Abstract
Anthracycline‐based chemotherapy represents a milestone in the treatment of breast cancer. We previously demonstrated in an in vitro model that cyclin E overexpression is associated with increased expression of manganese superoxide dismutase (MnSOD) and resistance to doxorubicin. In the present study, immunohistochemical expression of cyclin E and MnSOD was evaluated in 134 early breast cancer patients receiving adjuvant epirubicin‐based chemotherapy regimens containing epirubicin. Both parameters were correlated with the available clinicopathological parameters and with the outcome of patients. Overexpression of cyclin E and MnSOD was detected in 46 (34.3%) and 56 (41.8%) patients, respectively, and expression levels of the two proteins were related. Disease‐free and alive patients displayed a lower mean percentage of cyclin E‐expressing cells than relapsed and dead patients, respectively. Kaplan–Meier survival analysis demonstrated a significant separation between high versus low cyclin E‐expressing tumors in terms of overall survival (P = 0.038 by log‐rank). Similar results were obtained considering the subset of node‐negative patients separately. No significant relationship with patient outcome was observed for MnSOD expression levels. At multivariate analysis cyclin E failed to demonstrate an independent prognostic value. In conclusion, the results of the present study support previous evidence that increased cyclin E expression is associated with higher MnSOD expression levels and poorer outcome, at least as evaluated in terms of overall survival. Further studies are warranted to evaluate the usefulness of cyclin E as a prognostic marker to identify breast cancer patients at higher risk of death from the disease when treated with adjuvant anthracycline‐based therapy. (Cancer Sci 2009; 100: 1026–1033)
Breast cancer is the leading cause of cancer and the second leading cause of cancer death in women in the USA( 1 ) and its incidence is increasing in many countries, including Italy.( 2 ) Adjuvant treatments produce significant benefit in operable breast cancer. However, despite surgical resection and subsequent adjuvant therapy, approximately 20–25% of node‐negative breast cancer patients still develop recurrence or distant metastases within 10 years of diagnosis. Available risk factors are not able to clearly identify patients at high risk of disease recurrence and progression.( 3 ) Thus, the search for new molecular markers continues to be a hot topic in breast cancer research.
Anthracycline‐based chemotherapy represents a milestone in the treatment of both early and advanced breast cancer. The 1998 Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta‐analysis first demonstrated an absolute risk reduction in terms of disease relapse and death in operable breast cancer patients undergoing polychemotherapy and an absolute anthracycline‐related 3% risk reduction of recurrence and death in node‐positive patients compared to a cyclophosphamide, methotrexate, and 5‐fluorouracil regimen after 10 years of follow up.( 4 ) Risk reduction was substantially independent of age, node status, estrogen receptor (ER) status, and menopausal status, although the absolute advantage was proportional to baseline risk score of relapse and decreased with increasing age. The subsequent 2005 EBCTCG meta‐analysis confirmed that approximately 6 months of adjuvant anthracycline‐based polychemotherapy reduced the breast cancer‐related death rate( 5 ) and such an advantage has been recently confirmed by the last 2008 EBCTCG meta‐analysis in ER‐negative patients.( 6 )
Potential severe risks have to be taken into consideration when prescribing an anthracycline‐based chemotherapy regimen and in particular long‐term side effects have to be considered in the adjuvant setting.( 7 ) Indeed, two long‐term toxicities of epirubicin and doxorubicin treatment are of great clinical relevance: cardiac heart failure and secondary leukemia. The overall incidence of these side effects is quite low (~1%) and the impact on overall mortality is minimal, but they may significantly affect long‐term quality of life and so warrant consideration when selecting patients for anthracycline‐based chemotherapy,( 8 , 9 , 10 ) especially considering the fact that more than half of node‐positive and approximately one‐third of node‐negative patients relapse within 10 years.( 11 )
In mammalian cells, the cell cycle is tightly regulated by a family of kinases (cyclin‐dependent kinases, Cdk) that are activated by different cyclins and regulate cell cycle progression. Deregulated expression of cell cycle regulatory proteins is a frequent event in human malignancies and has been reported to have prognostic significance in several human tumors.( 12 , 13 , 14 ) Cyclin E plays a pivotal role in cell cycle control, driving mammalian cells through the G1 to S phase transition by binding to and activating CDK2.( 15 ) Transgenic mice overexpressing cyclin E develop breast cancers( 16 ) and cyclin E has been reported as an important player in the human multistep tumorigenesis process, increasing proliferation and genetic instability.( 17 , 18 ) Moreover, it has been suggested as a promising new prognostic and predictive marker in breast as well as other human cancers.( 12 , 14 , 19 , 20 , 21 ) We previously reported that cyclin E‐overexpressing rat fibroblasts display an increased resistance to doxorubicin and related this effect to an increased expression of manganese superoxide dismutase (MnSOD), which was also evident in the same cyclin E‐overexpressing derivatives. The overexpression of MnSOD was able to reduce reactive oxygen species‐derived cell damage associated with doxorubicin treatment.( 22 ) Thus, it was of interest to verify whether a relationship also exists between cyclin E expression and the response to anthracycline in tumor cells in vivo.
