Abstract
Mitomycin C (MMC), a chemotherapeutic agent in breast cancer treatments, inhibits tumor growth through DNA cross‐linking and breaking, but it has severe side effects. Here we examined whether and how curcumin reduced the side effects of MMC. We found that combination treatment with MMC and curcumin reduced tumor weight by 70% and 36% compared with saline and curcumin‐treated groups, respectively. The combination treatment reduced weight loss and improved kidney function and bone marrow suppression compared with MMC treatment alone. Moreover, the combination treatment inhibited glucose regulatory protein (GRP58)‐mediated DNA cross‐linking. The combination treatment inhibited GRP58 through the ERK/p38 MAPK pathway. In conclusion, the current study provided evidence that MMC and curcumin combination treatment reduced MMC side effects by inhibiting GRP58‐mediated DNA cross‐linking through the ERK/p38 MAPK pathway. (Cancer Sci 2009)
Breast cancer accounts for about 30% of all cancers among women and is becoming the most prevalent type of cancer among women in the Western world.( 1 ) Breast cancer incidence is currently low in China. However, reproductive and lifestyle risk factors for breast cancer among Chinese women are rapidly increasing. Currently, China is on the cusp of a breast cancer epidemic.( 2 ) Although early stage breast cancer is highly treatable, the incidence of this disease has increased and mortality rates are also altered.( 1 )
Chemotherapy as well as surgery and radiation are available for treating the primary tumor in breast cancer. Mitomycin C (MMC), a broad‐spectrum chemotherapeutic, is able to inhibit tumor growth through DNA cross‐linking and breaking.( 3 ) It has been reported that glucose regulatory protein (GRP58) mediates the activation of MMC, leading to DNA cross‐linking. In breast cancer treatments, MMC is a third‐line chemotherapeutic agent.( 4 ) MMC easily induces drug resistance,( 5 ) and its usage is often accompanied with severe side effects such as renal toxicity.( 6 ) Therefore MMC offers no firm expectation for curing breast cancer.
Previous research has addressed the chemotherapeutic potential of curcumin (diferuloylmethane),( 7 ) which is a relatively nontoxic, plant‐derived polyphenol. Moreover, it has been reported that curcumin normalizes the elevation of tumor‐related enzymes when combined with cisplatin. Therefore, curcumin is a potential adjuvant for treating tumors.( 8 )
Glucose regulatory protein GRP58, also known as ERp57 or ERp60, is localized in the endoplasmic reticulum, cytosol, and nucleus.( 9 , 10 , 11 ) In the endoplasmic reticulum, GRP58 specifically interacts with glycoproteins such as calnexin and calreticulin, playing an important role as a molecular chaperone of glycoprotein biosynthesis and assembly of newly synthesized MHC class I molecules, a process that also involves the chaperones calnexin and calreticulin.( 9 ) GRP58 is also implicated as a component of a subset of nuclear matrix proteins that are responsible for DNA attachment to the nuclear matrix and for the formation of DNA loops due to GRP58 binding to DNA.( 10 , 11 )
Glucose regulatory protein (GRP58) is markedly expressed in human breast, lung, uterus, and stomach tumors compared with normal tissues.( 12 ) Previous studies have shown that MMC‐induced DNA cross‐linking and cytotoxicity is mediated by cytosolic GRP58.( 13 , 14 ) However, it remains unknown whether curcumin is involved in GRP58‐mediated DNA cross‐linking.
Mitogen‐activated protein kinase (MAPK) pathways are implicated in the response to chemotherapeutic drugs.( 15 ) MAPK pathway signaling modules consist of a three‐tiered kinase core where a MAPKKK activates a MAPKK that activates a MAPK.( 16 ) The primordial MAPK cascades are ubiquitously expressed and respond to a wide variety of external cues and drugs. MAPK p38 had been implicated in the regulation of various cellular processes.( 17 ) Previous reports have shown that MEK activation by mitogenic stimuli contributes to cell differentiation, proliferation, and survival.( 18 ) The effect of extracellular signal‐regulated protein kinase (ERK) and MAPK has been studied in breast cancer cells. Some responsiveness is regulated by ERK and MAPK in breast cancer cells. However, whether or not GRP58 is involved in ERK/p38 MAPK signal transduction has not been investigated.
