Abstract
Although the introduction of screening mammography in Japan would be expected to reduce mortality from breast cancer, the optimal screening modality in terms of cost‐effectiveness remains unclear. We compared the cost‐effectiveness ratio, defined as the cost required for a life‐year saved, among the following three strategies: (1) annual clinical breast examination; (2) annual clinical breast examination combined with mammography; and (3) biennial clinical breast examination combined with mammography for women aged 30–79 years using a hypothetical cohort of 100 000. The sensitivity, specificity and early breast cancer rates were derived from studies conducted from 1995 to 2000 in Miyagi Prefecture. The treatment costs were based on a questionnaire survey conducted at 13 institutions in Japan. We used updated parameters that were needed in the analysis. Although the effectiveness of treatment in terms of the number of expected survival years was highest for annual combined modality, biennial combined modality had a higher cost‐effectiveness ratio, followed by annual combined modality and annual clinical breast examination in all age groups. In women aged 40–49 years, annual combined modality saved 852.9 lives and the cost/survival duration was 3 394 300 yen/year, whereas for biennial combined modality the corresponding figures were 833.8 and 2 025 100 yen/year, respectively. Annual clinical breast examination did not confer any advantages in terms of effectiveness (815.5 lives saved) or cost‐effectiveness (3 669 900 yen/year). While the annual combined modality was the most effective with respect to life‐years saved among women aged 40–49 years, biennial combined modality was found to provide the highest cost‐effectiveness. (Cancer Sci 2006; 97: 1242–1247)
- CBE
clinical breast examination
- RCT
randomized controlled trial
- SMG
screening mammography.
Breast cancer is the most common cancer in Japanese women. According to estimates for 1999, 36 139 new cases of breast cancer were diagnosed and these accounted for 16.1% of all new cases of cancer in women.( 1 ) As the prognosis of breast cancer is closely associated with the clinical stage of the disease, early discovery of the cancer (i.e. secondary prevention) is important for disease control, as well as primary prevention by lifestyle modification.( 2 ) Women aged 40–49 years, having the highest incidence rate of breast cancer, should be targeted by an appropriate screening modality in Japan.
The first national guidelines for breast cancer screening, established in 1987, endorsed the use of without mammography for women aged 30 years or over, although there had been no evidence of the effectiveness of breast cancer screening using CBE alone.( 3 , 4 Subsequent studies conducted in Japan indicated that SMG leads to a better sensitivity and disease stage distribution at diagnosis.( 5 , 6 , 7 , 8 , 9 , 10 ) Work to introduce mammography into breast cancer screening has been continuing in Japan, and mammography for women aged 50 years or over was endorsed by the Ministry of Health, Labour and Welfare in 2000, and expanded to include women aged 40 years or over in 2004. It is recommended that mammography screening should be conducted at 2‐yearly intervals and that CBE be used at the same time.
Although the introduction of SMG in Japan would be expected to reduce mortality due to breast cancer, as in Western countries,( 11 ) the cost‐effectiveness of such screening in Japan remains unclear, as the incidence of breast cancer is comparatively lower.( 1 , 2 Higher cost‐effectiveness of SMG has been observed in studies from Western countries,( 12 , 13 , 14 , 15 , 16 ) whereas only three studies have been conducted to evaluate the cost‐effectiveness of SMG in Japan in 1991, 1997 and 1999.( 17 , 18 , 19 ) The studies in Japan had some methodological limitations including usage of American SMG sensitivity and specificity data,( 17 ) no evaluation of the cost‐effectiveness of a biennial screening interval,( 18 ) no usage of actual biennial SMG sensitivity data,( 19 ) or usage of the same sensitivity and specificity for every age group.( 18 , 19 Ohuchi et al. reported that the sensitivity and specificity of biennial screening were not seriously inferior to those of annual screening.( 20 ) Therefore, cost‐effectiveness analysis of biennial SMG is needed to obtain better screening strategies in terms of effects and costs. In addition, because the highest incidence of breast cancer is observed among Japanese women aged 45–49 years,( 1 ) and these women have relatively higher breast density than women aged 50 years or older,( 8 , 9 , 10 ) cost‐effectiveness analysis requires data on actual sensitivity and specificity obtained by separating women into at least those aged 49 years or younger and those aged 50 years or older.
