Abstract
(Cancer Sci 2010; 101: 646–651)
Estrogen‐related receptor (ERR) is a nuclear receptor that modulates the estrogen‐signaling pathway. Here, we investigated the expression of both ERRβ and ERRγ in human prostate tissues. Using original rabbit polyclonal anti‐ERRβ and anti‐ERRγ antibodies, the expression of ERRβ and ERRγ was evaluated by immunohistochemical analysis of cancerous lesions (n = 107) and benign foci (n = 92), obtained by radical prostatectomy. Stained slides were evaluated for the proportion of immunoreactive cells and their staining intensity. Total immunoreactivity scores (IR scores; range, 0–8) were calculated as the sum of the proportion and intensity scores. The relationship between the clinicopathological characteristics of the patients and the expression of the three ERRs (ERRα, ERR β, and ERR γ) was evaluated. IR scores for ERRβ and ERRγ were significantly lower in cancerous lesions than that in benign foci (P < 0.0001, for both). Clinicopathological analyses revealed that the patients with low ERRγ IR scores (≤4) tended to show poor cancer‐specific survival (P = 0.07). Then, we used data from our previous study (Fujimura T., Int J Cancer, 2007; 120: 2325–30). Patients with a high IR score for ERRα and a low score for ERRγ showed significantly poorer cancer‐specific survival than those with a low IR score for ERRα and a high score for ERRγ (P = 0.0003). We demonstrated the differential expression of ERRβ and ERRγ in prostate tissue. The combined evaluation of the expression of ERRα and ERRγ could be a significant prognostic factor for prostate cancer.
Estrogen‐signaling pathways in addition to androgen‐signaling pathways are implicated in the development of prostate cancer (PCa).( 1 ) Diethylstilbestrol (DES) was previously used for endocrine therapy in the treatment of PCa. Presently, selective estrogen receptor modulators (SERMs) are being used for this therapy. Toremifene, an estrogen receptor α (ERα) modulator, is used in patients with high‐grade intraepithelial neoplasia (HGPIN) for the prevention of PCa, while raloxifene, an ERβ modulator, is used in patients with hormone‐refractory PCa.( 2 , 3 ) The biological activities of these chemicals are mediated by two ERs – ERα and ERβ. Since ERα is predominantly localized in the stromal cells of the prostate,( 4 , 5 , 6 , 7 ) the ERα‐mediated effects of estrogens on the prostatic epithelium are thought to be mediated by paracrine pathways. In contrast, ERβ is predominantly localized in the epithelial cells of the normal human prostate. ERβ expression is lesser in PCa than in benign epithelium.( 4 , 8 ) Thus, ERβ exhibits a protective effect against aberrant cell proliferation and carcinogenesis.( 9 , 10 , 11 , 12 )
Recent studies have focused on additional estrogen‐related signaling pathways that are mediated by three estrogen‐related receptors (ERRs), namely, ERRα, ERRβ, and ERRγ, in the estrogen‐targeted organs.( 13 , 14 , 15 ) The ERRs (α, β, and γ) are orphan members of the nuclear receptor superfamily. ERRα (NR3B1) and ERRβ (NR3B2) were identified in the kidney and heart in a screen designed to clone steroid hormone receptors closely related to ERα.( 12 ) ERRγ (NR3B3) was cloned from the fetal brain by rapid amplification of cDNA ends (RACE)‐polymerase chain reaction (PCR).( 16 ) The expression of ERRα is more abundant than those of the other two subtypes of ERR and is detected in tissues with high metabolism, such as the heart, kidney, intestinal tract, skeletal muscle, and brown adipose tissue.( 17 ) The expression patterns of ERRβ and ERRγ are more restricted, but they are abundant in the heart and kidneys where they play a central role in regulating energy metabolism.( 17 , 18 ) Apart from energy metabolism, ERRs are involved in the development of cancer. Several studies have implicated ERRα in the development of human breast cancer and colorectal cancer.( 19 , 20 , 21 ) Further, in the case of human breast cancer, ERRα and ERRγ are associated with an unfavorable and favorable prognosis, respectively.( 22 )
The characteristics of ERRs should be clarified for a better understanding of the estrogen‐ and estrogen‐related signaling pathways in the prostate. In a previous study, we reported that increased ERRα expression is a negative prognostic predictor in human PCa.( 23 ) Reduced expression of ERRβ and ERRγ in some prostatic carcinomas has been reported, suggesting that these receptors perform antiproliferative or tumor‐suppressing functions in PCa cells.( 24 , 25 ) However, clinicopathological analyses for the expression of ERRβ and ERRγ would be required in human PCa. In the present study, we evaluated the expression of ERRβ and ERRγ in human prostate tissues by using immunohistochemistry, and investigated the correlation between the three ERRs and its clinical significance.
