Abstract
Human CYP2A13, which is expressed in the respiratory tract, is the most efficient enzyme for the metabolic activation of tobacco‐specific nitrosamines such as 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK). The relevance of CYP2A13 in carcinogenicity and toxicity in the respiratory tract has been suggested, but the expression of CYP2A13 protein in lung cancer tissues remains to be determined. We first prepared a mouse monoclonal antibody against human CYP2A13. The antibody showed no cross reactivity with the other CYP isoforms including CYP2A6. Using the specific antibody, we performed immunohistochemical analysis for human lung carcinomas. In adenocarcinomas (n = 15), all specimens were positive for the staining and five samples showed strong staining. In squamous cell carcinomas (n = 15) and large cell carcinomas (n = 15), each 14 samples were positive for the staining and two and three samples showed strong staining, respectively. In small cell carcinoma samples (n = 15), eight samples were negative for the staining and five samples showed weak or moderate staining. In conclusion, we first found that the expression of CYP2A13 was markedly increased in non‐small cell lung carcinomas. The high expression might be associated with the tumor development and progression in non‐small cell lung carcinomas.
(Cancer Sci 2010; 101: 1024–1028)
The human cytochrome P450 2A (CYP2A) subfamily comprises three members: CYP2A6, CYP2A7, and CYP2A13.( 1 ) Among them, CYP2A6 and CYP2A13 are functional enzymes.( 2 , 3 ) They are composed of 494 amino acids with a high degree of identity (93.5%). It has been reported that CYP2A6 is expressed in liver, whereas CYP2A13 is predominantly expressed in the respiratory tract, with the highest level in the nasal mucosa, followed by the lung and trachea.( 3 , 4 , 5 ) The expression has been analyzed at the mRNA level. The tissue distribution of CYP2A13 at the protein level has not been determined because a specific antibody against CYP2A13 is not commercially available.
CYP2A13 is the most efficient enzyme in the metabolism of nicotine and cotinine as well as the metabolic activation of 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK) with similar substrate specificity as CYP2A6.( 3 , 6 ) Recently, we found that CYP2A13 efficiently metabolizes various environmental chemicals in air pollutants or tobacco smoke such as 4‐aminobiphenyl, naphthalene, styrene, and toluene.( 7 , 8 ) Since these chemicals are easily absorbed by inhalation, CYP2A13 in the lung plays an important role in the local metabolism of the chemicals. It is considered that CYP2A13 would be relevant to carcinogenicity and toxicity in the lung.
The human P450 isoforms of which the expression in lung cancer has been most studied are CYP1A1 and CYP1B1, because cigarette smoking containing polycyclic aromatic hydrocarbons induces their expression.( 9 ) Especially, CYP1B1 is highly expressed in lung non‐small cell carcinomas compared with normal tissues.( 10 , 11 ) CYP1A1 and CYP1B1 catalyze the metabolic activation of polycyclic aromatic hydrocarbons, which would be one of the causal factors of lung cancer. Since CYP2A13 is involved in the metabolic activation of environmental chemicals, it is important to know the expression level of CYP2A13 protein in lung cancer. Previously, Zhu et al. reported that CYP2A13 protein could not be detected in lung cancers by immunohistochemical analysis using a polyclonal antibody they made.( 12 ) In the present study, we originally prepared an antibody against CYP2A13. Using the antibody, we evaluated the expression of CYP2A13 protein in various types of human lung cancers.
Materials and Methods
Chemicals and reagents. Normal mouse IgG and biotinylated goat anti‐mouse IgG were obtained from Santa Cruz biotechnology (Santa Cruz, CA, USA) and Zymed (South San Francisco, CA, USA), respectively. Mayer’s hematoxylin solution was purchased from Wako Pure Chemical Industries (Osaka, Japan).