To this aim the expression of cyclin E and MnSOD were evaluated in a group of early breast cancer patients who received an epirubicin‐based adjuvant chemotherapy regimen, and their relationship with clinicopathological parameters and patient outcome was analyzed. The results obtained demonstrate that increased expression levels of cyclin E correlated with high MnSOD expression and were associated with reduced overall survival. The implications of these findings are discussed.
Materials and Methods
Patient characteristics and tissue samples. Tumor samples were obtained from breast cancer patients who underwent surgery at the Santa Chiara University Hospital in Pisa and Versilia Hospital in Lido di Camaiore (Italy) from 1994 to 2001. Patients undergoing conservative breast surgery received complementary radiotherapy. A total of 134 early breast cancer patients receiving an epirubicin‐based adjuvant chemotherapeutic regimen were retrospectively selected for the study. Patients receiving pre‐operative chemotherapy or having a family history of breast cancer were excluded as well as those for whom follow‐up data were missing. In particular, 108 out of 134 patients (80.6%) received six cycles of a combination of cyclophosphamide, epirubicin, and 5‐fluorouracil, three patients (2.2%) received four cycles of a doublet containing cyclophosphamide and epirubicin, 19 patients (14.2%) received a sequence of four cycles of epirubicin followed by four cycles of cyclophosphamide, methotrexate, and 5‐fluorouracil, and four patients (3.0%) received a sequence of four cycles of epirubicin followed by four cycles of paclitaxel (Table 1).
Table 1.
Baseline patient characteristics
| Characteristic | n (%) |
|---|---|
| Number of patients | 134 |
| Age (years) | |
| Median [range] | 56 [32–77] |
| Histotype † | |
| Invasive ductal carcinoma | 122 (91.0) |
| Invasive lobular carcinoma | 2 (1.5) |
| Mixed (ductal and lobular) | 2 (1.5) |
| Adenoid cystic carcinoma | 4 (3.0) |
| Undifferentiated | 2 (1.5) |
| Medullary carcinoma | 2 (1.5) |
| Tumor stage § | |
| I | 22 (16.4) |
| IIa | 40 (29.9) |
| IIb | 8 (6.0) |
| IIIa | 28 (21.0) |
| IIIb | 2 (1.5) |
| IIIc | 34 (25.2) |
| Tumor grade | |
| G2 | 45 (33.6) |
| G3 | 83 (61.9) |
| Unknown | 6 (4.5) |
| Chemotherapy | |
| CEF | 108 (80.6) |
| E→CMF | 19 (14.2) |
| E→TXL | 4 (3.0) |
| Cyclophosphamide and epirubicin | 3 (2.2) |
According to World health Organization histological typing of breast tumors (see text for complete reference( 25 )).
According to UICC – TNM classification of malignant tumors, sixth edition 2002.( 23 )
According to Elston and Ellis classification (see text for complete reference( 24 )).CEF, cyclophosphamide, epirubicin, and 5‐fluorouracil; E→CMF, epirubicin followed by cyclophosphamide, methotrexate, and 5‐fluorouracil; E→TXL, epirubicin followed by paclitaxel.
The median age at diagnosis was 56 years (range 32–77 years) and the median follow‐up time was 72 months (range 21–122 months). Seventy (52.2%) out of 134 patients experienced a disease relapse during the follow‐up period and 32 (23.9%) died of the disease. The selection did not require the approval of the Institutional Ethical Committee because the samples were coded and the names of the patients were not revealed. All available clinicopathological data were collected and stored in an appropriate database. Age, tumor grade and stage,( 23 , 24 ) size, histotype,( 25 ) ER status, and progesterone receptor (PgR) status were considered.
Immunohistochemistry. Cyclin E and MnSOD expression were evaluated by immunohistochemistry (IHC). All analyses were carried out on routinely processed, formalin‐fixed and paraffin‐embedded tissue samples as previously described.( 26 , 27 , 28 ) Briefly, representative tumor sections (3 µm) were deparaffinized, rehydrated, and immunostained using antigen retrieval by microwave technique at 750 W for 15 min in 10 mM citrate buffer (pH 6). After cooling down to room temperature the endogenous peroxidase was blocked with 1% H2O2 in methanol for 5 min. Sections were incubated for 1 h at room temperature with anti‐human cyclin E (clone HE‐12; PharMingen, San Diego, CA, USA) and anti‐MnSOD (Calbiochem‐Merck Eurolab, Darmstadt Germany) monoclonal antibodies diluted 1 : 100. After washing, sections were immunostained using the avidin–biotin–peroxidase complex method (Vectastain Elite ABC kit; Vector, Burlingame, CA, USA). The peroxidase activity was visualized with 0.05% 3,3‐diaminobenzidine (Sigma Chemical Co., St Louis, MO, USA) and 0.2% H2O2. The slides were rinsed with phosphate‐buffered saline and counterstained with hematoxylin. Sections of known positive mammary carcinoma were used as positive controls. Negative controls were obtained by omitting the primary antibodies.
