Abstract
To investigate the relationship between the degree of liver dysfunction and the pharmacokinetics of docetaxel, a population pharmacokinetic model was developed in an oncology practice without excluding patients with moderate to severe liver dysfunction. Two hundred patients were treated with docetaxel as a single agent or in combination chemotherapy. The plasma concentration–time course data were analyzed using a three‐compartment open model with zero‐order administration and first‐order elimination on the NONMEM program. Sixty‐one had elevated transaminase levels, and alkaline phosphatase was elevated in 40. Body surface area, albumin, α1‐acid glycoprotein, and liver function were found to be significant covariates for the systemic clearance of docetaxel. Compared to patients with normal or minimal impairment of liver function, patients with grade 2 and 3 elevations of transaminases at baseline in conjunction with elevation of alkaline phosphatase had 22 and 38% lower clearances, respectively. Goodness‐of‐fit plots indicated that the model was fitted well with the observed data, and the bootstrap method guaranteed robustness of the model. We developed a population pharmacokinetic model for docetaxel, which can be used in the setting of an oncology practice. Based on the model, dose reduction by approximately 20 and 40% should be considered for patients with grade 2 and 3 elevations of transaminases at baseline in conjunction with elevation of alkaline phosphatase, respectively. (Cancer Sci 2009; 100: 144–149)
Anticancer drugs have a narrow therapeutic window, and interpatient variabilities in pharmacokinetics and pharmacodynamics may results in serious toxicities.( 1 ) Elucidating the factors causing these interpatient variabilities is helpful for avoiding serious toxicities and augmenting antitumor activity. Population pharmacokinetics represent a means to investigate the effect of patients’ variables on the pharmacokinetics of drugs.( 2 , 3 , 4 ) In this approach, pharmacokinetics are analyzed in many patients with different backgrounds as a population, and the effect of these backgrounds on the pharmacokinetics are investigated. Pharmacokinetic information on patients with small numbers of drug concentration data can also be analyzed by population pharmacokinetic methodology.( 2 , 3 , 4 , 5 ) Thus, it is a useful tool for investigating pharmacokinetics of drugs in a population including elderly patients or patients with organ dysfunctions.
Docetaxel has been used widely to treat breast, non‐small‐cell lung, ovarian, head and neck, gastric, esophageal, and prostate cancers.( 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 ) The drug is eliminated from the body mainly by hepatic metabolism. Population pharmacokinetic models of docetaxel have been developed using data obtained from patients treated in clinical trials prior to its drug registration,( 16 , 17 , 18 ) where body surface area, albumin, age, α1‐acid glycoprotein, and liver function were found to be significant covariates for the systemic clearance of docetaxel. In clinical studies for the development of anticancer drugs, unfit patients including those with moderate to severe liver dysfunction or poor performance status are commonly excluded, and information on pharmacokinetics and pharmacodynamics for such patients is therefore lacking.
Therefore, in the present study, we developed a population pharmacokinetic model of docetaxel in cancer patients treated in our oncology practice, including unfit patients who would have been excluded from the past clinical studies during drug development, and investigated the pharmacokinetic alterations of docetaxel in relation to the extent of liver function impairment. In the previous population pharmacokinetic study, which was carried out as part of the clinical trial program for drug approval, only 3% of patients had pharmacokinetically relevant liver dysfunction compared with 9% in our study.( 16 )
Materials and Methods
Patient selection. Patients with different cancers receiving docetaxel as a single agent or in combination chemotherapy in medical practice were eligible for this population pharmacokinetic study. Other eligibility criteria included being 20 years old or older, performance status of 3 or better, white blood cell count ≥3000/mL, and platelet count ≥75 000/mL. The dose and schedule of docetaxel were set according to the approved usage in Japan, that is, intravenous 60‐min infusion at a dose of 60 mg/m2 every 3 weeks. However, the dose and schedule were modified in combination chemotherapy or based on the extent of liver impairment or performance status in each patient at the discretion of attending physicians. All patients gave written informed consent, and this study was approved by the Institutional Review Board at the National Cancer Center, Japan.