Recombinant human P450 enzymes. Recombinant human CYP1A1, CYP1B1, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 expressed in baculovirus‐infected insect cells (Supersomes) were purchased from BD Gentest (Woburn, MA, USA). E. coli membranes expressing human CYP2A6 and CYP2A13 were previously prepared.( 13 , 14 ) The expression system of human CYP2S1 in E. coli was constructed according to the method by Wu et al. ( 15 ) and E. coli membranes expressing CYP2S1 were also prepared. The P450 content and protein concentration were determined according to the method described previously.( 13 )
Preparation of antibody against CYP2A13. Recombinant human CYP2A13 expressed in E. coli was purified according to the method described previously.( 16 ) Mouse monoclonal antibody against human CYP2A13 was prepared by Kohjin Bio (Saitama, Japan). The hybridomas producing the antibodies were screened by ELISA with the purified recombinant CYP2A13 and CYP2A6. The clones reacted with CYP2A13, but not with CYP2A6 were selected. Among them, a clone showing highest reactivity was expanded by intraperitoneal injection into mineral oil‐primed mice. Monoclonal antibodies from mouse ascitic fluids were partially purified by precipitation with 33% ammonium sulfate. Finally, the specificity of the antibody was confirmed by immunoblot analysis as described below.
Immunoblot analysis. SDS‐polyacrylamide gel electrophoresis and immunoblot analysis were performed according to Laemmli.( 17 ) The recombinant CYP1A1, CYP1B1, CYP2A6, CYP2A13, CYP2B6, CYP2D6, CYP2E1, CYP2S1, and CYP3A4 (each 1 pmol) were separated on 10% polyacrylamide gel and transferred electrophoretically to a polyvinylidene difluoride membrane, Immobilon‐P (Millipore, Billerica, MA, USA). The membrane was blocked in 3% non‐fat dry milk in phosphate‐buffered saline (PBS) containing 0.1% Tween 20 for 12 h at room temperature. The membranes were incubated with the prepared mouse monoclonal anti‐CYP2A13 antibody (1:2000, PBS) for 1 h at room temperature. Biotinylated anti‐mouse IgG and the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA, USA) were used for diaminobenzidine staining.
Tissue samples. Fifteen specimens each of adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma obtained from surgically removed lung tissues were used. These specimens were collected between 1997 and 2005 at the Pathology Departments of Kanazawa University Hospital, Japan. The age and sex of the patients were as follows: adenocarcinomas, 44–75 years, 10 men and five women; squamous cell carcinomas, 36–83 years, 12 men and three women; large cell carcinomas, 36–80 years, 14 men and one woman; small cell carcinomas, 56–83 years, 14 men and one woman. These specimens were fixed in neutral formalin and then embedded in paraffin for immunohistochemistry. This study was approved by the Ethics Committee of Kanazawa University (Kanazawa, Japan).
Immunohistochemistry. The sections described above were first deparaffinized with xylene three times for 5 min each and hydrated gradually through a series of graded ethanol (100%, 99.5%, 90%, 70%). After the washes with distilled water for 5 min, the sections were treated with a liberated antibody binding solution (Polysciences, Warrington, PA, USA) for 8 min to liberate the antigen‐epitope site. Endogenous peroxidase activity in the sections was blocked with 3% hydrogen peroxide in PBS for 30 min. Nonspecific binding was blocked with 1.5% normal rabbit serum for 30 min at room temperature. The sections were then incubated with the mouse monoclonal anti‐CYP2A13 antibody at 4°C for 16 h. The sections were then rinsed in PBS for 5 min and incubated with biotinylated goat anti‐mouse IgG as the second antibody for 30 min at room temperature. After rinsing with PBS, staining reactions were performed using the ABC‐elite kit. After counterstaining with Mayer’s hematoxylin solution, the sections were mounted. As a negative control, normal mouse IgG was used instead of the anti‐CYP2A13 antibody. The staining intensity of tumor cells was estimated as follows: 0, no staining; 1, weakly staining; 2, moderately staining; 3, strongly staining. The estimated percentages of positive tumor cells were classified as follows: 0, none; 1, <10%; 2, 10–50%; 3, >50%. The products of the scores 0–1, 2–4, 6–9 were defined as −, +, ++, and +++, respectively.
