49 | 5.88 | 42.48 | 23.47 |
| Patient 4 | Peritoneal wash | I | − (I) | 29.49 | 2.08 | 35.61 | 32.82 |
| Patient 5 | Peritoneal wash | II | − (I) | 50.13 | 12.38 | 11.01 | 26.48 |
| Healthy 1‡ | Peripheral blood | NC | NC | 77.90 | 9.54 | 6.60 | 5.96 |
| Healthy 2 | Peripheral blood | NC | NC | 72.57 | 14.39 | 12.57 | 0.74 |
| Healthy 3 | Peripheral blood | NC | NC | 80.84 | 8.23 | 9.03 | 1.90 |
| Healthy 4 | Peripheral blood | NC | NC | 85.23 | 0.86 | 12.60 | 0.54 |
†Peritoneal wash from gastric cancer patients; ‡peripheral blood from healthy volunteers. NC, not classified; OML, oligomannose‐coated liposome; PBMs, peripheral blood monocytes; PEMs, peritoneal macrophages.
To understand the molecular bases of enhanced uptake of OMLs by PBMs, we examined the expression of CD206 in human PBMs. We found that most CD14‐positive PBMs were CD206‐positive on their cell surface (Fig. 2a). The uptake rate of OML and BLs by CD206‐positive PBMs was 66 and 2.9%, respectively. This increased uptake of OMLs by PBMs was strongly inhibited by α‐methylmannoside (uptake rate = 2.9%), but not by galactose. In another experiment, uptake rates of OMLs (76%) by PBMs was found to be decreased to 9.1 and 35% by the treatment with α‐methylmannoside and α‐methylgalactoside, respectively. However, 62% of CD 14‐positive, but CD206‐negative PBMs uptook OMLs (Fig. 2b).
Figure 2.
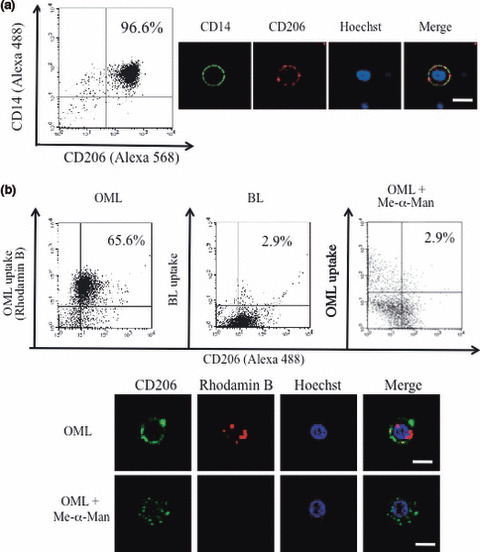
Uptake of oligomannose‐coated liposomes (OMLs) by CD206‐positive human peripheral blood monocyte (PBM) from healthy volunteers. (a) Human PBMs attached to culture dish were harvested and stained with the combination of anti‐CD14 and anti‐CD206 antibodies, and their expression was measured. (b) Rhodamine B‐labeled OMLs and bare liposomes (BLs) were incubated with the human PBMs for 1 h at 37°C. After washing with PBS, PBMs were stained with anti‐CD206 antibody and OML uptake by human PBMs was measured. Efficient uptake of OMLs (left panel), but not BLs (center panel), and inhibition of OML uptake by α‐methylmannoside (right panel) are evident. Bars = 10 μm.