For cyclin E, only clear nuclear staining was considered positive (Fig. 1a,b). A minimum of 1000 cells was counted for each tumor and immunoreactivity was expressed as the percentage of positive cells over the total number of tumor cells. A value of 3% positive cells, corresponding to the median value of cyclin E‐expressing tumor cells, was used as the cut‐off value to distinguish high‐ and low‐expressing tumors. For MnSOD, a score system ranging from 0 to 3 was used to differentiate tumor cell expression. In positive cells, a near homogeneous cytoplasmic staining was detected whereas nuclei showed no staining (Fig. 1c,d). Specimens showing no staining were considered negative (score 0). In positive cells, the intensity of staining was graded as follows: weak (score 1), moderate (score 2), and strong (score 3). Score 0 samples were considered negative whereas scores from 1 to 3 were considered positive. The immunostained specimens were evaluated by two observers independently (AS and PC) without knowledge of clinical characteristics or follow‐up information and the discrepant cases were jointly re‐evaluated.
Figure 1.
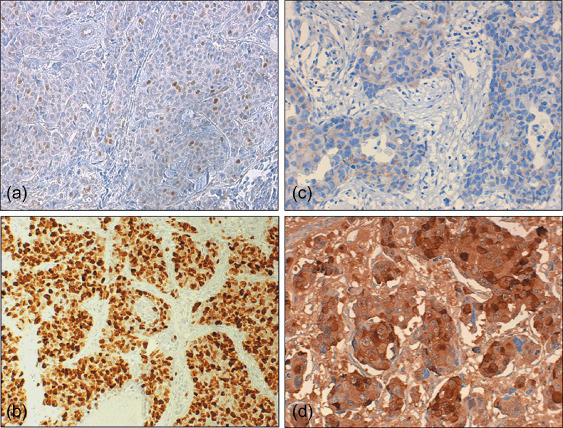
Immunohistochemical analysis of (a,b) cyclin E and (c,d) manganese superoxide dismutase (MnSOD) in primary breast cancers. Shown are examples of (a) low and (b) high cyclin E‐expressing tumors, and (c) weak and (d) strong staining for MnSOD. Original magnification, (a, b) ×120 and (c,d) ×200.
Statistical analysis. The relationships between cyclin E, MnSOD, and other molecular and clinical parameters were assessed by contingency table methods and tested for significance using Pearson's χ2‐test. Mean values were compared using Student's t‐test. Disease‐free survival (DFS) was defined as the interval between surgery and the first documented evidence of disease in local‐regional area, distant sites, contralateral breast, or a combination of the above. Relapses were accurately assessed by clinical, radiological and, whenever feasible, histopathological examination. Overall survival (OS) was defined as the interval between surgery and death from the disease. Patients who died of causes unrelated to disease were not included in the survival analyses. Survival curves were calculated using the Kaplan–Meier method and tested for significance using the log‐rank test. Univariate and multivariate relative risks were calculated using the Cox proportional hazards regression. Statistical analyses were carried out using SPSS (Chicago, IL, USA) software. All tests were two‐tailed, and P < 0.05 was considered to be significant.
Results
Expression of cyclin E and MnSOD were evaluated by IHC in a series of 134 breast cancers. For cyclin E, only clear nuclear staining in the absence of cytoplasmic background coloration was considered positive and a value of 3% of positive cells, corresponding to the median value of cyclin E‐expressing tumor cells (mean = 11%; range 0–80%), was used as the cut‐off value to distinguish high‐ and low‐expressing tumors. For MnSOD, near‐homogeneous cytoplasmic staining was detected (when present) in all tumor cells whereas nuclei showed no staining. Specimens showing no staining were considered negative (score 0). In positive cells, the intensity of staining was graded as weak (score 1), moderate (score 2), and strong (score 3).
Cyclin E expression was considered high in 46 (34.3%) out of 134 tumors in our series. No relationship was observed between cyclin E expression and other clinicopathological parameters such as age, ER status, PgR status, and tumor grading (Table 2). However, high cyclin E expression levels were significantly more frequent in higher‐size (pT2–3) compared to lower‐size (pT1) tumors (P = 0.03). For MnSOD, staining was detected in 56 (42%) cases and showed no relationship with the aforementioned parameters whereas only a trend for significance was observed between positive staining for MnSOD and higher tumor size (P = 0.08) (Table 2). We preferred to stratify patients using 50 years of age as a cut‐off value as this age is usually considered the threshold for entering menopause, which is an important prognostic factor for breast cancer patients, but similar results were obtained in all of the analyses using the median age (56 years) as the cut‐off value.
Table 2.