Statistical analyses. The statistical significance of differences in the extent of staining between different types of lung carcinoma was tested by Fisher’s exact method. A value of P < 0.05 was considered statistically significant.
Results
Specificity of the anti‐human CYP2A13 antibody. The specificity of the raised monoclonal antibody against human CYP2A13 was evaluated with immunoblot analysis. A panel of recombinant CYP isoforms, CYP1A1, CYP1B1, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 expressed in baculovirus‐infected insect cells as well as CYP2A6, CYP2A13, and CYP2S1 expressed in E. coli membrane, were separated on SDS‐PAGE (Fig. 1). The antibody prepared in this study specifically reacted with CYP2A13. It did not react with the other P450 isoforms including CYP2A6.
Figure 1.
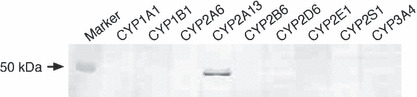
Immunoblot analysis using the mouse monoclonal antibody against human CYP2A13 prepared in this study. One pmol each of recombinant P450 isoforms, CYP1A1, CYP1B1, CYP2B6, CYP2D6, CYP2E1, and CYP3A4 expressed in baculovirus‐infected insect cells and CYP2A6, CYP2A13, and CYP2S1 expressed in E. coli, were separated on 10% SDS‐PAGE.
Immunohistochemical analysis of CYP2A13 for human lung cancer. The antibody was used for the immunohistochemical analysis of a total of 60 lung cancer tissues. In adjacent noncancerous tissues, strong staining was observed in the epithelial cells of the bronchus (Fig. 2a), but no staining was observed in peripheral lung tissues (Fig. 2b). We confirmed the absence of staining in human liver (data not shown), supporting the specificity of this antibody. Next, we evaluated the staining of different types of lung carcinoma (Fig. 2c–f). All of 15 adenocarcinomas showed positive immunostaining, and five and three samples were judged as +++ and ++, respectively (Table 1). Each 14 samples of 15 squamous cell carcinomas and 15 large cell carcinomas were positive for the staining, and eight squamous cell carcinomas and six large cell carcinomas were judged as +++ or ++. In contrast, in 15 small cell carcinomas, eight samples were negative for the staining and five samples were judged as + or ++. These results suggest that CYP2A13 is highly expressed in non‐small cell carcinomas.
Figure 2.
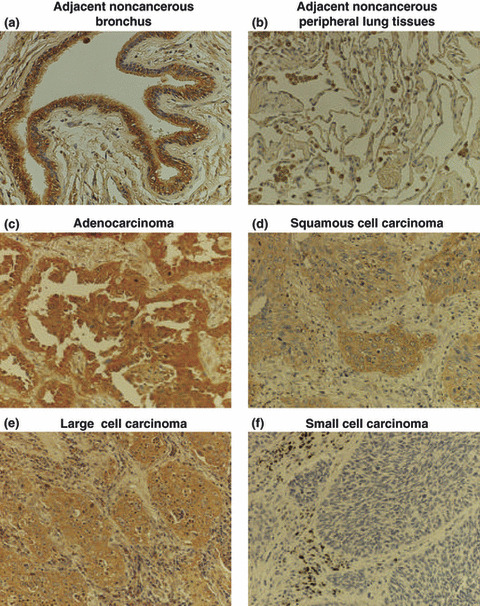
Immunohistochemical analysis of CYP2A13 in human lung cancer tissues. Adjacent noncancerous (a) bronchus and (b) peripheral lung tissues; (c) adenocarcinoma; (d) squamous cell carcinoma; (e) large cell carcinoma; (f) small cell carcinoma. Strong immunostaining was observed in the epithelial cells in bronchus, but not in peripheral lung tissues. In lung carcinomas, immunostaining was positive in most of non‐small cell carcinomas. In contrast, the staining was mostly negative in small cell carcinoma. Original mag‐nification, ×200.
Table 1.