Materials and Methods
Tissue selection and patient characteristics. Formalin‐fixed, paraffin‐embedded sections were obtained from 107 consecutive patients who underwent radical prostatectomy for the treatment of prostate adenocarcinoma between 1987 and 2001. This study was approved by our institutional ethical committee. The age of the patients ranged from 52 to 76 years (mean, 66.8 ± 6.0 years); before treatment, the serum prostate‐specific antigen (PSA) level ranged from 2.2 to 136 ng/dL (mean, 16.9 ± 19.5 ng/dL). According to the evaluations of two trained pathologists, the cancerous lesions consisted of tumors with Gleason scores (GS) of 6 (n = 22), 7 (n = 42), 8 (n = 20), 9 (n = 22), and 10 (n = 1). The pathological primary tumor (pT) stages were 2a (n = 14), 2b (n = 13), 2c (n = 8), 3a (n = 38), 3b (n = 31), and 4 (n = 3). The pathological regional lymph node (pN) stages were 0 (n = 93) and 1 (n = 14). The prostate tissue and lymph node sections examined in this study comprised 107 cancerous and 92 benign foci. Thirty‐three patients (31%) were treated with surgery alone. The remaining 74 (69%) patients, who had pT3 cancer and/or experienced a postoperative PSA nadir of >0.2 ng/mL, received adjuvant androgen deprivation and/or radiation therapy. The patients were followed up postoperatively by their surgeons at 3‐month intervals for 5 years and yearly thereafter. The mean patient follow‐up period was 91 ± 40 months (range, 10–209). At the end of the follow‐up period, 77 patients (73%) were alive with no evidence of the disease, and 12 (11%) were alive with biochemical or clinical recurrence. Twelve patients (11%) died of PCa, and six (6%) died of other diseases during the follow‐up period.
Plasmid construction. Human ERRβ cDNA (ERRβ amino acids 2–500) and ERRγ (ERRγ amino acids 2–458) were N‐terminally Flag‐tagged and subcloned into pcDNA3 vector (pcDNA3‐FLAG‐hERRβ and pcDNA3‐FLAG‐hERRγ, respectively).
Cell culture. The 293T cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere. Transfections of hERRβ and hERRγ were performed using 5 μg of pcDNA3‐FLAG‐hERRβ and pcDNA3‐FLAG‐hERRγ and Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The cell extracts were analyzed after 48 h.
Antibodies. Anti‐FLAG M2 antibody was purchased from Sigma‐Aldrich (St. Louis, MO, USA). Anti‐ERRβ and anti‐ERRγ antibodies were generated from rabbit serum using a glutathione S‐transferase fusion protein with amino acids 41–90 and 2–51 of human ERRβ and ERRγ protein, respectively, as antigens. These antisera were then purified using an affinity column filled with GST protein‐coupled Affi‐Gel 10 (Bio‐Rad, Hercules, CA, USA) to remove the anti‐GST antibody.
The characterization of these antibodies was confirmed by western blot analysis in hERRβ‐ and hERRγ‐ transfected 293T cells.
Western blot analyses. Whole‐cell lysates were prepared using a sodium dodecyl sulfate (SDS) sample buffer from 293T cells transfected with pcDNA3‐FLAG‐hERRβ or pcDNA3‐FLAG‐hERRγ and resolved by 10% denaturing SDS–polyacrylamide gel electrophoresis. Blotted membranes were incubated with anti‐FLAG M2 antibody, anti‐ERRβ, or anti‐ERRγ (1:1500 dilution, both) followed by reaction with antimouse IgG or antirabbit IgG (Amersham Bioscience, Uppsala, Sweden). Bands were visualized with an enhanced chemiluminescence system (Amersham Bioscience).
Immunohistochemical analysis. Immunohistochemical analyses for ERRβ and ERRγ were performed with the streptavidin–biotin amplification method using an EnVision+ visualization kit (Dako, Carpinteria, CA, USA), as previously described.( 23 ) Tissue sections (6 μm) were deparaffinized, rehydrated through graded ethanol, and rinsed in phosphate‐buffered saline (PBS). In order to retrieve the antigens, the sections were heated in an autoclave at 120°C for 10 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate [pH 6.0]). The sections were blocked with endogenous peroxidase using 0.3% H2O2 and incubated in 10% bovine serum for 30 min. The primary antibody, a polyclonal antibody against ERRβ (1:200 dilution) or a polyclonal antibody against ERRγ (1:200 dilution), was applied and incubated at 4°C overnight. The sections were rinsed in PBS and incubated at room temperature with EnVision+ and antirabbit IgG for 1 h. The antigen–antibody complex was visualized with 3, 3′‐diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris‐HCl buffer [pH 7.6], and 0.006% H2O2).