The objectives of the present study were to compare the cost‐effectiveness ratio, defined as the cost required for a life‐year saved, among different screening modalities (CBE alone, CBE and SMG, or no screening) and screening intervals (annual or biennial) for subjects in different age groups from their thirties to seventies. The screening strategies we chose were annual CBE alone, annual CBE and SMG, and biennial CBE and SMG from a practical perspective. We used updated parameters to ascertain the cost‐effectiveness of using SMG for Japanese women.
Patients and Methods
Models for cost‐effectiveness analysis. The analytical models used in the present study for cost‐effectiveness analysis of screening strategies were described in 1997.( 18 ) The models were developed to allow comparison of the cost‐effectiveness of various cancer screenings. We developed two mathematical models for estimation of cost‐effectiveness, namely the ‘annual model’ and the ‘biennial model’. Using these models, we established a hypothetical cohort of 400 000 asymptomatic women aged 30, 40, 50, 60 and 70 years. Of these women, 300 000 underwent examination for breast cancer (Fig. 1) using the following strategies: (1) annual CBE alone; (2) annual CBE and SMG; and (3) biennial CBE and SMG, for 10 years and were followed for 5 years for all strategies. The remaining 100 000 women had no examination and were followed for 15 years. We assumed that each woman would participate in the program annually for the annual model or biennially for the biennial model, unless breast cancer was detected or the woman died of causes other than breast cancer. Women in whom cancer was detected were observed for 5 years thereafter.
Figure 1.
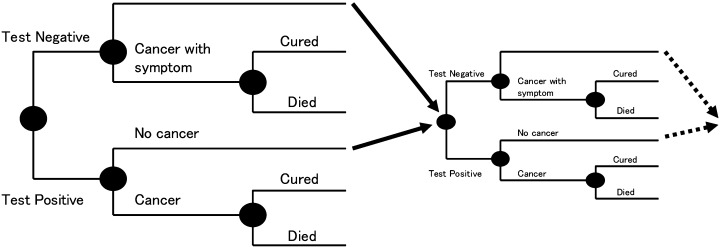
Structure of the analytical model (cohorts with screening). In a theoretical cohort, 100 000 subjects participated in the first screening. Resubmitting those who did not contract breast cancer to the next screening (excluding those who died of other diseases) by simulation allows the calculation of costs and effects for participation in screening at any age (for example, from 30 to 79 years). It was assumed that 100% of subjects underwent further examination and that the sensitivity of such examinations was 100%.
In each screening modality cohort, the number of subjects taking work‐up examinations and the number of subjects in whom breast cancer was detected was calculated from the sensitivity and specificity of the screening modality. We also estimated the life‐years for which the patient would survive based on early breast cancer rates, the 5‐year survival rate for early stage or other stages of breast cancer, and life expectancy. The number of life‐years of survival was adopted as the parameter of effectiveness. We hypothesized that when asymptomatic women undergo breast cancer screening, the proportion of early stage breast cancers would be those determined by screening modalities. A difference in the effects of annual and biennial screening emerged in the rate of false‐negative breast cancers. Furthermore, as women false‐negative for breast cancer would experience symptoms during the period between screenings, it was hypothesized that the proportion of early stage breast cancers among women with a false‐negative screening result would be similar to that among women who were not screened.
These models defined the costs from the payers’ viewpoint. Only direct costs were determined. The costs comprised those for the screening test, work‐up examinations for recalled women, diagnostic tests for outpatients, initial treatment of breast cancer and terminal care. Women with cancer in the ‘no screening’ program visited a hospital only after developing symptoms. We analyzed the effectiveness and cost of the ‘no screening’ program in the same way as for the screening programs.
Cost‐effectiveness ratios were defined as the cost required for a life‐year saved. Both costs and life‐years were discounted at an annual rate of 3%.
Data resource. For each of the screening strategies, sensitivity, specificity and proportion of early stage breast cancer were derived from studies conducted in Miyagi prefecture. Between 1995 and 1998, 15 271 women aged 40–49 years and 17 755 women aged 50–69 years in Miyagi prefecture underwent annual single‐view mammography for breast cancer screening, combined with CBE.( 9 ) Between 1996 and 2000, annual or biennial breast cancer screening using CBE, either with or without single‐view mammography, was conducted in Miyagi prefecture, and the past records and stage of cancer detected for 87 screenees aged 40–49 years and 117 screenees aged 50–69 years were investigated.( 21 ) We used sensitivity, specificity and proportion of early stage breast cancer from the above studies for each of the screening strategies (Table 1). Annual CBE and SMG had the highest sensitivity, followed by biennial CBE and SMG, and then annual CBE. The sensitivity and early breast cancer detection rates were better for CBE and SMG than for CBE alone. Diagnostic accuracy for women aged 30–39 and 70–79 years was assumed to be similar to that in women aged 40–49 or 50–69 years, respectively. Early breast cancer was defined as stage 0 or I according to the classification of the International Union Against Cancer.( 22 )
Table 1.