CYP2A13‐specific staining in lung adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma
| Lung carcinoma type | Immunoreactive score | |||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| Adenocarcinoma (n = 15) | 0 | 7 | 3 | 5 |
| Squamous cell carcinoma (n = 15) | 1 | 6 | 6 | 2 |
| Large cell carcinoma (n = 15) | 1 | 8 | 3 | 3 |
| Small cell carcinoma (n = 15) | 8 | 4 | 1 | 2 |
The statistical significance of differences in the extent of staining in the different lung carcinoma types was tested by Fisher’s exact method (P < 0.05).
Relationship between the CYP2A13 levels and smoking status or clinical characteristics of lung cancer. The relationship between the CYP2A13 staining levels and smoking status was evaluated (Table 2). In 15 adenocarcinomas and 15 squamous cell carcinomas, 10 and 12 samples were smokers, respectively. All of 15 large cell carcinomas and small cell carcinomas were smokers. No relationship was observed between the CYP2A13 immunostaining levels and the pack‐years. We also investigated the relationship between the CYP2A13 staining levels and the extents of the primary tumor (T‐factor), regional lymph node metastasis (N‐factor), and distant metastasis (M‐factor), and the clinical stage was also evaluated (Table 2). In adenocarcinomas, squamous cell carcinomas, and large cell carcinomas, no relationship was observed. In small cell carcinomas, significant differences were observed in the extent of CYP2A13 staining with the values of N‐factor and the clinical stage. However, we could not draw definitive conclusions because of the limited number of samples.
Table 2.
Characteristics of patients with lung adenocarcinoma, squamous cell carcinoma, large cell carcinoma, or small cell carcinoma in relation to expression level of CYP2A13
| Adenocarcinoma | Squamous cell carcinoma | Large cell carcinoma | Small cell carcinoma | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | P | − | + | ++ | +++ | P | − | + | ++ | +++ | P | − | + | ++ | +++ | P | |
| Smoking status | ||||||||||||||||||||
| Never smokers | 0 | 2 | 1 | 2 | 1.000 | 1 | 1 | 1 | 0 | 0.446 | 0 | 0 | 0 | 0 | 1.000 | 0 | 0 | 0 | 0 | 1.000 |
| Smokers | 0 | 5 | 2 | 3 | 0 | 5 | 5 | 2 | 1 | 8 | 3 | 3 | 8 | 4 | 1 | 2 | ||||
| Pack‐years | ||||||||||||||||||||
| <35 | 4 | 2 | 3 | 1.000 | 1 | 2 | 0 | 1.000 | 0 | 2 | 0 | 2 | 0.351 | 3 | 0 | 0 | 0 | 0.434 | ||
| 35–64 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 5 | 1 | 1 | 3 | 3 | 0 | 1 | ||||||
| 65–94 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | ||||||
| 95< | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ||||||
| T‐factor | ||||||||||||||||||||
| 1 | 0 | 2 | 1 | 3 | 0.670 | 1 | 2 | 1 | 1 | 0.914 | 0 | 1 | 0 | 1 | 0.990 | 4 | 4 | 0 | 0 | 0.119 |
| 2 | 0 | 4 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 3 | 1 | 0 | 3 | 0 | 1 | 2 | ||||
| 3 | 0 | 0 | 1 | 1 | 0 | 2 | 3 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | ||||
| 4 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | ||||
| N‐factor | ||||||||||||||||||||
| 0 | 0 | 3 | 2 | 5 | 0.400 | 0 | 4 | 5 | 1 | 0.188 | 1 | 4 | 1 | 1 | 1.000 | 6 | 0 | 0 | 0 | 0.004 |
| 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | ||||
| 2 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 2 | 1 | 2 | 3 | 1 | 0 | ||||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| M‐factor | ||||||||||||||||||||
| 0 | 0 | 6 | 2 | 5 | 0.667 | 1 | 5 | 6 | 2 | 1.000 | 1 | 8 | 3 | 3 | 1.000 | 8 | 4 | 1 | 2 | 1.000 |
| 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Clinical Stage | ||||||||||||||||||||
| IA | 0 | 1 | 1 | 3 | 0.908 | 0 | 1 | 1 | 1 | 0.752 | 0 | 1 | 0 | 1 | 1.000 | 3 | 0 | 0 | 0 | 0.052 |
| IB | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | ||||
| IIA | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||||
| IIB | 0 | 1 | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 0 | 0 | 0 | 2 | ||||
| IIIA | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 3 | 1 | 0 | ||||
| IIIB | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | ||||
| IV | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| I | 0 | 3 | 1 | 4 | 0.907 | 0 | 1 | 2 | 1 | 0.717 | 0 | 2 | 0 | 1 | 0.930 | 5 | 0 | 0 | 0 | 0.012 |