Relationship between cyclin E and manganese superoxide dismutase (MnSOD) expression and main tumor characteristics in a series of 134 early breast cancer patients receiving adjuvant epirubicin‐based regimens
| Characteristic | Cyclin E expression | MnSOD expression | |||||
|---|---|---|---|---|---|---|---|
| Total | Low | High | P‐value | Negative | Positive | P‐value | |
| Age (years) | |||||||
| ≤50 | 65 | 40 | 25 | 35 | 30 | ||
| >50 | 69 | 48 | 21 | 0.4 | 43 | 26 | 0.4 |
| Node status | |||||||
| Negative | 64 | 47 | 17 | 34 | 30 | ||
| Positive | 70 | 41 | 29 | 0.1 | 44 | 26 | 0.3 |
| ER expression | |||||||
| Negative | 60 | 39 | 21 | 34 | 26 | ||
| Positive | 68 | 43 | 25 | 0.8 | 44 | 24 | 0.4 |
| PgR expression | |||||||
| Negative | 74 | 48 | 26 | 46 | 28 | ||
| Positive | 52 | 35 | 17 | 0.8 | 26 | 26 | 0.2 |
| Grading † , ‡ | |||||||
| G2 | 45 | 29 | 16 | 22 | 23 | ||
| G3 | 83 | 56 | 27 | 0.8 | 51 | 32 | 0.2 |
| Size § | |||||||
| pT1 | 70 | 52 | 18 | 46 | 24 | ||
| pT2–3 | 64 | 36 | 28 | 0.03 | 32 | 32 | 0.08 |
High cyclin E expression was strongly related to MnSOD expression. Thirty‐one (67%) of the 46 tumors that overexpressed cyclin E also showed positive staining for MnSOD, and 63 (72%) out of 88 specimens that did not overexpress cyclin E were not stained by the anti‐MnSOD antibody (P = 0.008). We did not accurately analyze the expression of MnSOD and cyclin E in normal mammary tissue. However, the few cases of normal glands present in the analyzed sections stained positively for MnSOD and were considered negative for cyclin E expression. Thus, we can conclude that expression of MnSOD was mainly lost in tumors that did not display increased expression of cyclin E. Moreover, a statistically significant relationship was observed between cyclin E expression levels (evaluated as the percentage of positive cells) and MnSOD expression (evaluated as staining intensity) (P = 0.009, r = 0.46) (data not shown) and a progressive significant increase in the percentage of cyclin E‐positive cells was observed with increasing MnSOD staining intensity (Fig. 2).
Figure 2.
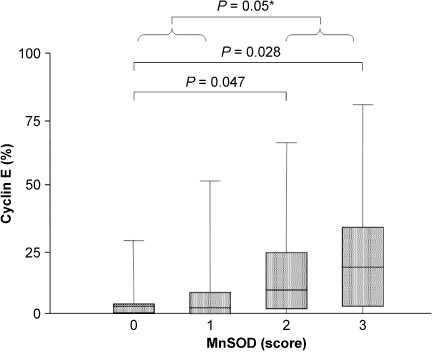
Box‐plot of cyclin E expression levels in breast cancers stratified according to manganese superoxide dismutase (MnSOD) staining intensity. For each group, the bottom and top edges of the box are the 25th and 75th percentiles and the bottom and top parts of the external lines are 2.5th and 97.5th percentiles, respectively. Median values are shown by the lines within the boxes. *Group 0/1 versus 2/3.
Seventy (52%) of the 134 patients relapsed from initial diagnosis whereas 64 (48%) did not. The mean percentage of cyclin E‐positive cells was lower in not relapsed (6.6 ± 9.9%) compared to relapsed (14.6 ± 22.19%) patients and this difference reached significance (P = 0.048).
Using 3% of positive cells as the cut‐off value, cyclin E appeared to be expressed at high levels in 28 (40%) cases that recurred during the follow‐up period and in only 18 (28%) of the cases that did not. Thus, recurrence was more frequent in high cyclin E‐expressing tumors but this difference was not significant (P = 0.2). Similarly, a shorter median length of DFS was observed in high cyclin E‐expressing tumors (75 months, 95% confidence interval [CI] 68–81) compared to low‐expressing tumors (76 months, 95% CI 68–83) but this difference was not significant and DFS plots were not significantly different in patients with high levels of cyclin E versus low‐expressing tumors (P = 0.13 by log‐rank test) (Fig. 3a).
Figure 3.
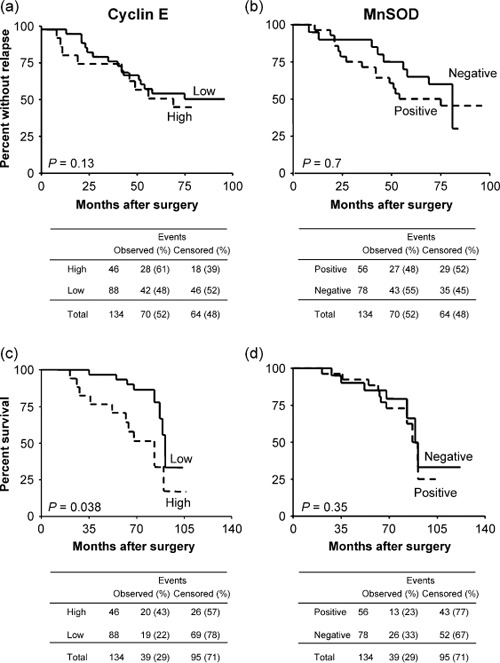
Kaplan–Meier curves for (a,b) disease‐free and (c,d) overall survival in 134 breast cancer patients stratified according to (a,c) cyclin E and (b,d) manganese superoxide dismutase (MnSOD) expression.