Uptake of OMLs by human PEMs. We next examined uptake of OMLs and BLs by PEMs obtained from peritoneal washes of five gastric cancer patients. These included two stage I early gastric cancer patients and three stages II–VI advanced gastric cancer patients (Table 1). Flow cytometric analysis demonstrated that an average 64% (range, 48–76%) of whole peritoneal cells were CD14‐positive, and an average 62.7% (range, 45–81%) of the CD14‐positive PEMs successfully took up OMLs (Fig. 3a, left). The average uptake rate (9.6%) of BLs by CD14‐positive PEMs was much smaller than that of OMLs (Fig. 3a, right). Comparison of the proportion of CD14‐positive cells and the uptake rate of OMLs between CD14‐positive PBMs and PEMs is summarized in Table 2. Percentages of CD14‐positive cells in peritoneal washes and peripheral blood were 64.3 and 89.5%, respectively. In addition, the OML uptake rate by CD14‐positive macrophages from peritoneal wash and peripheral blood was 62.8 and 88.6%. No toxicity of OMLs to PBMs in terms of viability was observed.
Figure 3.
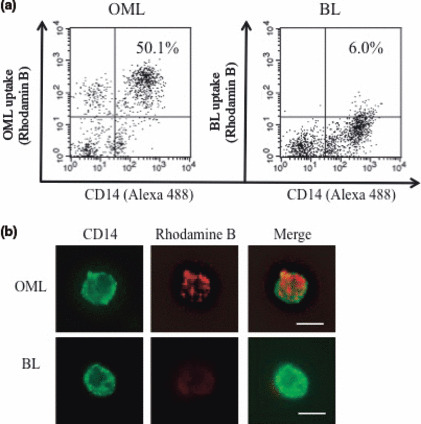
Uptake of oligomannose‐coated liposomes (OMLs) by human peritoneal macrophages (PEMs) from gastric cancer patients. (a) Flow cytometric analysis of OML uptake by human PEMs from a gastric cancer patient, the representative case of Patient 5 is shown. Rhodamine B‐labeled OMLs (left panel) and bare liposomes (BLs) (right panel) were incubated with the cell fractions obtained after centrifugation of peritoneal washes from patients for 1 h at 37°C. After washing with PBS, peritoneal cells were stained with anti‐CD14 antibody and measured by flow cytometer. (b) Confocal laser scanning microscopic analysis of uptake of Rhodamine B‐labeled OML and BL (red) by CD14‐positive PEMs (green). Bars = 10 μm.
Table 2.
Summary of OML uptake by PBMs and PEMs according to CD14 status
| Samples | CD14 (+) OML (+) (%) | CD14 (−) OML (+) (%) | CD14 (+) OML (−) (%) | CD14 (−) OML (−) (%) | OML(+)/CD14 (+) (ratio) (%) |
|---|---|---|---|---|---|
| PEM† | 40.36 | 7.94 | 23.95 | 27.82 | 62.76 |
| PBM‡ | 79.14 | 8.26 | 10.20 | 2.29 | 88.58 |
†Peritoneal macrophages (PEM) from gastric cancer patients; ‡peripheral blood monocytes (PBM) from healthy volunteers.
Accumulation of human PBMs and PEMs in the mouse omentum. We next examined the delivery of OMLs by human monocytes/macrophages to the omental milky spots. Milky spots were identified as spots stained black after i.p. injection of CH40 (Fig. 4a). Oligomannose‐coated liposome (OML)‐incorporated human PBMs successfully accumulated in the omental milky spots (Fig. 4b), whereas BLs were not substantially delivered to the mouse omentum (data not shown). Similarly, OML‐incorporated PEMs from patients succeeded to accumulate in the milky spot of the omentum (Fig. 4c). When GFP‐tagged MKN‐28 gastric cancer cells were pre‐injected into the mouse peritoneal cavity 1 day before injection of OMLs, OML‐incorporated human PBMs successfully accumulated in the micrometastatic foci in the mouse omentum (Fig. 4d, upper panels). Confocal fluorescence microscopy showed that OML‐incorporated PBMs infiltrated to the metastatic foci and accumulated around individual tumor cells (Fig. 4d, lower panels).
Figure 4.