Sensitivity, specificity and proportion of early stage breast cancer according to screening strategies from studies in Miyagi prefecture ( 9, 21
| Group | Sensitivity | Specificity | Proportion of early stage breast cancer |
|---|---|---|---|
| Women aged 40–49 years | |||
| Annual CBE | 0.873 | 0.918 | 0.620 |
| Annual CBE and SMG | 0.938 | 0.898 | 0.800 |
| Biennial CBE and SMG | 0.810 | 0.898 | 0.800 |
| Women aged 50–69 years | |||
| Annual CBE | 0.932 | 0.961 | 0.500 |
| Annual CBE and SMG | 0.950 | 0.930 | 0.740 |
| Biennial CBE and SMG | 0.910 | 0.930 | 0.740 |
CBE, clinical breast examination; SMG, screening mammography.
Because data on the incidence of breast cancer for the whole of Japan were lacking, data from the Miyagi Prefectural Cancer Registry (1993–1997) were substituted for breast cancer incidence.( 23 ) Mortality from breast cancer and total mortality were derived from the annual report on Vital Statistics of Japan in 2001,( 24 ) and life expectancy was derived from the 19th Life Table.( 25 )
The proportion of early stage breast cancers among breast cancers detected at outpatient departments, the 5‐year survival rate by clinical stage, screening costs, further examination costs, diagnostic costs for outpatients and treatment costs were based on a questionnaire survey carried out by the Grant‐in‐Aid for Cancer Research( 7 , 8 ) from the Ministry of Health and Welfare (Chief Investigator, Noriaki Ohuchi) in 1996 at 13 institutions in Japan where breast cancer screening had been actively conducted.( 18 ) After excluding the maximum and minimum values, an average of the remaining values was utilized (Table 2).
Table 2.
Results from a questionnaire survey to 13 institutions in Japan ( 18 )
| Variable | Average | Minimum–maximum |
|---|---|---|
| Proportion of early stage breast cancer for breast cancers found at outpatient (%) | 37.2 | 28–60 |
| 5‐year survival rate for women with early stage breast cancer (%) | 95.2 | 92–99 |
| 5‐year survival rate for women with advanced breast cancer (%) | 73.8 | 60–82 |
| Screening costs (yen) | ||
| CBE | 2 276 | 2 000–2935 |
| CBE and SMG | 4 791 | 3 720–5366 |
| Further examination costs (yen) | ||
| CBE | 18 931 | 12 000–30 000 |
| CBE and SMG | 18 000 | 10 000–30 000 |
| Diagnosis costs at outpatient (yen) | 30 000 | |
| Treatment costs for early stage breast cancer (yen) | 1 050 000 | 750 000–1 340 000 |
| Treatment costs for advanced breast cancer (yen) | ||
| Not including terminal care costs | 2 030 000 | 1 000 000–2 700 000 |
| Including terminal care costs | 6 910 000 | 4 000 000–12 000 000 |
CBE, clinical breast examination; SMG, screening mammography.
Sensitivity analysis. We carried out sensitivity analysis to assess the effects of changes in our assumptions about the sensitivity and specificity of the screening strategies and the costs of the screening on the cost‐effectiveness ratios.
Results
Cost‐effectiveness analysis. Results derived from analysis of costs and the effects of the three screening strategies for patients aged 40–49 years are shown in Table 3. All forms of screening appeared to prolong survival in terms of the number of expected survival years. Whereas annual CBE and SMG was the most effective modality, biennial CBE and SMG was found to require the lowest total cost and provide the highest cost‐effectiveness. For annual CBE and SMG, the number of lives saved was 852.9 and the cost/survival duration was 3 394 300 yen/year, whereas for biennial CBE and SMG, the corresponding figures were 833.8 and 2 025 100 yen/year, during 15 years of follow up among 100 000 women aged 40–49 years. Annual CBE did not confer any advantages in terms of effectiveness (815.5 lives saved) or cost‐effectiveness (3 669 900 yen/year).