Treatment and follow up. For the measurement of docetaxel concentrations in plasma, heparinized blood was collected. Blood sampling at the end of docetaxel infusion, and 0.17, 1, 5, 10, and 24 h thereafter was recommended, but this was allowed to be rather flexible depending on clinical situations. However, exact infusion time and sampling times were recorded accurately. Plasma concentrations of docetaxel were determined by a high‐performance liquid chromatographic method as reported previously.( 19 )
Population pharmacokinetic analysis. Population pharmacokinetic analyses were carried out using a non‐linear mixed‐effect modeling program, NONMEM (version V, level 1.1; ICON DEVELOPMENT Solutions, Ellicott City, MD, USA). NONMEM was running with a Compaq Visual FORTRAN 6.6 compiler (Hewlett‐Packard, Palo Alto, CA, USA) on a Pentium 4 central processing unit, under the Windows XP operating system (Microsoft Corporation, Redmond, WA, USA). After one‐ and two‐compartment models were tested, a three‐compartment open model with zero‐order administration and first‐order elimination (ADVAN 11 and TRANS 4) was selected to describe the plasma concentration–time course for docetaxel in the entire population based on goodness of fit to the data. The pharmacokinetic model was parameterized in terms of clearance (CL), the volume of distribution of the central compartment (V 1) as well as those of two peripheral compartments (V 2 and V 3), and intercompartment clearances (Q 2 and Q 3). Assuming a log‐normal distribution for interindividual variability in pharmacokinetic parameters, the interindividual variability was modeled as (e.g. for clearance):
where CLj and 2 are the estimated values in an individual j and the population mean for clearance, respectively, and η jCL is the individual random perturbation with a mean of zero and a variance ω2. Intraindividual residual variability was also described by a log‐normal distribution model. The first‐order conditional estimation method was used to estimate the pharmacokinetic parameters.
Relationships between covariates and pharmacodynamic parameters. The following covariates were tested to improve the population pharmacokinetic model: age, sex, body surface area, performance status, albumin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatinine, and coadministered anticancer agents. Forward selection and backward elimination were used to select covariates to be included in the model. Statistical discrimination between hierarchial models was based on difference in objective function (Obj) in NONMEM analyses, equal to minus twice the log likelihood of the data. Covariates were inserted sequentially into the basic model by forward selection. During this process, P < 0.001 was considered significant, corresponding to a decrease in Obj of 10.83 and 13.82 for degrees of freedom of 1 and 2, respectively. Continuous variables were normalized by their population median and were expressed by multiplicative models. Multiplicative models were used to enhance convergence and were coded as:
where P is the individual's estimate of the parameters, β1 represents the typical value of the parameter, β2 represents the effect of the covariate, and COV is the ratio of the individual's covariate value to the median value. For convenience in clinical application, hepatic function was categorized according to grade by the National Cancer Center Institute Common Toxicity Criteria. Each liver function test (e.g. AST, ALT, ALP, bilirubin) and their combinations (e.g. the maximum grade of AST and ALT) were tested as covariates. After all significant variables were included in the model, each covariate was removed in a stepwise backward elimination procedure to determine whether it was significant in the final model.
Bootstrap validation. The accuracy and robustness of the final model were assessed by using a bootstrap method.( 20 , 21 , 22 ) A bootstrap sample was generated by repeated random sampling from the original data set, and the size of bootstrap sample was the same as the original sample size. Two hundred bootstrap samples were reconstructed, and the final model is fitted repeatedly to the 200 bootstrap samples. The mean parameter estimates obtained from bootstrap replications that were calculated normally were compared with those obtained from the original data set.
Results
We analyzed pharmacokinetic data from 200 cancer patients with different backgrounds, including 18 patients older than 75 years and seven patients with a performance status of 3 (Table 1). Docetaxel was given in combination chemotherapy with cisplatin, doxorubicin, or irinotecan in 103 patients, with the dose ranging from 15 to 60 mg/m2. Hypoalbuminemia was observed in 137 patients at baseline, and AST or ALT levels were elevated in 61 patients (Table 2), including 17 with grade 2 or greater elevation of AST or ALT in combination with elevated ALP levels. Serum bilirubin was increased in five patients but was associated with elevated transaminase levels in only two patients.
Table 1.