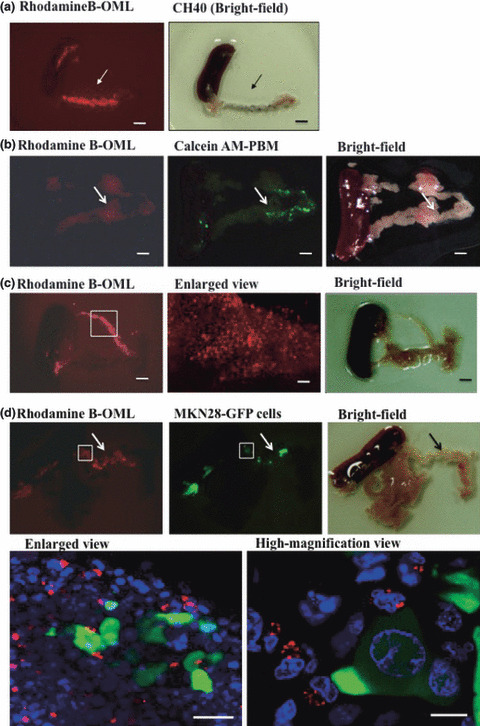
Accumulation of oligomannose‐coated liposome (OML)‐incorporated human peripheral blood monocytes (PBMs) and peritoneal macro‐phages (PEMs) in the mouse omentum. (a) Identification of milky spots by intraperitoneal injection of activated carbon particles (CH40). Note colocalization of Rhodamine B‐labeled OMLs (red) and milky spots (black) in the omentum. Bars = 1 mm. (b) Rhodamine B‐labeled OMLs were incubated with the human peripheral blood leukocytes from healthy subjects for 1 h at 37°C. After washing with PBS, OMLs‐bearing PBMs were stained with Calcein‐AM and injected into the mouse peritoneal cavity. Mice were sacrificed 24 h after injection and omentum (arrow) with spleen was isolated from mice (right panel). Accumulation of OML‐incorporated PBMs in the omentum is seen (left and center panel). Bars = 1 mm. (c) OMLs were incubated with the peritoneal cells from gastric cancer patients for 1 h at 37°C. After washing with PBS, OML‐bearing PEMs without Calcein‐AM staining were injected into the mouse peritoneal cavity. Mice were sacrificed 24 h after injection and omentum (arrow) with spleen was isolated from mice (right panel). Accumulation of OML‐incorporated PEMs is seen in the omentum (left and center panel). Bars = 1 mm. (d) Green fluorescent protein (GFP)‐tagged MKN‐28 gastric cancer cells were pre‐injected into mouse peritoneal cavity. Twenty‐four hours later, OML‐bearing human peripheral blood leukocytes from healthy subjects were injected into mouse peritoneal cavity. Mice were sacrificed 24 h after injection and omentum (arrow) with spleen and pancreas was isolated from mice (upper right panel). Co‐localization of OML‐incorporated PBMs (red) and metastatic tumor cells (green) was observed (upper left and center panel). Bars = 1 mm. In high magni‐fication view of the omentum, localization of OML‐incorporated PBMs (red) around tumor cells (green) is seen (lower panels). Bars = 50 μm (left), 10 μm (right).
Accumulation of human PBMs in resected human omentum ex vivo. We further examined whether OML‐incorporated PBMs accumulated in the milky spots of surgically resected patient omentum ex vivo. After overnight incubation of OML‐bearing PBMs with small pieces of patient omentum, OML‐bearing PBMs successfully accumulated in the milky spots of patient omentum (data not shown). When patient omentum was pre‐incubated with GCIY‐EGFP gastric cancer cells overnight ex vivo, OML‐incorporated human PBMs accumulated in the tumor cell foci in the omentum pre‐incubated with tumor cells (Fig. 5a). Microscopically, OML‐incorporated human PBMs infiltrated to the tumor cell nest and accumulated around individual tumor cells (Fig. 5b).
Figure 5.