Table 3.
Results from cost‐effectiveness analysis for three screening strategies in women aged 40–49 years
| Variable | No screening | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|---|
| CBE alone | CBE and SMG | |||
| Number of lives saved | 771.8 | 8 15.5 | 852.9 | 833.8 |
| Survival duration (years) | 15 963.9 | 16 756.8 | 17 434.2 | 17 098.0 |
| Screening costs (× 106 yen) | 1 985.1 | 4 178.7 | 2 122.2 | |
| Further examination costs (× 106 yen) | 1 366.2 | 1 613.6 | 822.5 | |
| Diagnosis costs at outpatient (× 106 yen) | 24.5 | 3.0 | 1.5 | 6.4 |
| Initial treatment costs (× 106 yen) | 1 360.0 | 1 186.8 | 1 038.7 | 1 094.4 |
| Terminal treatment costs (× 106 yen) | 971.0 | 724.3 | 513.6 | 606.6 |
| Total costs (× 106 yen) | 2 355.5 | 5 265.3 | 7 346.1 | 4 652.1 |
| Costs/number of lives saved (× 103 yen/capita) | 66 536.6 | 61 540.3 | 37 002.4 | |
| Costs/survival duration (× 103 yen/year) | 3 669.9 | 3 394.3 | 2 025.1 | |
CBE, clinical breast examination; SMG, screening mammography.
Comparison of the number of lives saved among age groups. Table 4 shows a comparison of the number of lives saved by the different screening strategies among the different age groups. For all age groups, it was projected that most lives would be saved with the use of annual CBE and SMG, followed by biennial CBE and SMG, and then annual CBE. However, annual CBE and SMG and biennial CBE and SMG were very similar in terms of the numbers of lives saved for patients in their thirties, fifties, sixties and seventies, whereas the difference for patients in their forties tended to be slightly larger.
Table 4.
Comparison of number of lives saved among age groups
| Age group (years) | No screening | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|---|
| CBE alone | CBE and SMG | |||
| 30–39 | 236.3 | 249.7 | 261.0 | 253.7 |
| 40–49 | 771.8 | 815.5 | 852.9 | 833.8 |
| 50–59 | 741.6 | 764.7 | 809.4 | 800.6 |
| 60–69 | 683.8 | 705.2 | 746.4 | 737.9 |
| 70–79 | 588.6 | 607.0 | 642.5 | 636.3 |
CBE, clinical breast examination; SMG, screening mammography.
Comparison of cost‐effectiveness among age groups. Table 5 shows a comparison of the ratio of cost to survival duration (years) among the different age groups. Regardless of the screening strategy employed, a J‐shaped curve was seen when age was plotted against the ratio of cost to survival duration. The bottom of the J reflected patients aged 40–49 years. In all age groups, the smallest ratio of cost to survival duration was observed for biennial CBE and SMG. The ratios for annual CBE and annual CBE and SMG were larger than that for biennial CBE and SMG, and the ratio for annual CBE and SMG was slightly smaller than that for annual CBE.
Table 5.
Comparison of ratios of costs to survival duration among age groups
| Age group (years) | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|
| CBE alone | CBE and SMG | ||
| 30–39 | 12 228 280 | 11 387 100 | 7 967 840 |
| 40–49 | 3 669 880 | 3 394 310 | 2 025 070 |
| 50–59 | 6 371 330 | 4 179 810 | 2 064 810 |
| 60–69 | 8 866 350 | 5 837 010 | 2 848 130 |
| 70–79 | 13 734 380 | 9 079 140 | 4 009 740 |
CBE, clinical breast examination; SMG, screening mammography.
Sensitivity analysis. A sensitivity analysis using the best case and worst case for the sensitivity of each screening strategy showed that even the lowest cost‐effectiveness ratio for annual CBE alone (sensitivity = 1.000) was similar to the highest ratio for biennial CBE and SMG (sensitivity = 0.600) (Table 6). A sensitivity analysis for specificity showed that the cost‐effectiveness ratio was reversed when the sensitivity of annual CBE alone was equal to or greater than 0.950 and that of biennial CBE and SMG was equal to or less than 0.700 (Table 7). A sensitivity analysis for screening costs showed that the cost‐effectiveness ratio was reversed when the screening cost of annual CBE alone was equal to or less than 1000 yen, and that of biennial CBE and SMG was equal to or greater than 6000 yen (Table 8).