On the other hand, MnSOD expression was detected in 27 (39%) recurrent cases and in 29 (45%) of the cases that did not recur. Thus, recurrence was more frequent in tumors displaying no staining for MnSOD but this difference was not significant (P = 0.5). Patients whose tumors did not express MnSOD also showed a shorter median length of DFS (56.5 months, 95% CI 42–76) compared to positive cases (74 months, 95% CI 56–79) but this difference was not significant and DFS plots were not significantly different in patients with positive staining for MnSOD versus negative tumors (P = 0.7 by log‐rank test) (Fig. 3b). DFS analysis by the Kaplan–Meier method only showed a significant separation in our series when tumors were stratified according to node status (DFS was shorter in node‐positive cases, P = 0.03), PgR status (DFS was shorter in PgR‐negative cases, P = 0.04), and tumor stage (stage III tumors displayed a shorter DFS compared to lower‐stage cases, P = 0.04) (data not shown).
During the follow‐up period, disease‐related death occurred in 39 (29%) of the 134 patients. The median percentage of cyclin E‐positive cells was lower in alive (3%, 95% CI 0–3%) compared to dead (5.5%, 95% CI 2–40%) patients and this difference was significant (P = 0.02). Correspondingly, 20 (43%) of the 46 patients with high cyclin E‐expressing tumors and only 19 (22%) of the 88 low‐expressing patients died of disease during the follow‐up period (Table 3) and this difference was significant (P = 0.01). Moreover, the median survival of patients whose tumors expressed high levels of cyclin E (68 months, 95% CI 52–80 months) was significantly shorter compared to low‐expressing patients (76 months, 95% CI 71–83 months) (P = 0.019). Thus, patients with tumors displaying high staining for cyclin E were more likely to die of the disease compared with patients who had tumors expressing low levels of the protein, as confirmed by the Kaplan–Meier curves that displayed a significant separation between the two groups of patients (P = 0.038 by log‐rank test) (Fig. 3c). Hence, death occurred more frequently when breast cancers in our series displayed high staining for cyclin E.
Table 3.
Relationship between disease relapse, patient survival and various clinical and molecular parameters in a series of 134 early breast cancer patients receiving adjuvant epirubicin‐based regimens
| Paramater | Total | Disease relapse | Patient death | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | P‐value | No | Yes | P‐value | ||
| Age (years) | |||||||
| ≤50 | 65 | 27 | 38 | 48 | 17 | ||
| >50 | 69 | 37 | 32 | 0.77 | 47 | 22 | 0.66 |
| Node status | |||||||
| Negative | 64 | 37 | 27 | 47 | 17 | ||
| Positive | 70 | 27 | 43 | 0.037 | 48 | 22 | 0.3 |
| Cyclin E expression | |||||||
| Low | 88 | 46 | 42 | 69 | 19 | ||
| High | 46 | 18 | 28 | 0.2 | 26 | 20 | 0.01 |
| MnSOD expression | |||||||
| Negative | 78 | 35 | 43 | 26 | 52 | ||
| Positive | 56 | 29 | 27 | 0.48 | 43 | 13 | 0.24 |
| ER expression | |||||||
| Negative | 60 | 30 | 30 | 41 | 19 | ||
| Positive | 68 | 32 | 36 | 0.8 | 49 | 19 | 0.74 |
| PgR expression | |||||||
| Negative | 74 | 32 | 42 | 50 | 24 | ||
| Positive | 52 | 30 | 24 | 0.33 | 38 | 14 | 0.45 |
| Grading † , ‡ | |||||||
| G2 | 45 | 21 | 24 | 29 | 16 | ||
| G3 | 83 | 44 | 29 | 0.71 | 56 | 27 | 0.88 |
| Size § | |||||||
| pT1 | 70 | 42 | 28 | 54 | 16 | ||
| pT2–3 | 64 | 22 | 42 | 0.035 | 41 | 23 | 0.17 |
On the other hand, no relationship was observed between MnSOD expression and OS in our series of patients. Indeed, 26 (33%) of the 78 patients negative for MnSOD expression and only 13 (23%) of the 56 positive patients died of disease during the follow‐up period, but this difference was not significant (P = 0.2) and the median survival of patients was comparable in both negative (75 months, 95% CI 68–81 months) and positive (76 months, 95% CI 76–83 months) cases. Thus, MnSOD expression was not related to patient survival as confirmed by the Kaplan–Meier curves (P = 0.35 by log‐rank test) in our series of breast cancers patients (Fig. 3d).
Node‐positive status (P = 0.04), PgR‐negative status (P = 0.007), ER‐negative status (P = 0.05), and higher tumor grade (P = 0.025) and stage (P = 0.048) were also associated with shorter OS in our series of breast cancer patients (data not shown). We then carried out a multivariate analysis by building a Cox proportional hazard model that included all variables demonstrating a significant association with OS at univariate analysis. As shown in Table 4, only node involvement (P = 0.041; relative risk 5.13) was confirmed to be an independent predictor of OS, whereas high cyclin E expression was not an independent predictor of survival, although it was associated with a relative risk of death of 1.439. Tumor stage was not included in the model because of the high collinearity with lymph node involvement.
Table 4.