Rabbit IgG was used instead of the primary antibodies as a negative control. Sections of the human kidney were immunoassayed as positive controls by using the primary antibodies in the same manner as described above.
Immunohistochemical assessment. The slides were evaluated for the proportion (0, none; 1, <1/100; 2, 1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5, >2/3) and staining intensity (0, none; 1, weak; 2, moderate; and 3, strong) of positively stained cells. The total immunoreactivity score (IR score) (0, 2–8) was determined as the sum of the proportion and intensity.( 26 ) Two investigators (T.F. and J.K.) evaluated the tissue sections independently. If the IR score differed between the two investigators, a third investigator (S.T.) evaluated the tissue sections, and the average IR score was considered. Since almost all benign foci showed IR scores of ≥5 for ERRβ and ERRγ, we defined an IR score of 4 as the cut‐off for high ERRβ and ERRγ in order to identify a potential correlation between ERRβ and ERRγ expressions in malignant epithelium and the clinicopathological characteristics of patients with PCa.
Statistical analyses. The correlation between the IR score, age, and pretreatment serum PSA level was evaluated by Mann–Whitney U‐test. The analyses between the IR score, pathological stage, and the GS was estimated by χ2‐square test. The comparison between IR score in the benign foci and that in cancerous lesions was analyzed by Wilcoxon’s singed‐rank sum test. Cancer‐specific survival curves were obtained using the Kaplan–Meier method and verified by the log‐rank (Mantel–Cox) test. We used JMP 8.0 software (SAS Institute, Cary, NC, USA) and regarded P‐values <0.05 as statistically significant.
Results
Western blot analysis. Using the polyclonal anti‐ERRβ and anti‐ERRγ antibodies, a 56‐kD and a 51‐kD band, which corresponded to the molecular weight of ERRβ and ERRγ, respectively, were detected in 293T‐pcDNA3‐FLAG‐ERRβ and 293T‐pcDNA3‐FLAG‐ERRγ, respectively (Fig. 1).
Figure 1.
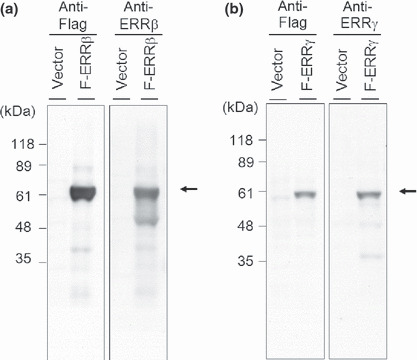
Western blot analysis showing the estrogen‐related receptor (ERR)‐β and ERRγ proteins in 293T cells transfected with pcDNA3‐FLAG‐ERRβ (F‐ERRβ) and pcDNA3‐FLAG‐ERRγ (F‐ERRγ). All the cell extracts were subjected to immunoblotting with the generated anti‐ERRβ (a), anti‐ERRγ (b), and anti‐FLAG antibodies. Anti‐ERRβ and ERRγ antibodies were detected bands, which corresponded to the molecular weight of ERRβ (56 kD) and ERRγ (51 kD), respectively (arrows).
Immunoreactivity of ERRβ and ERRγ in benign and malignant prostate tissue. Strong IRs of ERRβ and ERRγ were identified in the nuclei of human renal tissue (Fig. 2a,e). In prostatic tissue, staining was abundant in the benign epithelium (Fig. 2b,f) but less in cancerous cells (Fig. 2c,d,g,h). IRs of ERRβ and ERRγ in the human prostate are summarized in Figure 3(a and b). Positive IR for ERRβ and ERRγ were observed in 84 of 92 (91%) and 91 of 92 cases (98%) of benign epithelium, respectively, and in 72 of 107 (67%) and 88 of 107 (82%) cases of cancer, respectively. The benign foci showed significantly higher ERRβ and ERRγ IR scores than the cancerous lesions (P < 0.0001, for both).
Figure 2.