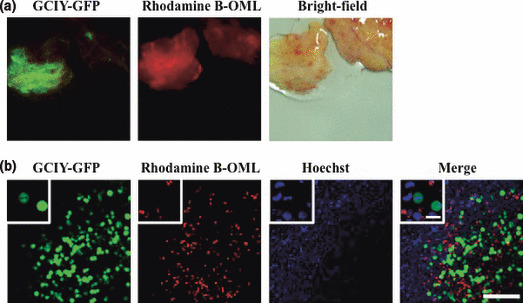
Accumulation of oligomannose‐coated liposome (OML)‐incorporated human peripheral blood monocytes (PBMs) in surgically resected patient omentum ex vivo. Green fluorescent protein (GFP)‐tagged GCIY cells were pre‐incubated with patient omentum for 12 h at 37°C. Rhodamine B‐labeled OML‐incorporated PBMs were then added to this omentum and further incubated for 12 h at 37°C. (a) Accumulation of OML‐incorporated PBMs (red) to the tumor cell nests (green) on the omentum. (b) Localization of OML‐incorporated PBMs (red) around the tumor cells (green). Inset: high‐magnification view of PBMs and tumor cells. Bar = 50 μm.
Discussion
We previously reported that when OMLs were injected into the mouse peritoneal cavity, OMLs were efficiently taken up by the mouse peritoneal macrophages and successfully transferred to the micrometastatic foci at the omentum in mice.( 17 ) In the present study, we demonstrated for the first time that human monocytes/macrophages derived from peripheral blood of healthy volunteers and peritoneal washes of gastric cancer patients efficiently took up OMLs and accumulated not only in the micrometastasis at the mouse omentum, but also in patient omentum ex vivo at a level comparable to that in mouse peritoneal macrophages as described above. These results suggest that DDS using OMLs and human PEMs have a potential to target peritoneal micrometastasis in gastric cancer patients and, therefore, would be a platform for new intraperitoneal chemotherapy with minimum dose of drug administration that should be translated to the clinical trials.
We found that the OML uptake rate by CD14‐positive PBMs was 88% on average, whereas the uptake rate of CD14‐positive PEMs from gastric cancer patients averaged 63%. We previously reported that the OML uptake rate by F4/80‐positive mouse macrophages was 72.8%.( 17 ) Therefore, the uptake rate of PEMs derived from gastric cancer patients seems to be slightly lower than that of PBMs from healthy volunteers and mouse PEMs. In two stage I patients, the OML uptake rate by PEMs was especially low (45 and 47%), whereas the uptake rate (an average 76%) in three advanced gastric cancer patients (stages II, II, and IV) was 66, 80, and 81%, respectively, which are comparable to that of mouse PEMs. Peritoneal washes from patients, especially from early gastric cancer patients, contain a small number of PEMs relative to other adherent cells such as mesothelial cells and stromal cells. Therefore, enrichment of macrophages by preferential attachment to a culture dish was difficult to perform. In addition, PEM samples are not as fresh as those of human PBMs and mouse PEMs, which can be isolated immediately after sampling. Indeed, it takes several hours to harvest the cell fraction from peritoneal washes of patients after sampling during an operation.( 21 ) Therefore, the slightly low OML uptake rate by PEMs from early gastric cancer patients may be due to the small number of viable and healthy macrophages harvested, although cytokine‐induced or a cell lineage‐related functional difference between human PEMs and other monocytes/macrophages cannot be ruled out. There is putative indication of intraperitoneal chemotherapy using DDS with OMLs for advanced gastric cancer patients with stages II–III,( 22 ) and the cases with low OML uptake rate were limited to the early gastric cancer patients. Therefore, low OML uptake rates by PEMs from patients may not always pose a major problem for future clinical application of this DDS.