Contribution of various potential prognostic factors to overall survival by Cox regression analysis in early breast cancer patients receiving epirubicin‐based adjuvant chemotherapy
| Variable | Hazard ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Nodes status† | 5.130 | 1.063–24.754 | 0.041 |
| Estrogen receptor status‡ | 1.017 | 0.317–3.259 | 0.97 |
| Progesterone receptor status‡ | 0.406 | 0.121–1.358 | 0.14 |
| Grading§ | 4.058 | 0.870–18.923 | 0.07 |
| Cyclin E¶ | 1.439 | 0.484–4.275 | 0.51 |
The risk ratio is given as node‐positive versus node‐negative.
The risk ratio is given as negative versus positive tumors.
The risk ratio is given as G3 versus G2 tumors.
The risk ratio is given as high‐ versus low‐expressing tumors.
To analyze a more homogeneous group of patients, the relationship between cyclin E expression levels and clinical outcome was analyzed within the node‐negative patients (n = 64). In this subgroup, recurrence was observed in 27 patients and 17 of them died of disease during the follow‐up period. When these tumors were stratified according to cyclin E expression, 12 (63%) of the 19 high‐expressing tumors recurred during the follow‐up period whereas only 15 (33%) recurred among the remaining 45 cases and this difference was significant (P = 0.03). Moreover, the median DFS (56 months) of patients whose tumors expressed high levels of cyclin E was shorter compared to low‐expressing patients (75.5 months) but this difference was not significant (P = 0.08). This was confirmed by the Kaplan–Meier curves of DFS, which did not display a significant separation within the group of node‐negative breast cancer patients (P = 0.14 by log‐rank test) (Fig. 4a).
Figure 4.
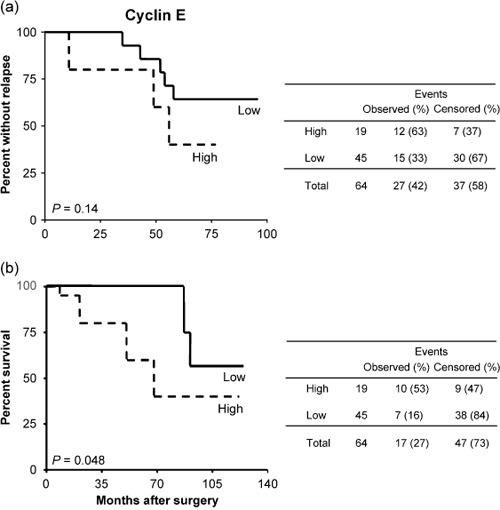
Kaplan–Meier curves for (a) disease‐free and (b) overall survival in the subset (n = 64) of node‐negative breast cancer patients stratified according to cyclin E expression.
On the other hand, death of disease occurred in 10 (53%) of the 19 high cyclin E‐expressing tumors and in only 7 (15%) of the remaining 45 low‐expressing tumors and this difference was significant (P = 0.001). Moreover, the median OS of patients whose tumors expressed high levels of cyclin E (68 months, 95% CI 60–78 months) was shorter compared to low‐expressing patients (82 months, 95% CI 72–91 months) and this difference showed a trend toward significance (P = 0.1). In a univariate setting, patients with tumors displaying high cyclin E staining were more likely to die of disease compared with low‐expressing tumors, as confirmed by the Kaplan–Meier curves of OS, which displayed a significant separation between the two groups of patients (P = 0.048 by log‐rank test) (Fig. 4b). Cox multivariate analysis including cyclin E expression, grading, and ER expression (variables associated with survival in the univariate analyses) only identified cyclin E as a weak independent OS risk predictor (Table 5).
Table 5.
Contribution of various potential prognostic factors to overall survival by Cox regression analysis in node‐negative early breast cancer patients receiving epirubicin‐based adjuvant chemotherapy
| Variable | Hazard ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Estrogen receptor status † | 1.005 | 0.461–2.192 | 0.98 |
| Grading ‡ | 1.597 | 0.737–3.460 | 0.23 |
| Cyclin E § | 2.085 | 0.958–4.539 | 0.046 |
The risk ratio is given as negative versus positive tumors.
The risk ratio is given as G3 versus G2 tumors.
The risk ratio is given as high versus low expressor tumors.
No relationship was observed between MnSOD expression and other clinicopathological parameters as well as the outcomes of patients in the same subsets (data not shown). Neither MnSOD nor cyclin E expression levels displayed a relationship with patient outcome in the subset of node‐positive patients.
Discussion
In the present study, the expression levels of cyclin E and MnSOD were evaluated by IHC (Fig. 1) in a series of early breast cancer patients who received an epirubicin‐based adjuvant chemotherapy regimen and their relationship and potential prognostic significance were analyzed.