Demographics of patients
| Demographic | No. patients |
|---|---|
| Age (years) | |
| Median 57 | |
| Range 21–86 | |
| Sex | |
| Female | 114 |
| Male | 86 |
| Performance status | |
| 0 | 46 |
| 1 | 130 |
| 2 | 17 |
| 3 | 7 |
| Combination chemotherapy | |
| Cisplatin | 66 |
| Doxorubicin | 6 |
| Irinotecan | 31 |
| Cancer | |
| Breast cancer | 79 |
| Non‐small cell lung cancer | 68 |
| Head and neck cancer | 31 |
| Others 22Dose of docetaxel (mg/m2) | |
| –25 | 30 |
| 35 | 59 |
| 45 | 9 |
| 55 | 16 |
| 60 | 86 |
| Infusion time (h) | |
| 0.5 | 52 |
| 1.0 | 128 |
| 1.5 | 20 |
| Body surface area (mg/m2) | |
| Median 1.53 | |
| Range 1.17–1.99 | |
| Liver function | |
| HEP1 | 183 |
| HEP2 | 10 |
| HEP3 | 7 |
HEP1, normal liver function (normal alkaline phosphatase [ALP] or <grade 2 elevation of aspartate aminotransferase [AST] or alanine aminotransferase [ALT]); HEP2, mild liver dysfunction (increased ALP in combination with grade 2 elevation of AST or ALT); HEP3, moderate liver dysfunction (increased ALP in combination with grade 3 or greater elevation of AST or ALT).
Table 2.
Blood chemistry of patients
| Liver function | Grade | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Albumin | ||||
| Concentration (g/dL) | ≥4.0 | 3.0–3.9 | 2.0–2.9 | ≤1.9 |
| No. patients | 63 | 126 | 10 | 1 |
| Total bilirubin | ||||
| Concentration (mg/dL) | ≤1.2 | 1.3–1.5 | 1.6–3.6 | 3.7–12 |
| No. patients | 195 | 3 | 1 | 1 |
| Aspartate aminotransferase | ||||
| Concentration (IU/L) | ≤33 | 34–82 | 83–165 | 166–660 |
| No. patients | 157 | 27 | 10 | 6 |
| Alanine aminotransferase | ||||
| Concentration (IU/L) | ≤27 | 28–67 | 68–135 | 136–540 |
| No. patients | 145 | 43 | 6 | 6 |
| Alkaline phosphatase | ||||
| Concentration (IU/L) | ≤359 | 360–897 | 898–1795 | 1796–7140 |
| No. patients | 160 | 26 | 11 | 3 |
| α1‐Acid glycoprotein | ||||
| Concentration (mg/dL) | ≤93 † | 94–232 ‡ | 233–465 § | |
| No. patients | 90 | 107 | 3 | |
≤Upper limit of normal range (ULN);
>ULN and ≤2.5 × ULN;
>2.5 × ULN and ≤5 × ULN.
The actual number of plasma concentration data per patient ranged from two to nine with a median of six. Concentration–time curves were best described by a three‐compartment linear model (Fig. 1). First, population pharmacokinetic parameters were computed using a simple structural model without any covariates, and the influence of covariates on the clearance of docetaxel was investigated. Body surface area, albumin, liver function index, and α1‐acid glycoprotein improved the model when included as covariates (Table 3). Among the different indices of liver function investigated, the combination of ALP and the maximum grade of AST or ALT improved the model to the highest extent. In this model, patients were classified into three groups: seven patients with elevated ALP (i.e. grade ≥ 1) in combination with grade 3 or greater elevation of AST or ALT (HEP3), 10 with elevated ALP in combination with grade 2 elevation of AST or ALT (HEP2), and 183 with normal or minimum liver dysfunction (HEP1).
Figure 1.
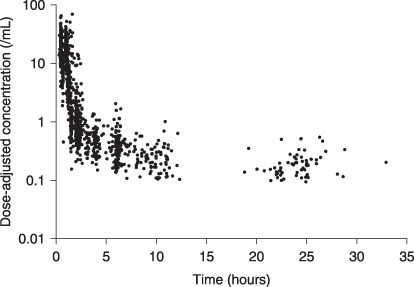
Observed plasma concentration of docetaxel. Concentrations were normalized by the actual dose of docetaxel in each patient.
Table 3.