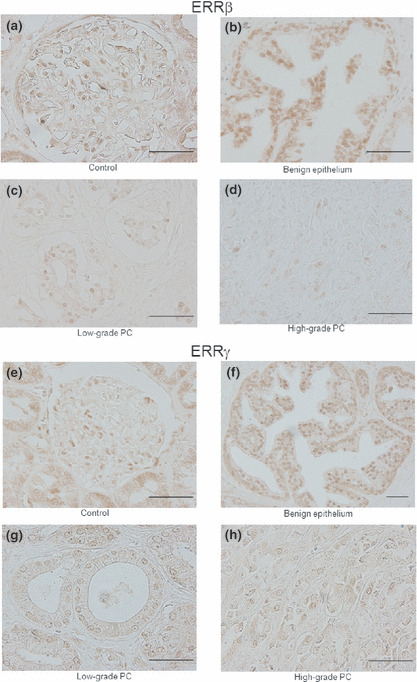
Immunohistochemical staining for estrogen‐related receptor (ERR)‐β (a–d) and ERRγ (e–h) in human renal and prostatic tissues. Positive staining for ERRβ and ERRγ was observed in the nuclei of renal glomerular and epithelial cells, respectively (a,e). Immunoreactivities (IRs) for anti‐ERRβ and ERRγ antibodies were abundant in benign prostatic epithelium (IR score, 7; immunointensity, strong) (b,f). Decreased ERRβ and ERRγ IRs were observed in low‐grade prostate cancer (PCa) (Gleason score [GS], 6) (IR score, 5; immunointensity, moderate) (c,g), and weak expression of ERRβ and ERRγ was also observed in high‐grade PCa (GS 9) (IR score, 4; immunointensity, moderate) (d,h). Original magnification, ×400; Scale bar = 50 μm.
Figure 3.
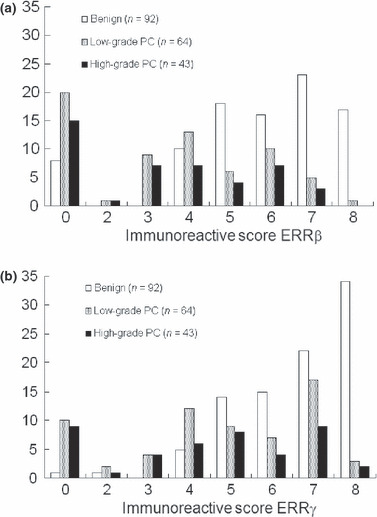
Immunoassaying of estrogen‐related receptor (ERR)‐β (a) and ERRγ (b) in the human prostate. We evaluated 107 cancerous and 92 benign foci. ERRβ and ERRγ immunoreactivities (IRs) were positive in 84 of 92 (91%) and 89 of 92 cases (95%) of benign epithelium, respectively, and in 71 of 106 (67%) and 85 of 106 (80%) cancer cases, respectively. Over 80% and 92% cases of benign foci showed IR scores of ≥5 for ERRβ and ERRγ, respectively, whereas IR scores of ≥5 were obtained in the case of 35% and 55% of patients with cancerous lesions, respectively.
The immunointensities of ERRβ in the atrophic glands and high‐grade PIN were significantly lower than that of the hyperplastic lesions (P = 0.0047 and 0.0067, respectively). In addition, the immunointensities of ERRγ in the atrophic glands and high‐grade PIN tended to be low compared with that of the hyper plastic lesions (P = 0.083, both). We described these results of IR scores in hyperplastic lesions as those in the benign foci.
Correlation of ERRβ and ERRγ expression with the clinicopathological characteristics of patients with PCa. Almost all the benign foci had IR scores of ≥5 for ERRβ and ERRγ, and we defined an IR score of 4 as the cut‐off value above which the foci were classified as having high IR. No significant correlation was found between ERRβ and ERRγ expression and clinicopathological characteristics such as age, serum PSA level, and pathological stage (Table 1).
Table 1.
Relationship between immunoreactivity of ERRb, ERRg, and clinicopathological findings in PCa (n = 107)
| Clinical findings | ERRβ immunoreactive score† | ERRγ immunoreactive score† | |||||
|---|---|---|---|---|---|---|---|
| Low (n = 71) | High (n = 36) | P‐values | Low (n = 47) | High (n = 60) | P‐values | ||
| Serum PSA (ng/mL) | 14.7 ± 13.9 | 19.7 ± 26.2 | 0.2 | 13.0 ± 13.7 | 18.9 ± 21.9 | 0.11 | |
| Gleason score | ≤7 | 42 | 22 | 0.99 | 27 | 37 | 0.68 |
| ≥8 | 29 | 14 | 20 | 23 | |||
| Pathological T stage | ≤3a | 48 | 24 | 0.89 | 27 | 45 | 0.08 |
| ≥3b | 23 | 12 | 20 | 15 | |||
| Pathological N stage | 0 | 61 | 32 | 0.99 | 39 | 54 | 0.44 |
| 1 | 10 | 4 | 8 | 6 | |||
†ERRb and ERRg immunoreactive scores of 0–4 and 5–8 were defined as low and high immunoreactivity, respectively. ERR, estrogen‐related receptor; PCa, prostate cancer; PSA, prostate‐specific antigen.