Table 6.
Impact of screening sensitivity on ratios of costs to survival duration in women aged 40–49 years
| Sensitivity | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|
| CBE alone | CBE and SMG | ||
| 0.600 | 5 584 810 | 5 608 030 | 3 137 280 |
| 0.650 | 5 113 770 | 5 135 460 | 2 789 780 |
| 0.700 | 4 710 010 | 4 730 400 | 2 504 070 |
| 0.750 | 4 360 100 | 4 379 350 | 2 265 010 |
| 0.800 | 4 053 920 | 4 072 180 | 2 062 040 |
| 0.850 | 3 783 760 | 3 801 140 | 1 887 560 |
| 0.900 | 3 543 620 | 3 560 230 | 1 735 970 |
| 0.950 | 3 328 760 | 3 344 670 | 1 603 040 |
| 1.000 | 3 135 390 | 3 150 670 | 1 485 520 |
CBE, clinical breast examination; SMG, screening mammography.
Table 7.
Impact of screening specificity on ratios of costs to survival duration in women aged 40–49 years
| Specificity | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|
| CBE alone | CBE and SMG | ||
| 0.700 | 8 205 240 | 5 506 520 | 3 414 780 |
| 0.750 | 7 165 020 | 4 973 130 | 3 063 840 |
| 0.800 | 6 124 800 | 4 439 750 | 2 712 910 |
| 0.850 | 5 084 580 | 3 906 360 | 2 361 970 |
| 0.900 | 4 044 360 | 3 372 970 | 2 011 030 |
| 0.950 | 3 004 140 | 2 839 580 | 1 660 090 |
| 1.000 | 1 963 920 | 2 306 200 | 1 309 160 |
CBE, clinical breast examination; SMG, screening mammography.
Table 8.
Impact of screening costs on ratios of costs to survival duration in women aged 40–49 years
| Screening costs (yen) | Annual screening | Biennial screening CBE and SMG | |
|---|---|---|---|
| CBE alone | CBE and SMG | ||
| 1000 | 2 266 300 | 1 145 450 | 544 420 |
| 2000 | 3 366 280 | 1 738 660 | 934 990 |
| 3000 | 4 466 270 | 2 331 870 | 1 325 560 |
| 4000 | 5 566 260 | 2 925 080 | 1 716 130 |
| 5000 | 6 666 250 | 3 518 290 | 2 106 700 |
| 6000 | 7 766 240 | 4 111 500 | 2 497 270 |
CBE, clinical breast examination; SMG, screening mammography.
Discussion
In the present study, the cost‐effectiveness of carrying out SMG for Japanese women was investigated by analyzing the cost‐effectiveness of three screening strategies and comparing them among age groups. Whereas annual CBE and SMG was the most effective modality, biennial CBE and SMG was found to require the lowest total cost and provide the highest cost‐effectiveness. For annual CBE and SMG, the number of lives saved was 852.9 and the cost/survival duration was 3 394 300 yen/year, whereas for biennial CBE and SMG the corresponding figures were 833.8 lives saved and 2 025 100 yen/year during 15 years of follow up among 100 000 women aged 40–49 years. Annual CBE did not confer any advantages in terms of effectiveness (815.5 lives saved) or cost‐effectiveness (3 669 900 yen/year). Similar results were observed among all of the age groups.