Model building
| Model | Covariates | Objective function(Obj) | Difference in objective function | P |
|---|---|---|---|---|
| 1 | None | –5072 | ||
| 2 | BSA | –5303 | –230 | <0.0001* |
| 3 | BSA, ALB | –5538 | –235 | <0.0001* |
| 4 | BSA, ALB, HEP | –5554 | –16 | <0.0001* |
| 5 | BSA, ALB, HEP, AGP | –5574 | –20 | <0.0001* |
| 6 | BSA, ALB, AGP | –5556 | +18 | <0.0001** |
| 7 | BSA, HEP, AGP | –5469 | +105 | <0.0001** |
| 8 | BSA, ALB, HEP | –5543 | +30 | <0.0001** |
Compared to the previous model.
Compared to Model 5 (final model). AGP, α1‐acid glycoprotein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; HEP, normal, mildly (increased ALP in combination with grade 2 elevation of AST or ALT) or moderately elevated liver function tests (increased ALP in combination with grade 3 or greater elevation of AST or ALT).
The predicted values obtained by Bayesian estimation are plotted versus the observed values in Figure 2a. Weighted residual plots for the population pharmacokinetic model are shown in Figure 2b. The values were generally distributed around zero and were relatively symmetrical. No obvious bias pattern was apparent in the plot of the predicted concentration versus the weighted residual. Pharmacokinetic parameters in the population pharmacokinetic model are summarized in Table 4. Among the 200 bootstrap samples, 153 samples were converged. All structural parameters (θi) and variance parameters (ω, σ) were within 19.5% of the bootstrapped mean out of the 153 samples (Table 4). Systemic clearance of docetaxel was positively correlated with body surface area and albumin, and negatively correlated with α1‐acid glycoprotein. In patients with mild (HEP2) and moderate (HEP3) liver dysfunction, clearance was reduced by 22 and 38%, respectively. The difference in the reduction of systemic clearance between each category of liver dysfunction was highly significant (P < 0.001). These reductions were apparent when the systemic clearance of docetaxel for individuals was calculated by Bayesian estimation and compared in relation to liver function (Fig. 3).
Figure 2.
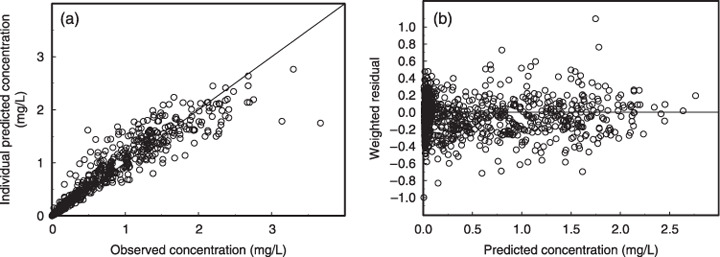
(a) Observed docetaxel concentration versus predicted docetaxel concentration from a Bayesian post hoc analysis of the model. The solid line represents the unit line. (b) Weighted residuals versus predicted concentration. The horizontal line represents the zero level.
Table 4.
Estimation and precision of parameters in population pharmacokinetic model of docetaxel and bootstrap validation
| Parameter | θ | Estimated parameters (precision † ) | Difference § | |
|---|---|---|---|---|
| Original analysis | Bootstrap validation ‡ | |||
| Clearance (L/h) | θ1 | 29.3 (4) | 28.3 (9) | 3.31 |
| Body surface area | θ2 | 1.11 (26) | 1.15 (29) | –3.87 |
| Albumin | θ3 | 2.00 (26) | 1.94 (26) | 2.80 |
| α1‐Acid glycoprotein | θ4 | 0.251 (29) | 0.260 (35) | –4.20 |
| LIV | θ5 | 0.776 (14) | 0.759 (21) | 2.18 |
| θ6 | 0.623 (24) | 0.616 (31) | 1.17 | |
| V 1 (L) | θ7 | 7.75 (5) | 7.63 (4) | 1.57 |
| Q 2 (L/h) | θ8 | 5.46 (9) | 5.67 (14) | –3.81 |
| V 2 (L) | θ9 | 8.69 (14) | 9.55 (26) | –9.91 |
| Q 3 (L/h) | θ10 | 19.0 (10) | 19.7 (17) | –3.52 |
| V 3 (L) | θ11 | 660 (14) | 789 (41) | –19.5 |
| Interindividual variability (%) | ||||
| ωCL | 31 (23) | 31 (12) | –0.65 | |
| ωV1 | 19 (38) | 18 (27) | 2.69 | |
| ωQ3 | 31 (22) | 32 (9) | –3.02 | |
| ωV3 | 38 (35) | 37 (35) | 0.566 | |
| Intraindividual variability (%) | ||||
| σ | 29 (19) | 29 (11) | 1.46 | |
| † Expressed as Coefficient of variation. ‡ Calculated from 200 bootstrap replicates (153 convergence). § (Original value – bootstrap value)/original value × 100 (%). The equation used to estimate the population parameters was Clearance = θ1 × (body surface area/1.53)θ2 × (albumin/3.7)θ3 × (97/α1‐acid glycoprotein)θ4 × LIV × EXP(η1), where LIV = 1 for normal ALP or <grade 2 elevation of AST or ALT, LIV = θ5 for increased ALP in combination with grade 2 elevation of AST or ALT, and θ6 for increased ALP in combination with ≥grade 3 elevation of AST or ALT. | ||||
Figure 3.