| II | 0 | 2 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | 2 | ||||
| III | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 3 | 2 | 0 | 3 | 3 | 1 | 0 | ||||
| IV | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
Discussion
In previous studies, the tissue distribution of CYP2A13 was analyzed at the mRNA level, demonstrating the expression in the respiratory tract including the nasal mucosa, trachea, and lung.( 3 , 4 , 5 ) Recently, Wong et al. successfully detected CYP2A13 protein in fetal nasal microsomes by immunoblotting using a polyclonal anti‐mouse Cyp2a5 antibody.( 18 ) Although this antibody reacted with CYP2A6, they could separate CYP2A13 from CYP2A6 by high resolution SDS‐PAGE using a DNA sequencing apparatus. Zhang et al. detected CYP2A13 protein in lung microsomes by immunoblotting using the anti‐Cyp2a5 antibody, in which immunoprecipitants from lung microsomes using the anti‐Cyp2a5 antibody were separated.( 19 ) Thus, since a specific antibody to CYP2A13 has not been available, great effort has been required to detect CYP2A13 protein. This background prompted us to prepare a specific antibody against human CYP2A13.
The amino acid identities of CYP2A13 with CYP2A6, CYP2B6, and CYP2S1 are 93.5%, 53.4%, and 47.5%, respectively. It has been demonstrated that a monoclonal antibody against CYP2A6 from BD Gentest reacts with not only CYP2A6 but also CYP2A13 and CYP2E1.( 12 ) It seems that anti‐rat CYP2A antibody reacted with CYP2S1 protein in human hepatic stellate cells.( 20 ) We investigated whether our antibody against CYP2A13 may react with other P450 isoforms including CYP2A6, CYP2B6, and CYP2S1. In the results, it was demonstrated that the antibody specifically reacted with CYP2A13 (Fig. 1). Thus, we succeeded in preparing an antibody specific to human CYP2A13.
Using the specific antibody against CYP2A13, we performed immunoblot analysis using human normal lung microsomes, but we observed no band (data not shown). Then, we performed immunohistochemical analysis using lung cancer tissues. In adjacent noncancerous tissues, strong staining was observed in the epithelial cells of the bronchus, but no staining was observed in peripheral lung tissues (Fig. 2a,b). Thus, the expression of CYP2A13 is not uniform in the lung, supporting the negative results of the immunoblot analysis using microsomes prepared from whole lung tissues. The distribution of CYP2A13 in the epithelial cells of the bronchus would be physiologically reasonable, because CYP2A13 plays a role in the metabolism of environmental chemicals. When four kinds of lung carcinomas were evaluated for the CYP2A13 expression, we found differences in the extent of the immunostaining. The strong staining was observed in most samples of non‐small cell carcinomas. In general, it is well known that squamous cell carcinoma and small cell carcinoma are associated with tobacco smoking.( 21 , 22 ) Adenocarcinoma is the most common type of lung cancer in female nonsmokers, and it is increasingly associated with smoking as well. In contrast, no association has been reported for large cell carcinoma with smoking.( 23 ) Recently, we found that CYP2A13 metabolically activates 4‐aminobiphenyl, naphthalene, and styrene which are carcinogenic components in tobacco smoke.( 7 , 8 ) CYP2A13 metabolically activates NNK, a causal factor of lung adenocarcinoma.( 24 ) In support, there are studies showing a relationship between the genetic polymorphism of the CYP2A13 gene and the risk of lung adenocarcinoma.( 25 , 26 ) Thus, higher expression of CYP2A13 in adenocarcinoma and squamous cell carcinoma may be associated with the tumor development and progression. However, we have no conclusive explanation for low expression of CYP2A13 in small cell carcinoma. The regulation mechanism of CYP2A13 may be different in different cancer types. Furthermore, we cannot speculate the reason at the present why the cancer cells still need the function protein of CYP2A13 after carcinogenic events. Clarifying this issue would contribute to our understanding for physiological and biological significance of CYP2A13.