Our findings are in strong agreement with three previously published cost‐effectiveness studies in Japan,( 17 , 18 , 19 ) confirming that introduction of SMG is a cost‐effective modality in Japan. Okubo et al.( 17 ) compared five annual screening strategies: (1) no screening; (2) CBE alone; (3) SMG alone; (4) CBE followed by SMG if CBE findings were abnormal (CBE followed by SMG); and (5) CBE and SMG. They reported that the additional costs of each modality, as compared with no screening, per additional year of life saved per cohort of 100 000 Japanese women would be US$49 700 for CBE alone, US$40 400 for CBE followed by SMG, US$14 300 for SMG alone, and US$18 200 for CBE and SMG. The study used American SMG sensitivity and specificity data, and the treatment costs were based on expert opinion. Thus, the study had methodological limitations. Ohnuki et al., with the use of cost‐effectiveness analysis, reported that in every age group from thirties to seventies, annual CBE and SMG was superior to annual CBE alone in terms of cost/survival duration.( 18 ) For example, for women in their forties, the cost/survival duration was 1 458 000 yen/year for annual CBE alone and 1 088 000 yen/year for annual CBE and SMG. The study used the same sensitivity and specificity data for every age group and did not evaluate biennial screening cost‐effectiveness. Iinuma et al. also conducted cost‐effectiveness analysis among Japanese women and reported that biennial CBE and SMG required the lowest total cost and provided the highest cost‐effectiveness, followed by annual CBE and SMG, and annual CBE alone.( 19 ) For example, in women aged 45–49 years, the cost/survival duration was 1 410 000 yen/year for biennial CBE and SMG, 2 460 000 yen/year for annual CBE and SMG, and 3 260 000 yen/year for annual CBE alone. Although the study examined the cost‐effectiveness of biennial screening, it used the same biennial sensitivity and specificity data as those for annual screening. Furthermore, they used the same sensitivity and specificity data for every age group. We confirmed that biennial CBE and SMG required the lowest total cost and provided the highest cost‐effectiveness using actual sensitivity and specificity data for Japanese women separated into those aged 49 years or younger and those aged 50 years or older.
There were, however, several methodological limitations in our study. First, it was not a RCT, but utilized the next best alternative (i.e. the use of simulation models) to estimate the reduction in mortality and the cost‐effectiveness of SMG. Generally, a RCT is required for evaluating the effects of a screening intervention; however, no such RCT have been reported previously in Japan. Therefore, if SMG is implemented in addition to other screening strategies, it will be necessary to establish a system to observe the reduction in mortality simultaneously. Second, we assumed that all women allocated to the screening group would undergo examination for 10 years. The actual examination participation rate for women in Japan was 12.4% in 2002,( 26 ) and biennial examination might have led to a reduction in the participation rate. To obtain the cost‐effectiveness of biennial CBE and SMG, there is a need to introduce a government policy to improve the participation rate. Third, for women aged 30–39 and 70–79 years, we used sensitivity data from women aged 40–49 and aged 50–69 years, respectively, due to a lack of available data in Japan. Therefore, the results for women aged 30–39 and 70–79 years should be interpreted with caution, and should be re‐evaluated in a future study using actual data for women in these age groups. Finally, the sensitivities and specificities of CBE and SMG that we used in the present study might be different from the true values because they were similar. The data were derived from previous studies conducted in Miyagi prefecture.( 9 , 21 In these studies, CBE was carried out by a skilled surgeon with at least 5 years clinical experience in breast cancer diagnosis, and one‐view mammography was used to obtain the sensitivity, specificity and proportion of early stage breast cancer. We previously reported that two‐view mammography could improve the detection rate by 3% compared with one‐view mammography.( 27 ) If the sensitivities and specificities are generally lower and two‐view mammography is used, then in our model the cost per life‐year saved for CBE alone might become lower, and that for biennial SMG alone might become better than biennial CBE and SMG. Further detailed study to compare biennial SMG alone with biennial CBE and SMG using two‐view mammography should be conducted while considering the risk of increased radiation exposure.( 28 ) Nevertheless, our data in Table 3 also demonstrate that the number of lives saved by CBE was higher than in the ‘no screening’ group. Our results appear to indicate the effectiveness of CBE, at least among women who had no symptoms.
Shen and Parmigini reported that biennial mammography can be cost‐effective if coupled with annual CBE, using a microsimulation model incorporating age‐specific preclinical duration of the disease, sensitivities of the two modalities, breast cancer incidence and competing cause of mortality.( 29 ) Recent studies suggest that mammography combined with CBE may improve the overall screening sensitivity compared with mammography alone.( 30 , 31 , 32 ) Our study indicates the cost‐effectiveness of mammography combined with CBE, either annually or biennially, for women aged 40–49 years, although biennial combination is more cost‐effective than annual combination (3, 5, 8).