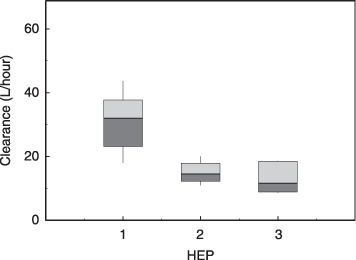
Box plot of estimated systemic clearance of docetaxel according to hepatic function calculated by Bayesian estimation. The top, middle, and bottom lines of each box correspond to the 75% (top quartile), 50% (median), and 25% (bottom quartile) values. The whiskers show the range values that fall between 10 and 90%. 1, 2, and 3 HEP denote normal (n = 183), mildly (increased alkaline phosphatase [ALP] in combination with grade 2 elevation of aspartate aminotransferase [AST] or alanine aminotransferase [ALT], n = 10), and moderately elevated liver function tests (increased ALP in combination with grade 3 or greater elevation of AST or ALT, n = 7), respectively.
Discussion
A population pharmacokinetic approach allows us to analyze data with small numbers of samples per patient, and can be used to investigate pharmacokinetics in unfit patients treated in oncology practice where full pharmacokinetic sampling may be difficult. Therefore, we used the methodology of population pharmacokinetics in Japanese patients treated in oncology practice, in order to investigate the influence of patients’ various backgrounds on the pharmacokinetics of docetaxel.
Goodness‐of‐fit plots (Fig. 2) indicated that the present population pharmacokinetic model was fitted well with the observed data. Table 4 indicates that a convergence ratio on bootstrap data was so high that the robustness of this model was sufficiently guaranteed. The differences between θi of the final model estimates and those of the bootstrap means were relatively small. Therefore, the parameter estimates on bootstrap samples corresponded well with the original data.
The present analysis indicated that the systemic clearance of the drug was significantly correlated to body surface area, albumin, α1‐acid glycoprotein, and liver function. Bruno et al. previously developed a population pharmacokinetic model for docetaxel in patients treated in clinical studies carried out for drug registration, and found the same factors to be significant determinants of clearance.( 16 ) Although age was incorporated as a covariate for clearance in their model, it was not applied to our study. The estimated coefficient of age in their model was small, and a difference of 20 years in age would yield less than a 10% difference in clearance of the drug. Furthermore, in two independent pharmacokinetic and pharmacodynamic studies of docetaxel comparing elderly and non‐elderly patients, pharmacokinetics were found not to be different between the two groups, although the same exposure to docetaxel resulted in more toxicities in elderly patients.( 23 , 24 )
In previous population pharmacokinetic studies of docetaxel,( 16 , 17 , 18 ) liver function was a significant covariate for clearance. A 33% reduction in clearance was observed for patients with AST or ALT > 60 IU together with ALP > 300 IU in a population pharmacokinetic model developed for patients in the USA and European countries,( 16 ) whereas patients with AST or ALT > 60 IU/L had 21% lower clearance in a model for Japanese patients.( 18 ) Liver function was incorporated as a binary covariate into these models because patients with clinically significant impairment of liver function had been excluded from these studies carried out for drug approval. In contrast, patients with significant liver dysfunction were included in our study, although the number of patients with liver dysfunction was small compared to those with normal liver function, and reductions in clearance could be estimated in relation to the extent of liver function impairment. Thus, 22 and 38% reductions were observed for mild and moderate liver dysfunction, respectively (Table 4). Dividing patients into three groups based on their liver function yielded better results than classifying them into two groups (data not shown), and the difference in the reduction of systemic clearance of docetaxel between patients with mild and moderate liver dysfunction was highly significant.