To date, the mechanism of efficient OML uptake by macrophages still remains unclear. CD206, a macrophage mannose receptor, expressed on macrophages and dendritic cells, recognizes mannose, fucose, and N‐acetylglucosamine residues of glycoconjugates and is a well‐characterized endocytic receptor for uptake of bacteria and yeast.( 23 ) In this study, we found that most of the CD14‐positive PBMs express CD206 on their cell surface. In addition, 91% of CD206‐positive PBMs incorporate OMLs into their cytoplasm and this was efficiently inhibited by α‐methylmannoside. However, 62% of CD14‐positive/CD206‐negative cells can also uptake OMLs. These results suggest that CD206 is at least partly responsible for OML uptake by human PBMs and PEMs, although another carbohydrate‐dependent recognition system such as complement component C3, and complement receptor type 3 (CR3) may be also involved in the OML uptake by PBMs as reported previously.( 24 )
Another important finding in this study is the successful delivery of OMLs by human PBMs and PEMs to the milky spots and micrometastasis in the omentum of both mouse and human origin. When OML‐incorporated PBMs and PEMs were injected into the mouse peritoneal cavity, these macrophages migrated to the milky spots and micrometastasis in the mouse omentum at 100% (= 6/6 events) incidence. In addition, when OML‐incorporated human PBMs co‐cultured with small pieces of noncancerous omental tissues surgically resected from ovarian cancer patients, these macrophages successfully migrated to the milky spots in patient omentum and tumor cell nests in the omentum formed after pre‐incubation with gastric cancer cells ex vivo at 100% incidence (= 2/2 events). These results indicate the reproducibility and reliability of this DDS for targeting micrometastasis in the omentum. As revealed by confocal laser scanning microscopy, at the site of micrometastasis in the human omentum, OMLs were localized in the cytoplasm of macrophages, but not outside the cells, and accumulated around the tumor cells. No OMLs were found to directly incorporate into the tumor cells. This accumulation of OML‐incorporated PBMs or PEMs around tumor cells is not specific to tumor cells, but coincidental. However, this accumulation is close enough to kill tumor cells through the bystander effect. In this context, it is important to say that OMLs do not always accumulate in the disseminated metastatic foci in the peritoneal cavity. Therefore, indication of this OML is limited to micrometastasis at an early stage, but not to advanced metastasis, which extends to milky spot‐deficient organs.
The detailed mechanisms of accumulation of OML‐incorporated macrophages to micrometastatic foci at the milky spots in the omentum still remains unclear. It is clinically well known that omentectomy is widely done to reduce the risk of recurrence and to improve survival in ovarian cancer patients.( 25 ) Past experimental studies further demonstrated that the omentum seemed to act as an initial implantation site for malignant cells in the peritoneal cavity,( 26 ) and milky spots in the omentum seem to facilitate the adhesion and invasion of cancer cells.( 27 ) In the present study, we found that both OML‐incorporated human PBMs and PEMs reproducibly recruited in the omental milky spots like mouse PEMs. In addition, these phagocytic cells infiltrated the metastatic foci and accumulated around tumor cells in the omentum. These findings strongly suggest the possibility that residential and/or newly recruited monocytes/macrophages in the milky spots play some important role in the formation and progression of peritoneal metastases. Macrophages have long been suspected to stimulate tumor growth through pro‐inflammatory cytokines such as tumor necrosis factor (TNF)‐α and interleukin (IL)‐12.( 28 ) Therefore, the coincident accumulation of macrophages around metastatic tumor cells observed in this study suggests the increase in the risk of creating a microenvironment favorable for metastasis development by tumor‐associated macrophages (TAM).( 29 , 30 ) In fact, IL‐12 is known to be produced by PEMs in response to OML uptake.( 31 ) These findings suggest a potential limitation to the use of this DDS in a clinical setting. In this context, our strategy using OMLs as DDS and macrophages as a cellular vehicle seems to be paradoxical. However, if OML‐encapsulated anticancer drugs are released as the result of the macrophage killing, rather than physiological release by mild hyperthermia as reported previously,( 17 ) such a chemotherapy using this DDS in combination with the self‐killing mechanism would not only suppress growth of tumor cells, but also eliminate OML‐incorporated infiltrating macrophages, thus preventing the undesirable risk of promoting metastasis by TAMs. In a series of recent molecular biological studies, nuclear factor (NF)‐κB activation has been shown to play a pivotal role in these supportive functions of TAM.( 32 ) Further study is needed to clarify the molecular mechanisms of interaction between macrophages and tumor cells in the micrometastatic foci in the omentum and to develop a new method to control TAM.