Figure 4 shows a cancer‐specific survival curve prepared using the Kaplan–Meier method. Twelve (11%) patients died because of PCa during the follow‐up period. No significant relation was observed between ERRβ expression and the cancer‐specific survival rate (P = 0.29) (Fig. 4a). However, the patients with low ERRγ IR (IR score ≤4) tended to show poor cancer‐specific survival (P = 0.07) (Fig. 4b). Then, on the basis of these results and those of our previous study on ERRα, we evaluated ERRγ expression as a prognostic predictor.( 23 ) The patients were divided into three groups on the basis of the IR scores: those with high ERRα but low ERRγ IR, those with low ERRα but high ERRγ IR, and those who do not fall under either of the above groups. The patients in the first group showed a significantly poorer cancer‐specific survival rate than those of the second group (P = 0.0003, Fig. 4c).
Figure 4.
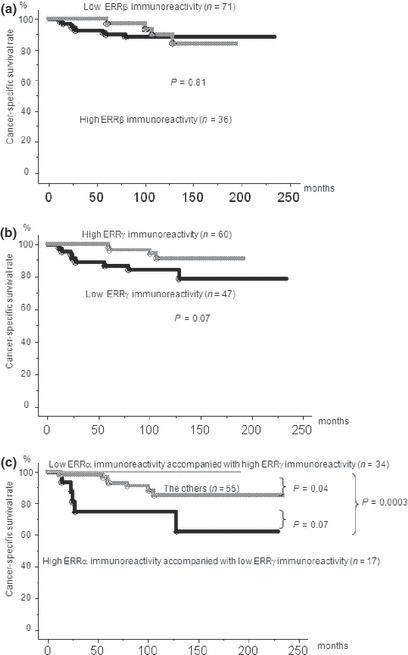
Cancer‐specific survival in patients with prostate cancer according to the immunoreactivity (IR) of estrogen‐related receptor (ERR)‐β and ERRγ (n = 107). No significant difference was observed in ERRβ IR (a), whereas patients with low ERRγ IR tended to show poor cancer‐specific survival (b). The patients with high ERRα IR and low ERRγ IRs (IR score, ≤4) showed significantly poorer cancer‐specific survival than patients with low ERRα IR and high ERRγ IRs and the other patients (P = 0.0003 and 0.07, respectively) (c).
Table 2 shows the results of univariate and multivariate proportional analyses of the cancer‐specific survival rates associated with ERRα and ERRγ IR and clinicopathological characteristics of the patients. GS, pathological T and N stages, and ERR IR were found to be significant prognostic predictors in the univariate analysis (P = 0.0001, 0.0002, 0.0094, and 0.0006, respectively). Multivariate analyses showed that among the five parameters evaluated, two ERRs (ERRα and ERRγ) were significantly poor prognostic predictors (P = 0.0015; hazard ratio, 15.2; 95% index, 1.65–186).
Table 2.
Univariate and multivariate proportional hazard analyses of cancer‐specific survival (n = 106)
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% index | P‐values | Hazard ratio | 95% index | P‐values | |
| Serum PSA (ng/dL) (≥10 vs <10) | 1.02 | 0.32–3.26 | 0.98 | 1.39 | 0.39–5.01 | 0.6 |
| Gleason score (≥8 vs≤7) | 17.2 | 3.34–314 | 0.0001 | 7.63 | 0.94–168 | 0.06 |
| Pathological T stage (≥3b vs≤3a) | 11.4 | 2.99–74.3 | 0.0002 | 2.38 | 0.42–20.9 | 0.34 |
| Pathological N stage (1 vs 0) | 5.29 | 1.56–16.6 | 0.0094 | 1.01 | 0.26–3.69 | 0.98 |
| ERRs (high ERRα and low ERRγvs low ERRα and high ERRγ or others) | 23.2 | 3.73–173 | 0.0006 | 15.2 | 1.65–186 | 0.015 |
We divided the data into three groups on the basis of immunoreactivity: high ERRα and low ERRγ, low ERRα and high ERR and other immunoreactivity groups. ERR, estrogen‐related receotor; PSA, prostate‐specific antigen.