Our population pharmacokinetic study was not designed to investigate pharmacodynamics; patients treated with docetaxel in various combination regimens were included and toxicities were not monitored in a uniform way. Therefore, relationships between liver function and toxicities could not be investigated. However, based on the alterations of observed docetaxel clearance, dose reductions by approximately 20 and 40% would be a reasonable strategy for patients with grade 2 and 3 elevations of AST or ALT in combination with elevated ALP, although variability in concentrations of docetaxel might be observed even with this dose adjustment because liver function is not the only source of pharmacokinetic variability. Furthermore, this recommendation requires further validation in a prospective study.
Population pharmacokinetics of many anticancer agents are currently being investigated as a part of clinical development;( 25 , 26 , 27 , 28 , 29 , 30 , 31 ) however, unfit patients, including those with organ dysfunction or poor performance status, are commonly excluded from clinical trials, resulting in a paucity of pharmacokinetic information for these groups. After drugs are approved, however, these patients are treated in medical practice, and dose reduction may be required at the discretion of attending physicians. It is therefore important to collect actual pharmacokinetic information in this context.
In conclusion, we developed a population pharmacokinetic model for docetaxel that can be used in the setting of an oncology practice. It was found that body surface area, albumin, α1‐acid glycoprotein, and liver function are significant covariates for the systemic clearance of docetaxel. According to the reductions of docetaxel clearance in patients with liver dysfunction predicted by our model, dose reduction by approximately 20 and 40% should be considered for patients with grade 2 and 3 elevations of transaminases at baseline in conjunction with elevation of alkaline phosphatase, respectively.
Acknowledgments
The present study was supported in part by a Grant‐in‐Aid for Cancer Research (17‐8) from the Ministry of Health, Labor, and Welfare of Japan and by Kobayashi Institute for Innovative Cancer Chemotherapy.
References
- 1. Ratain MJ, Schilsky RL, Conley BA, Egorin MJ. Pharmacodynamics in cancer therapy. J Clin Oncol 1990; 8: 1739–53. [DOI] [PubMed] [Google Scholar]
- 2. Minto C, Schnider T. Expanding clinical applications of population pharmacodynamic modelling. Br J Clin Pharmacol 1998; 46: 321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun H, Fadiran E, Jones C et al . Population pharmacokinetics. A regulatory perspective. Clin Pharmacokinet 1999; 37: 41–58. [DOI] [PubMed] [Google Scholar]
- 4. Samara E, Granneman R. Role of population pharmacokinetics in drug development: a pharmaceutical industry perspective. Clin Pharmacokinet 1997; 32: 294–312. [DOI] [PubMed] [Google Scholar]
- 5. Ludden T. Population pharmacokinetics. J Clin Pharmacol 1988; 28: 1059–63. [DOI] [PubMed] [Google Scholar]
- 6. Jones SE, Savin MA, Holmes FA et al . Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 2006; 24: 5381–7. [DOI] [PubMed] [Google Scholar]
- 7. Bear HD, Anderson S, Brown A et al . The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project protocol B‐27. J Clin Oncol 2003; 21: 4165–74. [DOI] [PubMed] [Google Scholar]
- 8. Georgoulias V, Ardavanis A, Tsiafaki X et al . Vinorelbine plus cisplatin versus docetaxel plus gemcitabine in advanced non‐small‐cell lung cancer: a phase III randomized rrial. J Clin Oncol 2005; 23: 2937–45. [DOI] [PubMed] [Google Scholar]
- 9. Georgoulias V, Ardavanis A, Agelidou A et al . Docetaxel versus docetaxel plus cisplatin as front‐line treatment of patients with advanced non small‐cell lung cancer: a randomized, multicenter phase III trial. J Clin Oncol 2004; 22: 2602–9. [DOI] [PubMed] [Google Scholar]