In conclusion, we provided basic preclinical evidence to support clinical application of a new intraperitoneal chemotherapy in combination with DDS using OMLs. Various OML‐encapsulating anticancer drugs utilizing macrophages as a cellular vehicle could be a powerful platform for a new targeting therapy against micrometastasis with minimum toxic side effects for prevention of peritoneal recurrence after curative surgery in gastric cancer patients.
Acknowledgments
We thank Miss N. Yoshimura for expert technical assistance. This work was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan, from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), and from New Energy and Industrial Technology Development Organization.
References
- 1. Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol 1992; 1: 341–6. [DOI] [PubMed] [Google Scholar]
- 2. Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology 2000; 58: 96–107. [DOI] [PubMed] [Google Scholar]
- 3. Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 2004; 5: 219–28. [DOI] [PubMed] [Google Scholar]
- 4. Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long‐term survival after surgery for advanced gastric cancer. J Clin Oncol 1994; 12: 970–4. [DOI] [PubMed] [Google Scholar]
- 5. Ohashi N, Kodera Y, Nakanishi H et al. Efficacy of intraperitoneal chemotherapy with paclitaxel targeting peritoneal micrometastasis as revealed by GFP‐tagged human gastric cancer cell lines in nude mice. Int J Oncol 2005; 27: 637–44. [PubMed] [Google Scholar]
- 6. Nakanishi H, Kodera Y, Yamamura Y et al. Rapid quantitative detection of carcinoembryonic antigen‐expressing free tumor cells in the peritoneal cavity of gastric‐cancer patients with real‐time RT‐PCR on the lightcycler. Int J Cancer 2000; 89: 411–7. [DOI] [PubMed] [Google Scholar]
- 7. Kodera Y, Nakanishi H, Ito S et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real‐time reverse transcriptase‐polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 2002; 235: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakanishi H, Ito S, Mochizuki Y et al. Peritoneal carcinomatosis; A new diagnostic and therapeutic strategy. In: Cardinni DC, ed. In Research Focus on Gastric Cancer. New York: Nova Scientific Publishers, 2007; 55–73. [Google Scholar]
- 9. Armstrong DK, Bundy B, Wenzel L et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43. [DOI] [PubMed] [Google Scholar]
- 10. Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intra‐peritoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev 2003; 22: 465–72. [DOI] [PubMed] [Google Scholar]
- 11. Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer 2005; 5: 355–66. [DOI] [PubMed] [Google Scholar]
- 12. Sakakura C, Hagiwara A, Shirasu M et al. Polymerase chain reaction for detection of carcinoembryonic antigen‐expressing tumor cells on milky spots of the greater omentum in gastric cancer patients: a pilot study. Int J Cancer 2001; 95: 286–9. [DOI] [PubMed] [Google Scholar]
- 13. Hagiwara A, Takahashi T, Sawai K et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res 1993; 53: 687–92. [PubMed] [Google Scholar]
- 14. Krist LF, Kerremans M, Broekhuis‐Fluitsma DM, Eestermans IL, Meyer S, Beelen RH. Milky spots in the greater omentum are predominant sites of local tumour cell proliferation and accumulation in the peritoneal cavity. Cancer Immunol Immunother 1998; 47: 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dullens HF, Rademakers LH, Doffemont M, Van Veen PT, Bulder R, Den Otter W. Involvement of the omental lymphoid organ in the induction of peritoneal immunity against tumor cells. Invasion Metastasis 1993; 13: 267–76. [PubMed] [Google Scholar]