Discussion
Androgen deprivation and estrogen therapies have been used for the treatment of PCa.( 4 , 27 , 28 ) The growth‐inhibitory effects of endocrine therapies are associated with the status of steroid receptors, such as androgen receptors (ARs) and ERs.( 27 , 28 ) In the 1980s, the emergence of techniques to clone orphan nuclear receptors prompted the investigation of the physiological functions of these receptors in target organs.( 14 , 15 ) Among the orphan nuclear receptors, ERRα, ERRβ, and ERRγ have functional links with the activities of the ERs. The primary function of ERRs seems to be the activation of fatty acid oxidation and mitochondrial biogenesis in tissues that have high energy requirements, for example cardiac and skeletal muscle.( 19 ) ERRs may also be involved in the transcriptional response to hypoxia and the growth of solid tumors. The adaptive response to hypoxia is mainly controlled by a transcriptional factor referred to as the hypoxia‐inducible factor (HIF). HIF regulates gene networks involved in glucose uptake and metabolism and tumor angiogenesis. The ERRs can directly interact with HIF1α, ‐2α, and ‐1βin vitro and in vivo to enhance HIF‐mediated gene transcription, while ERR inhibition attenuates hypoxic response.( 29 ) ERRα expression has also been associated with a negative outcome in breast and ovarian cancers.( 22 , 30 ) In the case of breast cancer, ERRα is a potential biomarker of unfavorable clinical outcome and hormonal insensitivity.( 22 ) In contrast, ERRγ overexpression is associated with a hormonally responsive (ER‐positive and progesterone receptor‐positive) status.( 22 ) Thus, in the case of breast cancer, ERRγ is a potential biomarker of a favorable clinical outcome and hormone sensitivity.( 22 ) In a previous study, we reported increased ERRα expression in PCa and showed its clinical significance.( 23 ) However, little is known about the distribution of ERRβ and ERRγ in human prostate tissue. In the present study, we found that the expression of ERRβ and ERRγ was lower in PCa tissue than in benign epithelium. Low ERRγ expression tended to correlate with poor prognosis in PCa, whereas ERRβ expression did not correlate with the clinical outcome. Recent studies have shown that the expression of ERRβ and ERRγ was lower in PCa lesions than in benign foci.( 24 , 25 ) Functional analyses using cell lines with stable expression of ERRβ and ERRγ and their agonists revealed that ERRβ and ERRγ perform antiproliferative or tumor‐suppressing functions in PCa.( 24 , 25 ) Thus, these findings suggest that ERRβ and ERRγ may regulate the proliferation of prostatic epithelial cells.
Interestingly, in the present study, combined analyses of the expression of two ERRs – ERRα and ERRγ– enhanced the clinical significance of these receptors in PCa as compared with the analysis of ERRα expression alone (hazard ratio, 5.24; 95% index, 1.11–25.7; P = 0.0367).( 23 ) We attempted to clarify how these receptors contribute to the development of PCa. All ERRs share the characteristic structural features of nuclear receptors, including a nonconserved amino terminal domain (NTD), a DNA‐binding domain (DBD), and a functional ligand‐binding domain (LBD), which embeds docking sites for nuclear receptor co‐regulators.( 18 ) NTD is a site for posttranslational modifications. The DBD contains two zinc finger domains that recognize the consensus estrogen‐related receptor responsive element (ERRE), and the LBD possesses a functional ligand‐binding pocket and an AF‐2 that interacts with co‐activator PGC‐1a and co‐repressor receptor‐interacting protein (RIP) 140.( 18 ) The functional mechanism of ERRs is complicated. First, ERRs bind to extended half‐sites with consensus sequence TCAAGGTA, referred to as an ERRE.( 14 , 18 ) However, like the ERs, the ERRs recognize the estrogen responsive element (ERE), which suggests that these receptors may control overlapping regulatory pathways.( 14 , 18 ) Second, ERRs have the potential to interact with co‐activators in a ligand‐independent manner.( 18 ) Third, there are more than 200 nuclear receptor co‐activator and co‐repressor proteins, such as PPARγ, steroid receptor co‐activator (SRC), and RIP140.( 18 ) Thus, it is currently unknown whether these splice variants that coexist in tissues play specific roles. In this study, we determined the correlation between the three ERRs. A weak correlation was found between ERRβ and ERRγ (index of correlation, 0.225; P = 0.022), whereas no correlation was found between ERRα and the other ERRs. Thus, in addition to ERα, ERβ, and ERβcx (splice variant of ERβ), the three ERRs studied here may also participate in regulating the development of PCa.( 8 , 23 )Further investigation is required to identify the entire mechanism that regulates the progression of PCa.