- 10. Vasey P, Jayson G, Gordon A et al . Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first‐line chemotherapy for ovarian carcinoma. J Natl Cancer Inst 2004; 96: 1682–91. [DOI] [PubMed] [Google Scholar]
- 11. Dreyfuss AI, Clark JR, Norris CM et al . Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol 1996; 14: 1672–8. [DOI] [PubMed] [Google Scholar]
- 12. Van Cutsem E, Moiseyenko VM, Tjulandin S et al . Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24: 4991–7. [DOI] [PubMed] [Google Scholar]
- 13. Petrylak DP, Tangen CM, Hussain MHA et al . Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20. [DOI] [PubMed] [Google Scholar]
- 14. Tannock IF, De Wit R, Berry WR et al . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. [DOI] [PubMed] [Google Scholar]
- 15. Muro K, Hamaguchi T, Ohtsu A et al . A phase II study of single‐agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 2004; 15: 955–9. [DOI] [PubMed] [Google Scholar]
- 16. Bruno R, Vivier N, Vergniol JC et al . A population pharmacokinetic model for docetaxel (Taxotere), model building and validation. J Pharmacokin Biopharm 1996; 24: 153–72. [DOI] [PubMed] [Google Scholar]
- 17. Bruno R, Hille D, Riva A et al . Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 1998; 16: 187–96. [DOI] [PubMed] [Google Scholar]
- 18. Tanigawara Y, Sasaki Y, Otsu T et al . Population pharmacokinetics of docetaxel in Japanese patients. Proc Am Soc Clin Oncol 1996; 15: 479. [Google Scholar]
- 19. Vergniol J, Bruno R, Montay G, Frydman A. Determination of Taxotere in human plasma by a semi‐automated high‐performance liquid chromatographic method. J Chromatogr 1992; 582: 273–8. [DOI] [PubMed] [Google Scholar]
- 20. Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 1997; 37: 486–95. [DOI] [PubMed] [Google Scholar]
- 21. Efron B. Bootstrap methods: another look at the jackknife. Ann Stat 1979; 7: 1–26. [Google Scholar]
- 22. Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986; 1: 54–77. [Google Scholar]
- 23. Minami H, Ohe Y, Niho S et al . Comparison of pharmacokinetics and pharmacodynamics of docetaxel and cisplatin in elderly and non‐elderly patients: why is toxicity increased in elderly patients? J Clin Oncol 2004; 22: 2901–8. [DOI] [PubMed] [Google Scholar]
- 24. Ten Tije AJ, Verweij J, Carducci MA et al . Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol 2005; 23: 1070–7. [DOI] [PubMed] [Google Scholar]
- 25. Williams PJ, Ette EI. The role of population pharmacokinetics in drug development in light of the Food and Drug Administration's ‘Guidance for industry: population pharmacokinetics.’ Clin Pharmacokinet 2000; 39: 385–95. [DOI] [PubMed] [Google Scholar]
- 26. Lee CKK, Rowinsky EK, Li J et al . Population pharmacokinetics of troxacitabine, a novel dioxolane nucleoside analogue. Clin Cancer Res 2006; 12: 2158–65. [DOI] [PubMed] [Google Scholar]
- 27. Blair EYL, Rivory LP, Clarke SJ, McLachlan AJ. Population pharmacokinetics of raltitrexed in patients with advanced solid tumours. Br J Clin Pharmacol 2004; 57: 416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruno R, Washington CB, Lu J‐F et al . Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 2005; 56: 361–9. [DOI] [PubMed] [Google Scholar]
- 29. Schmidli H, Peng B, Riviere GJ et al . Population pharmacokinetics of imatinib mesylate in patients with chronic‐phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 2005; 60: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boni JP, Leister C, Bender G et al . Population pharmacokinetics of CCI‐779: Correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther 2005; 77: 76–89. [DOI] [PubMed] [Google Scholar]
- 31. Van Kesteren C, Mathot RAA, Raymond E et al . Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation. J Clin Oncol 2002; 20: 4065–73. [DOI] [PubMed] [Google Scholar]