- 16. Burke B. Macrophages as novel cellular vehicles for gene therapy. Expert Opin Biol Ther 2005; 3: 919–24. [DOI] [PubMed] [Google Scholar]
- 17. Ikehara Y, Niwa T, Biao L et al. A carbohydrate recognition‐based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res 2006; 66: 8740–8. [DOI] [PubMed] [Google Scholar]
- 18. Hara M, Nakanishi H, Tsujimura K et al. Interleukin‐2 potentiation of cetuximab antitumor activity for epidermal growth factor receptor‐overexpressing gastric cancer xenografts through antibody‐dependent cellular cytotoxicity. Cancer Sci 2008; 99: 1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sobin LH. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol 2003; 21: 19–22. [DOI] [PubMed] [Google Scholar]
- 20. Nakanishi H, Ito S, Mochizuki Y, Tatematsu M. Evaluation of chemosensitivity of micrometastases with green fluorescent protein‐tagged tumor models in mice. In: Blumenthal RD, ed. Chemosensitivity. Totowa: Humana Press, 2005; 351–62. [DOI] [PubMed] [Google Scholar]
- 21. Ohashi N, Nakanishi H, Kodera Y et al. Intraoperative quantitative detection of CEA mRNA in the peritoneal lavage of gastric cancer patients with transcription reverse‐transcription concerted (TRC) method. A comparative study with real‐time quantitative RT‐PCR. Anticancer Res 2007; 27: 2769–77. [PubMed] [Google Scholar]
- 22. Ito S, Nakanishi H, Kodera Y, Mochizuki Y, Tatematsu M, Yamamura Y. Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer 2005; 93: 986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stahl PD, Ezekowitz RAB. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol 1998; 10: 50–5. [DOI] [PubMed] [Google Scholar]
- 24. Abe Y, Kuroda Y, Kuboki N, Matsushita M, Yokoyama N, Kojima N. Contribution of complement component C3 and complement receptor type 3 to carbohydrate‐dependent uptake of oligomannose‐coated liposomes by peritoneal macrophages. J Biochem 2008; 144: 563–70. [DOI] [PubMed] [Google Scholar]
- 25. Piver MS. Ovarian carcinoma. A decade of progress. Cancer 1984; 54: 2706–15. [DOI] [PubMed] [Google Scholar]
- 26. Tsujimoto H, Hagiwara A, Shimotsuma M et al. Role of milky spots as selective implantation sites for malignant cells in peritoneal dissemination in mice. J Cancer Res Clin Oncol 1996; 122: 590–5. [DOI] [PubMed] [Google Scholar]
- 27. Krist LF, Koenen H, Calame W et al. Ontogeny of milky spots in the human greater omentum: an immunochemical study. Anat Rec 1997; 249: 399–404. [DOI] [PubMed] [Google Scholar]
- 28. Mochizuki Y, Nakanishi H, Kodera Y et al. TNF‐alpha promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein (GFP)‐tagged human gastric cancer cell line. Clin Exp Metastasis 2004; 21: 39–47. [DOI] [PubMed] [Google Scholar]
- 29. Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF‐kappaB in cancer cells converts inflammation‐ induced tumor growth mediated by TNFalpha to TRAIL‐mediated tumor regression. Cancer Cell 2004; 6: 297–305. [DOI] [PubMed] [Google Scholar]
- 30. Pollard JW. Tumour‐educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–8. [DOI] [PubMed] [Google Scholar]
- 31. Takagi H, Furuya N, Kojima N. Preferential production of IL‐12 by peritoneal macrophages activated by liposomes prepared from neoglycolipids containing oligomannose residues. Cytokine 2007; 40: 241–50. [DOI] [PubMed] [Google Scholar]
- 32. Karin M, Cao Y, Greten FR, Li ZW. NF‐kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2: 301–10. [DOI] [PubMed] [Google Scholar]