The results obtained suggest that increased expression of cyclin E is associated with higher expression of the MnSOD protein in tumor cells (Fig. 2). This finding is consistent with previous in vitro data demonstrating that overexpression of exogenous cyclin E in rat fibroblasts is associated with increased expression of MnSOD.( 22 ) Cyclin E‐overexpressing cells also displayed increased resistance to doxorubicin, which was attributed to the increased ability of the overexpressed MnSOD to reduce reactive oxygen species‐derived cell damage.( 22 )
We did not find any relationship between cyclin E and MnSOD expression and the other clinicopathological parameters such as age, ER status, PgR status, and tumor grade (Table 2). Interestingly, higher expression of both molecules correlated with higher tumor size although the trend only reached significance for cyclin E (P = 0.01) and not for MnSOD (P = 0.08) (Table 2). MnSOD expression, as assessed by IHC, did not correlate with patient outcome (Fig. 3), either in terms of DFS or OS, whereas cyclin E overexpression was confirmed to be a prognostic factor in terms of OS but not DFS for breast cancer patients receiving epirubicin‐based adjuvant chemotherapy (Fig. 3). This was also true for the subset of node‐negative patients in terms of OS (Fig. 4; Table 5) Different tumor histotypes were included in the analyzed series of patients (Table 1). However, identical results were obtained both in terms of survival and correlation analyses when pure invasive ductal carcinomas (representing more than 90% of cases) were analyzed separately.
The present study was mainly aimed to verify whether a relationship exists between cyclin E and MnSOD expression in tumor cells in vivo, as observed in an in vitro model,( 22 ) and how and whether expression of these molecules correlate with patient outcome following anthracycline‐based therapy. Despite the strong relationship between the expression of the two proteins, only cyclin E and not MnSOD expression displayed prognostic significance in our series of breast cancer patients. We believe these findings suggest that cyclin E overexpression likely affects tumor behavior and, consequently, patient outcome through multiple mechanisms. Indeed, cyclin E promotes cell cycle progression by direct phosphorylation, as an active cyclin E–Cdk2 complex, of Retinoblastoma family proteins as well as other substrates, thus permitting G1 to S transition and progression through S phase.( 15 ) This function of cyclin E could be responsible for the observed relationship between increased expression of the protein and increased tumor size (Table 2), which might be a consequence of a higher proliferation rate of tumor cells due to deregulated activity of the cyclin E–Cdk2 complex. Moreover, increased cyclin E expression can promote tumor progression through increased genetic instability( 18 ) and has been shown to be associated with increased inactivation of the pRb protein( 17 ) and an increased rate of p53 gene mutations.( 21 ) Overall, these features associated with cyclin E overexpression might contribute to determining the final effects on the biological and clinical behavior of tumor cells and can explain the prognostic significance of cyclin E overexpression.
Our findings are in agreement with several studies that have reported a negative prognostic significance of cyclin E overexpression in breast cancer patients.( 14 , 19 , 20 , 29 ) In one of these studies, cyclin E expression was assessed by western blot analysis and high level of cyclin E was shown to be the strongest independent factor in predicting survival of breast cancer patients, even stronger than node status.( 14 ) Despite the reported strong association with patient outcome, western blotting evaluation of cyclin E levels is not suitable for routine application and would have a limited role from a clinical point of view. On the contrary, IHC is easily carried out in most hospitals and can be easily suggested as a routine practice. From this point of view, our findings are of great interest as they confirm previous evidence of the prognostic significance of IHC evaluation of cyclin E expression levels. Our series only included patients treated with epirubicin‐based adjuvant chemotherapy. However, cyclin E was shown to be a strong independent prognostic factor of breast cancer‐specific survival also among patients who did not receive adjuvant chemotherapy,( 29 ) thus further confirming the pivotal role of this protein in determining tumor behavior and patient outcome.
Cyclin E represents an attractive prognostic marker for breast cancer patients. In fact, its expression was not related to other known pathological indicators of aggressive breast cancer, such as node status, tumor grade and stage, and ER and PgR status (Table 2). Nonetheless, increased cyclin E expression was confirmed to be a negative prognostic indicator of shorter OS when patients were considered altogether (Fig. 3c), but also within the subset of node‐negative patients (Fig. 4b; Table 5) and only the limited sample size (n = 64) might have not allowed us to detect an effect in term of DFS in the latter group of patients. Moreover, immunohistochemical staining is a simple, inexpensive, and reliable assay that could be easily carried out routinely. Indeed, tumor tissue is routinely available for immunostaining and it is noteworthy that we did not observe significant variations between observers in assessing positive versus negative staining with the anti‐cyclin E antibody used for the analysis.
In conclusion, taken together with the data available in the literature, the results of the present study confirm that assessment of cyclin E expression by IHC might help to identify breast cancer patients who are at high risk of death of disease following adjuvant epirubicin‐based chemotherapy and might benefit from an alternative epirubicin‐free treatment (i.e. taxane‐based regimens). We believe these findings warrant further evaluation of this molecular marker in a larger series of breast cancer patients, especially within the node‐negative group of patients whose clinical outcome is not accurately predicted by the available prognostic markers.
Acknowledgments
This work was supported by the Università Cattolica del Sacro Cuore.