In addition to radical prostatectomy and radiotherapy, endocrine therapies play an important role in the treatment of PCa. The growth of PCa is androgen dependent; therefore, androgen‐deprivation therapy (ADT) is the standard treatment for PCa. Most PCs become hormone‐refractory after several years of therapy, which is a serious drawback for treatment.( 31 ) ARs were first evaluated as predictors of response to hormonal therapy.( 32 ) A recent study has shown that high levels of both AR mRNA and protein are required for the progression of PCa to the castration‐resistant stage.( 33 ) Donovan et al. have recently developed a model to predict both clinical failure and ADT sensitivity by using data obtained from 758 patients.( 34 ) These data included the AR levels, dominant GS at prostatectomy, lymph node involvement, and the quantitative characteristics obtained by hematoxylin–eosin staining of the prostate tissue.( 34 ) This model also suggested that high AR levels predict a PSA‐relapse rate after ADT. Moreover, the results of docetaxel‐based chemotherapy, such as TA327 and SWOG9916, show that chemotherapy is indicated for metastatic androgen‐independent PCa (AIPC).( 35 , 36 ) On the basis of the currently available data, multiple hormonal therapies can be administered to patients before the initiation of chemotherapy.( 37 ) Although tools such as Partin tables and Kattan postoperative nomograms are useful for predicting the prognosis of individual patients,( 38 , 39 ) few personalized tools are available for predicting the sensitivity of therapies (such as ADT, SERMs, radiation therapy, and chemotherapy) for recurrent PCa with or without metastasis. The availability of such personalized tools would enable us to adjust the therapeutic regimens for patients with recurrent PCa, according to the status of various receptors, including AR, ERα, ERβ, and ERRs.
In conclusion, we have shown the differential expression of ERRβ and ERRγ in prostate tissue and their clinical significance in human PCa. The combined analysis of ERRα and ERRγ expressions could be a useful prognostic indicator of PCa.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by Cell Innovation, the Genome Network Project, DECODE from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Japan Society for the Promotion of Science; Grants‐in‐Aid from the Ministry of Health, Labor, and Welfare of Japan; and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO).
References
- 1. Bonkhoff H, Bergs R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol 2009; 55: 533–42. [DOI] [PubMed] [Google Scholar]
- 2. Raghow S, Hooshdaran MZ, Katiyar S, Steiner MS. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res 2002; 62: 1370–6. [PubMed] [Google Scholar]
- 3. Shazer RL, Jain A, Galkin AV et al. Raloxifene, an oestrogen‐receptor‐β targeted therapy, inhibits androgen‐independent prostate cancer growth: results from preclinical studies and a pilot phase II clinical trial. BJU Int 2006; 97: 691–7. [DOI] [PubMed] [Google Scholar]
- 4. Ho SM. Estrogens and anti‐estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem 2004; 91: 491–503. [DOI] [PubMed] [Google Scholar]
- 5. Kirschenbaum A, Ren M, Erenburg I, Schacter B, Levine AC. Estrogen receptor messenger RNA expression in human benign prostatic hyperplasia: detection, localization, and modulation with a long‐acting gonadotropin‐releasing hormone agonist. J Androl 1994; 15: 528–33. [PubMed] [Google Scholar]
- 6. Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER) ‐α and ER‐β in normal and malignant prostate epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res 2000; 60: 3175–82. [PubMed] [Google Scholar]
- 7. Ehara H, Koji T, Deguchi T et al. Expression of estrogen receptor in diseased human prostate assessed by non‐radioactive in situ hybridization and immunohistochemistry. Prostate 1995; 27: 304–13. [DOI] [PubMed] [Google Scholar]
- 8. Fujimura T, Takahashi S, Urano T et al. Differential expression of estrogen receptor β (ERβ) and its C‐terminal truncated splice variant ERβcx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun 2001; 289: 692–9. [DOI] [PubMed] [Google Scholar]
- 9. Horvath LG, Henshall SM, Lee CS et al. Frequent loss of estrogen receptor‐beta expression in prostate cancer. Cancer Res 2001; 61: 5331–5. [PubMed] [Google Scholar]
- 10. Pasquali D, Rossi V, Esposito D et al. Loss of estrogen receptor β expression in malignant human prostate cells in primary cultures and in prostate cancer tissues. J Clin Endocrinol Metab 2001; 86: 2051–5. [DOI] [PubMed] [Google Scholar]
- 11. Latil A, Bieche I, Vidaud D et al. Evaluation of androgen, estrogen (ERα and ERβ), and progesterone receptor expression in human prostate cancer by real‐time quantitative reverse transcription‐polymerase chain reaction assays. Cancer Res 2001; 61: 1919–26. [PubMed] [Google Scholar]
- 12. Giguere V, Yang NA, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature 1988; 331: 91–4. [DOI] [PubMed] [Google Scholar]