While performing this study, another research group also generated a specific antibody against CYP2A13.( 12 ) In contrast to our study, they used a synthetic peptide covering C‐terminal amino acid residues 369 to 377 as an antigen. They confirmed that the antibody reacted with CYP2A13 but not with CYP2A6, CYP2S1, CYP3A4, and mouse CYP2A5 by immunoblot analysis using recombinant enzymes. Using this antibody, they have reported that immunostaining was observed in normal trachea but not in normal peripheral lung tissues, supporting our study. However, in contrast to our results, they reported that the expression of CYP2A13 protein was not detected in any adenocarcinomas (n = 6), squamous carcinomas (n = 3), large cell carcinomas (n = 3), alveolar carcinomas (n = 3), basaloid carcinoma (n = 1), or papillary bronchiolar carcinoma (n = 1). The reason for this discrepancy is not clear, but it may partly depend on differences in the numbers of samples or individual differences. Another possibility is that differences in the reactivity of the antibodies used may lead to conflicting results.
In summary, we prepared a specific monoclonal antibody against CYP2A13. The immunohistochemical analysis using this antibody demonstrated that, in lung tissues, the expression of CYP2A13 is localized in epithelial cells in the bronchus. We first found that the expression of CYP2A13 was markedly increased in lung non‐small cell lung carcinomas. The high expression might be associated with the tumor development and progression in non‐small cell lung carcinomas.
Disclosure Statement
None of the authors have any conflicts of interest to declare.
Acknowledgments
We acknowledge Brent Bell for reviewing the manuscript.
References
- 1. Fernandez‐Salguero P, Hoffman SMG, Cholerton S et al. A genetic polymorphism in coumarin 7‐hydroxylation: sequence of the human CYP2A genes and identification of variant CYP2A6 alleles. Am J Hum Genet 1995; 57: 651–60. [PMC free article] [PubMed] [Google Scholar]
- 2. Yamano S, Tatsuno J, Gonzalez FJ. The CYP2A3 gene product catalyzes coumarin 7‐hydroxylation in human liver microsomes. Biochemistry 1990; 29: 1322–9. [DOI] [PubMed] [Google Scholar]
- 3. Su T, Bao Z, Zhang QY et al. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco‐specific carcinogen, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone. Cancer Res 2000; 60: 5074–9. [PubMed] [Google Scholar]
- 4. Koskela S, Hakkola J, Hukkanen J et al. Expression of CYP2A genes in human liver and extrahepatic tissues. Biochem Pharmacol 1999; 57: 1407–13. [DOI] [PubMed] [Google Scholar]
- 5. Gu J, Su Y, Chen QY et al. Expression and biotransformation enzymes in human fetal olfactory mucosa: potential roles in developmental toxicity. Toxicol Appl Pharmacol 2000; 165: 158–62. [DOI] [PubMed] [Google Scholar]
- 6. Bao Z, He XY, Ding X et al. Metabolism of nicotine and cotinine by human cytochrome P450 2A13. Drug Metab Dispos 2005; 33: 258–61. [DOI] [PubMed] [Google Scholar]
- 7. Nakajima M, Itoh M, Sakai H et al. CYP2A13 expressed in human bladder metabolically activates 4‐aminobiphenyl. Int J Cancer 2006; 119: 2520–6. [DOI] [PubMed] [Google Scholar]
- 8. Fukami T, Katoh M, Yamazaki H et al. Human cytochrome P450 2A13 efficeintly metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem Res Toxicol 2008; 21: 720–5. [DOI] [PubMed] [Google Scholar]