References
- 1. Jemal A, Siegel R, Ward E et al . Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Grande E, Inghelmann R, Francisci S et al . Regional estimates of breast cancer burden in Italy. Tumori 2007; 93: 374–9. [DOI] [PubMed] [Google Scholar]
- 3. Lønning P. Breast cancer prognostication and prediction: are we making progress? Ann Oncol 2007; (Suppl 8): viii3–7. [DOI] [PubMed]
- 4. Group EBCTC . Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 1998; 352: 930–42. [PubMed] [Google Scholar]
- 5. Group EBCTC . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–717. [DOI] [PubMed] [Google Scholar]
- 6. Group EBCTC . Adjuvant chemotherapy in oestrogen‐receptor‐poor breast cancer: patient‐level meta‐analysis of randomised trials. Lancet 2008; 371: 29–40. [DOI] [PubMed] [Google Scholar]
- 7. Cardoso F, Atalay G, Piccart M. Optimising anthracycline therapy for node positive breast cancer. Am J Cancer 2002; 1: 257–68. [Google Scholar]
- 8. Campone M, Roche H, Kerbrat P et al . Secondary leukemia after epirubicin‐based adjuvant chemotherapy in operable breast cancer patients: 16 years experience of the French Adjuvant Study Group. Ann Oncol 2005; 16: 1343–51. [DOI] [PubMed] [Google Scholar]
- 9. Crump M, Tu D, Shepherd L, Levine M, Bramwell V, Pritchard K. Risk of acute leukemia following epirubicin‐based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21: 3066–71. [DOI] [PubMed] [Google Scholar]
- 10. Zambetti M, Moliterni A, Materazzo C et al . Long‐term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol 2001; 19: 37–43. [DOI] [PubMed] [Google Scholar]
- 11. Group EBCTC . Multi‐agent chemotherapy for early breast cancer. Cochrane Database Syst Rev 2002: CD000487. [DOI] [PubMed] [Google Scholar]
- 12. Donnellan R, Chetty R. Cyclin E in human cancers. FASEB J 1999; 13: 773–80. [DOI] [PubMed] [Google Scholar]
- 13. Sandhu C, Slingerland J. Deregulation of the cell cycle in cancer. Cancer Detect Prev 2000; 24: 107–18. [PubMed] [Google Scholar]
- 14. Keyomarsi K, Tucker S, Buchholz T et al . Cyclin E and survival in patients with breast cancer. N Engl J Med 2002; 347: 1566–75. [DOI] [PubMed] [Google Scholar]
- 15. Ohtsubo M, Theodoras A, Schumacher J, Roberts J, Pagano M. Human cyclin E, a nuclear protein essential for the G1‐to‐S phase transition. Mol Cell Biol 1995; 15: 2612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bortner D, Rosenberg M. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol 1997; 17: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielsen N, Emdin S, Cajander J, Landberg G. Deregulation of cyclin E and D1 in breast cancer is associated with inactivation of the retinoblastoma protein. Oncogene 1997; 14: 295–304. [DOI] [PubMed] [Google Scholar]
- 18. Spruck C, Won K, Reed S. Deregulated cyclin E induces chromosome instability. Nature 1999; 401: 297–300. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen N, Arnerlov C, Emdin S, Landberg G. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer 1996; 74: 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porter P, Malone K, Heagerty P et al . Expression of cell‐cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Med 1997; 3: 222–5. [DOI] [PubMed] [Google Scholar]
- 21. Lindahl T, Landberg G, Ahlgren J et al . Overexpression of cyclin E protein is associated with specific mutation types in the p53 gene and poor survival in human breast cancer. Carcinogenesis 2004; 25: 375–80. [DOI] [PubMed] [Google Scholar]
- 22. Sgambato A, Camerini A, Pani G et al . Increased expression of cyclin E is associated with an increased resistance to doxorubicin in rat fibroblasts. Br J Cancer 2003; 88: 1956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sobin LH, Wittekind C. UICC. TNM Classification of Malignant Tumours. New York: Wiley‐Liss, 2002. [Google Scholar]
- 24. Elston C, Ellis I. Pathological prognostic factors in breast cancer. I. The value of histologic grade in breast cancer: experience from a large study with long‐term follow‐up. Histopatology 1991; 19: 403–10. [DOI] [PubMed] [Google Scholar]
- 25. The World Health Organization . Histological typing of breast tumors. Neoplasma 1983; 30: 113–23. [PubMed] [Google Scholar]
- 26. Collecchi P, Passoni A, Rocchetta M, Gnesi E, Baldini E, Bevilacqua G. Cyclin‐D1 expression in node‐positive (N+) and node‐negative (N–) infiltrating human mammary carcinomas. Int J Cancer 1999; 84: 139–44. [DOI] [PubMed] [Google Scholar]
- 27. Baldini E, Camerini A, Sgambato A et al . Cyclin A and E2F1 overexpression correlate with reduced disease‐free survival in node‐negative breast cancer patients. Anticancer Res 2006; 26: 4415–21. [PubMed] [Google Scholar]
- 28. Sgambato A, Camerini A, Amoroso D et al . Expression of dystroglycan correlates with tumor grade and predicts survival in renal cell carcinoma. Cancer Biol Ther 2007; 6: 1840–6. [DOI] [PubMed] [Google Scholar]
- 29. Chappuis P, Donato E, Goffin J et al . expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment. Ann Oncol 2005; 16: 735–42. [DOI] [PubMed] [Google Scholar]