- 13. Horard B, Vanacker JM. Estrogen receptor‐related receptors: orphan receptors desperately seeking a ligand. J Mol Endocr 2003; 31: 349–57. [DOI] [PubMed] [Google Scholar]
- 14. Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab 2002; 13: 220–5. [DOI] [PubMed] [Google Scholar]
- 15. Yang N, Shigeta H, Shi H, Teng CT. Estrogen‐related receptor, hERR1, modulates estrogen receptor‐mediated response of human lactoferrin gene promoter. J Bio Chem 1996; 271: 5795–804. [DOI] [PubMed] [Google Scholar]
- 16. Eudy JD, Yao S, Weston MD et al. Isolation of a gene encoding a novel member of the nuclear receptors‐per family from the critical region of Usher syndrome type IIa at 1q41. Genomics 1998; 50: 382–4. [DOI] [PubMed] [Google Scholar]
- 17. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchial transcriptional network. Cell 2006; 126: 789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giguere V. Transcriptional control of energy homeostasis by the estrogen‐related receptors. Endocr Rev 2008; 29: 677–96. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki T, Miki Y, Moriya T et al. Estrogen‐related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res 2004; 64: 4670–6. [DOI] [PubMed] [Google Scholar]
- 20. Kraus RJ, Ariazi EA, Farrell ML, Mertz JE. Estrogen‐related receptor α actively antagonizes estrogen receptor‐regulated transcription in MCF‐7 mammary cells. J Bio Chem 2002; 277: 24826–34. [DOI] [PubMed] [Google Scholar]
- 21. Cavallini A, Notarnicola M, Giannini R et al. Oestrogen receptor‐related receptor alpha (ERRα) and oestrogen receptors (ERα and ERβ) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer 2005; 41: 1487–94. [DOI] [PubMed] [Google Scholar]
- 22. Ariazi EA, Clark GM, Mertz JE. Estrogen‐related receptor α and estrogen‐related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res 2002; 62: 6510–8. [PubMed] [Google Scholar]
- 23. Fujimura T, Takahashi S, Urano T et al. Increased expression of estrogen‐related receptor alpha (ERR alpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer 2007; 120: 2325–30. [DOI] [PubMed] [Google Scholar]
- 24. Yu S, Wang X, Ng CF, Chen S, Chan FL. ERRγ suppresses cell proliferation and tumor growth of androgen‐sensitive and androgen‐insensitive prostate cancer cells and its implication as a therapeutic target for prostate cancer. Cancer Res 2007; 67: 4904–14. [DOI] [PubMed] [Google Scholar]
- 25. Yu S, Wong YC, Ling MT, Ng CF, Chen S, Chan FL. Orphan nuclear receptor estrogen‐related receptor‐β suppresses in vitro and in vivo growth of prostate cancer cells via p21WAF1/CIP1 induction and as a potential therapeutic target in prostate cancer. Oncogene 2008; 27: 3313–28. [DOI] [PubMed] [Google Scholar]
- 26. Allred DC, Clark GM, Elledge R et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node‐negative breast cancer. J Natl Cancer Inst 1993; 85: 200–6. [DOI] [PubMed] [Google Scholar]
- 27. Culig Z, Steiner H, Bartsch G, Hobisch A. Mechanism of endocrine therapy‐responsive and – unresponsive prostate tumors. Endocr Relat Cancer 2005; 12: 229–44. [DOI] [PubMed] [Google Scholar]
- 28. Harkonen P, Makeda SI. Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol 2004; 92: 297–305. [DOI] [PubMed] [Google Scholar]
- 29. Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen‐related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci USA 2008; 105: 7821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujimoto J, Alam SM, Jahan I, Sato E, Sakaguchi H, Tamaya T. Clinical implication of estrogen‐related receptor (ERR) expression in ovarian cancers. J Steroid Biochem Mol Biol 2007; 104: 301–4. [DOI] [PubMed] [Google Scholar]
- 31. Huggins C, Hodges CV. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941; 1: 293–7. [Google Scholar]
- 32. Sadi MV, Barrack ER. Androgen receptors and growth fraction in metastatic prostate cancer as predictors of time to tumor progression after hormonal therapy. Cancer Surv 1991; 11: 195–215. [PubMed] [Google Scholar]
- 33. Chen CD, Welsbie DS, Tranc C et al. Molecular determinants of resistance to anti‐androgen therapy. Nat Med 2004; 10: 33–9. [DOI] [PubMed] [Google Scholar]
- 34. Donovan MJ, Hamann S, Clayton M et al. Systems pathology approach for the prediction of prostate cancer progression after radical prostatectomy. J Clin Oncol 2008; 26: 3923–9. [DOI] [PubMed] [Google Scholar]
- 35. Petrylak DP, Tangen CM, Hussain MH et al. Docetaxel and estramusutine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20. [DOI] [PubMed] [Google Scholar]
- 36. Tannock IF, De Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. [DOI] [PubMed] [Google Scholar]
- 37. Schnadig ID, Beer TM. Optimal timing of chemotherapy in androgen independent prostate cancer. Urol Oncol 2009; 27: 97–100. [DOI] [PubMed] [Google Scholar]
- 38. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281: 1591–7. [DOI] [PubMed] [Google Scholar]
- 39. Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol 1999; 17: 1499–507. [DOI] [PubMed] [Google Scholar]