The present results indicate that biennial CBE and SMG is an appropriate selection for replacement of the annual CBE because of its higher effectiveness and lower total costs. However, further consensus is required about whether biennial or annual CBE and SMG is preferable. In terms of effectiveness, which is equivalent to the number of lives saved, the difference between biennial and annual CBE and SMG was larger for women aged 40–49 years than for those in other age groups. For example, in our model, mortality reduction rates with annual and biennial CBE and SMG for women aged 50–59 years, compared with those in the non‐screening group, were 41.6% and 35.7%, respectively; hence, the difference between annual and biennial CBE and SMG was only 5.9%. However, the corresponding rates with annual and biennial CBE and SMG for women aged 40–49 years were 47.1% and 36.1%, respectively, the difference being 11.0%. Therefore, in terms of effectiveness, annual CBE and SMG appears better for women aged 40–49 years than for women in other age groups. The results of the present study indicate the cost‐effectiveness of CBE and SMG, either annually or biennially, for women aged 40–49 years; however, further investigation aimed at selecting the most appropriate screening modality, including additional ultrasound according to the tissue density of mammary glands, may be required.( 21 )
In conclusion, the findings of the present study appear to justify the use of the CBE and SMG modality in terms of cost‐effectiveness for women aged 40–49 years as well as for other age groups. The present results should provide useful basic data for the nationwide introduction of SMG for breast cancer screening in Japan in terms of cost‐effectiveness.
Acknowledgments
This study was supported by Grant‐in‐Aid for Cancer Research (15‐14), ‘Researches on quality and efficacy improvement of breast cancer screening’ (chief investigator: Noriaki Ohuchi) from the Ministry of Health, Labour and Welfare, Japan.
References
- 1. The Research Group for Population‐based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1999: estimates based on data from 11 population‐based cancer registries. Jpn J Clin Oncol 2004; 34: 352–6. [DOI] [PubMed] [Google Scholar]
- 2. Veronesi U, Boyle P, Goldhirsch A et al. Breast cancer. Lancet 2005; 365: 1727–41. [DOI] [PubMed] [Google Scholar]
- 3. Ota J, Horino T, Taguchi T et al. Mass screening for breast cancer: comparison of the clinical stages and prognosis of breast cancer detected by mass screening and in out‐patient clinics. Jpn J Cancer Res 1989; 80: 1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanemura S, Tsuji I, Ohuchi N et al. A case control study on the effectiveness of breast cancer screening by clinical breast examination in Japan. Jpn J Cancer Res 1999; 90: 607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohuchi N, Yoshida K, Kimura M et al. Improved detection rate of early breast cancer in mass screening combined with mammography. Jpn J Cancer Res 1995; 84: 807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohuchi N, Yoshida K, Kimura M et al. Comparison of false negative rates among breast cancer screening modalities with or without mammography: Miyagi trial. Jpn J Cancer Res 1995; 86: 501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morimoto T, Sasa M, Yamaguchi T et al. A comparison of mass screening for breast cancer using mammography and physical examination alone in Japan. Breast Cancer 1995; 30: 19–25. [DOI] [PubMed] [Google Scholar]
- 8. Morimoto T, Sasa M, Yamaguchi T et al. Breast cancer screening by mammography in women aged under 50 years in Japan. Anticancer Res 2000; 20: 3689–94. [PubMed] [Google Scholar]
- 9. Ohnuki K, Ohuchi N, Kimura M et al. Accuracy of mammography screening for breast cancer in women aged 40–49: a comparison with screening in women aged 50–69. J Jpn Assoc Breast Cancer Screen 2000; 9: 139–45. (In Japanese with English abstract.) [Google Scholar]
- 10. Ohnuki K. Mammographic screening for non‐palpable breast cancer in Japan. Breast Cancer 2005; 12: 258–66. [DOI] [PubMed] [Google Scholar]
- 11. Elmore JG, Armstrong K, Lehman CD et al. Screening for breast cancer. JAMA 2005; 293: 1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Oortmarssen GJ, Habbema JD, Van Der Maas PJ et al. A model for breast cancer screening. Cancer 1990; 66: 1601–12. [DOI] [PubMed] [Google Scholar]