- 9. Chang JT, Chang H, Chen PH et al. Requirement of aryl hydrocarbon receptor overexpression for CYP1B1 up‐regulation and cell growth in human lung adenocarcinomas. Clin Cancer Res 2007; 13: 38–45. [DOI] [PubMed] [Google Scholar]
- 10. Murray GI, Taylor MC, McFadyen MCE et al. Tumor‐specific expression of cytochrome P450 CYP1B1. Cancer Res 1997; 57: 3026–31. [PubMed] [Google Scholar]
- 11. Spivack SD, Hurteau GJ, Reilly AA et al. CYP1B1 expression in human lung. Drug Metab Dispos 2001; 29: 916–22. [PubMed] [Google Scholar]
- 12. Zhu LR, Thomas PE, Lu G et al. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide‐specific antibody. Drug Metab Dispos 2006; 34: 1672–6. [DOI] [PubMed] [Google Scholar]
- 13. Fukami T, Nakajima M, Yoshida R et al. A novel polymorphism of human CYP2A6 gene CYP2A6*17 has an amino acid substitution (V365M) that decreases enzymatic activity in vitro and in vivo. Clin Pharmacol Ther 2004; 76: 519–27. [DOI] [PubMed] [Google Scholar]
- 14. Yamanaka H, Nakajima M, Fukami T et al. CYP2A6 and CYP2B6 are involved in nornicotine formation from nicotine in humans: interindividual differences in these contributions. Drug Metab Dispos 2005; 33: 1811–8. [DOI] [PubMed] [Google Scholar]
- 15. Wu ZL, Sohl CD, Shimada T et al. Recombinant enzymes overexpressed in bacteria show broad catalytic specificity of human cytochrome P450 2W1 and limited activity of human cytochrome P450 2S1. Mol Pharmacol 2006; 69: 2007–14. [DOI] [PubMed] [Google Scholar]
- 16. Yamazaki H, Nakajima M, Nakamura M et al. Enhancement of cytochrome P450 3A4 catalytic activities by cytochrome b 5 in bacterial membranes. Drug Metab Dispos 1999; 27: 999–1004. [PubMed] [Google Scholar]
- 17. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriopharge T4. Nature 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 18. Wong HL, Zhang X, Zhang QY et al. Metabolic activation of the tobacco carcinogen 4‐(methylnitrosamino)‐(3‐pyridyl)‐1‐butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol 2005; 18: 913–8. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, D’Agostino J, Wu H et al. CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco‐specific carcinogen 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone. J Pharmacol Exp Ther 2007; 323: 570–8. [DOI] [PubMed] [Google Scholar]
- 20. Marek CJ, Tucker SJ, Koruth M et al. Expression of CYP2S1 in human hepatic stellate cells. FEBS Lett 2007; 581: 781–6. [DOI] [PubMed] [Google Scholar]
- 21. Wang SY, Hu YL, Wu YL et al. A comparative study of the risk factors for lung cancer in Guangdong, China. Lung Cancer 1996; 14 (Suppl 1): S99–105. [DOI] [PubMed] [Google Scholar]
- 22. Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking‐related risk of lung cancer. Epidemiology 1992; 3: 61–4. [DOI] [PubMed] [Google Scholar]
- 23. Morabia A, Wynder EL. Cigarette smoking and lung cancer cell types. Cancer (Phil) 1991; 68: 2074–8. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann D, Rivenson A, Murphy SE et al. Cigarette smoking and adenocarcinoma of the lung: the relevance of nicotine‐derived N‐nitrosamines. J Smok Rel Disord 1993; 4: 165–89. [Google Scholar]
- 25. Wang H, Tan W, Hao B et al. Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco‐specific carcinogen NNK. Cancer Res 2003; 63: 8057–61. [PubMed] [Google Scholar]
- 26. Kiyohara C, Takayama K, Nakanishi Y. CYP2A13, CYP2A6, and the risk of lung adenocarcinoma in a Japanese population. J Health Sci 2005; 51: 658–66. [Google Scholar]