- 13. De Koning HJ, Van Ineveld BM, Van Oortmarssen GJ et al. Breast cancer screening and cost‐effectiveness: policy alternatives, quality of life considerations and the possible impact of uncertain factors. Int J Cancer 1991; 49: 531–7. [DOI] [PubMed] [Google Scholar]
- 14. Beemsterboer PM, De Koning HJ, Warmerdam PG et al. Prediction of the effects and costs of breast‐cancer screening in Germany. Int J Cancer 1994; 58: 623–8. [DOI] [PubMed] [Google Scholar]
- 15. Lindfors KK, Rosenquist CJ. The cost‐effectiveness of mammographic screening strategies. (Published erratum appears in JAMA 1996; 275: 112.) JAMA 1995; 274: 881–4. [PubMed] [Google Scholar]
- 16. Wald NJ, Murphy P, Major P et al. UKCCCR multicentre randomised controlled trial of one and two view mammography in breast cancer screening. BMJ 1995; 311: 1189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okubo I, Glick H, Frumkin H et al. Cost‐effectiveness analysis of mass screening for breast cancer in Japan. Cancer 1991; 67: 2021–9. [DOI] [PubMed] [Google Scholar]
- 18. Ohnuki K, Tsuji I, Ohuchi N et al. Cost‐effectiveness analysis of annual breast cancer screening in Japan. J Jpn Assoc Breast Cancer Screen 1997; 6: 145–51. (In Japanese with English abstract.) [Google Scholar]
- 19. Iinuma T, Matsumoto T, Tateno Y. Cost‐effectiveness of mass screening for breast cancer, using mammography with physical examination, as a function of the screening interval. J Jpn Assoc Breast Cancer Screen 1999; 8: 23–30. (In Japanese with English abstract.) [Google Scholar]
- 20. Ohuchi N, Ohnuki K, Yoshida K et al. Appropriate interval of breast cancer screening with mammography based on the rates of early cancer and interval cancer. J Jpn Assoc Breast Cancer Screen 1996; 5: 245–8. (In Japanese with English abstract.) [Google Scholar]
- 21. Ohnuki K, Ohuchi N, Kimura M et al. Analysis of mammographic screening intervals for women aged 40–49. J Jpn Assoc Breast Cancer Screen 2002; 11: 143–8. (In Japanese with English abstract.) [Google Scholar]
- 22. International Union Against Cancer, Hermanek P, Sobin LH, eds. TNM Classification of Malignant Tumours, 4th edn. Berlin: Springer‐Verlag, 1987. [Google Scholar]
- 23. The Miyagi Prefectural Cancer Registry. Cancer incidence in women. In: The Miyagi Prefectural Cancer Registry, eds. Cancer in Miyagi Prefecture, 1993–1997. Sendai: The Miyagi Prefectural Cancer Registry, 2001; 24–5. (In Japanese.) [Google Scholar]
- 24. Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare. General mortality. In: Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare, eds. Vital Statistics of Japan 2001, Vol. 1. Tokyo: Health and Welfare Statistics Association, 2003; 130–317. (In Japanese.) [Google Scholar]
- 25. Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare. The 19th life tables. In: Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare, eds. The 19th Life Tables. Tokyo: Health and Welfare Statistics Association, 2002; 27–33. (In Japanese.) [Google Scholar]
- 26. Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare. Annual report of community health and geriatric health in 2002 (geriatric health). In: Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare, eds. Recall Rate of Cancer Screening. Tokyo, Health and Welfare Statistics Association, 2002; 510. (In Japanese.) [Google Scholar]
- 27. Ohnuki K, Ohuchi N, Yoshida H et al. Comparison of two views with one view procedures on the cancer detection rate in screening mammography. J Jpn Assoc Breast Cancer Screen 1993; 3: 33–7. (In Japanese.) [Google Scholar]
- 28. Iinuma T, Matsumoto T, Tateno Y. Quantitative comparison between benefit and risk of mammographic exposure in the mass screening of breast cancer for Japanese women. J Jpn Assoc Breast Cancer Screen 1994; 3: 227–36. (In Japanese with English abstract.) [Google Scholar]
- 29. Shen Y, Parmigiani G. A model‐based comparison of breast cancer screening strategies: mammograms and clinical breast examinations. Cancer Epidemiol Biomarkers Prev 2005; 14: 529–32. [DOI] [PubMed] [Google Scholar]
- 30. Barton MB, Harris R, Fletcher SW. The rational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: should it be done? How? JAMA 1999; 282: 1270–80. [DOI] [PubMed] [Google Scholar]
- 31. Bobo JK, Lee NC, Thames SF. Findings from 752 081 clinical breast examinations reported to a national screening program from 1995 through 1998. J Natl Cancer Inst 2000; 92: 971–6. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Zelen M. Screening sensitivity and sojourn time from breast cancer early detection clinical trials: mammograms and physical examinations. J Clin Oncol 2001; 19: 3490–9. [DOI] [PubMed] [Google Scholar]