In the present study, we demonstrated that the combination of curcumin and MMC inhibited the growth of tumors. We further showed that curcumin reduced the side effects of MMC in MCF‐7 breast cancer xenografts. We showed that curcumin inhibited GRP58‐mediated DNA cross‐linking, which was involved in ERK/p38 MAPK signaling in MCF‐7 cells.
Materials and Methods
Materials. MMC was purchased from ICN Company (Costa Mesa, CA, USA), dissolved in normal sodium as a 1‐mm/L stock solution and stored at 4°C away from light. Curcumin with a purity of more than 98% was obtained from National Institute for the Control of Pharmaceutical and Biological Products in Beijing, China, dissolved in dimethyl sulfoxide (DMSO) as a 40‐mm/L solution for treatment of cells, and dissolved in a physiological saline of 1% DMSO and 10% Tween‐80 for animal research. PD98059 and SB203580 were from Biomol (Philadelphia, PA, USA). The antibody against GRP58 was obtained from StressGen Biotechnologies Corp (Victoria, BC, Canada).
Cell culture. Human breast cancer MCF‐7 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in RPMI‐1640 medium (Gibco, San Francisco, CA, USA) supplemented with 10% heat‐inactivated (56°C, 30 min) fetal calf serum (PAA, A‐4061; Pasching, KA, Austria), 0.01 mg/mL insulin (Sigma, St. Louis, MO, USA), 2 mm/L glutamine (Gibco, San Francisco, CA, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL). The cell culture was maintained at 37°C with 5% CO2 in a humidified atmosphere.
Assay for cell growth inhibition. The inhibition of cell growth was determined by MTT assay. MCF‐7 cells were seeded into 96‐well culture plates (104 cells/well). After overnight incubation, various concentrations of MMC, curcumin, or MMC plus curcumin were added to the plates. DMSO was adjusted to the same final concentration of 0.01%. Following incubation, cell growth was measured at different time‐points by the addition of 20 μL MTT at 37°C for 4 h. Then DMSO (150 μL) was added to dissolve the formazan crystals. Absorbance was measured at 490 nm with an ELISA plate reader (BioTek, Winooski, VT, USA). The percentage of inhibition was calculated as follows:
Tumor xenograft modeling and treatment. Female nu/nu athymic mice (7 weeks of age) were obtained from Academia Sinica (Shanghai, China). The mice (five per cage) were housed in cages equipped with air filter lids and maintained under pathogen‐limiting conditions. MCF‐7 cells (1 × 107/mL) were inoculated into the mammary fat pad of the mice. Before inoculation, 17β‐estradiol was intraperitoneally injected. Once palpable tumors developed (about 2 weeks), the mice were randomized to six groups (n = 8). Treatment groups were divided to the curcumin (100 mg/kg), MMC (2 mg/kg), and combination curcumin (100 mg/kg) with MMC (2 mg/kg) groups. All drugs were received by intraperitoneal injection. Untreated groups were divided into the normal group, normal with 17β‐estradiol injection group, and model group injected with physiological saline of 1% DMSO and 10% Tween‐80 as a sham control. After 4 weeks of treatment, blood was collected through the eyes for serum determinations. Then animals were killed by cervical dislocation. The tumors were immediately removed, freed from connective and adipose tissue, and weighed. Bone marrow was extracted from the right femur of the animals. The animal protocols were preapproved by the ethical committee of our institution.
Colorimetric test. Serum creatinine (Cr), blood urea nitrogen (BUN), glutamic oxalacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) were measured using the colorimeter testing kit (Kangcheng, Nanjing, China). According to the manufacturer’s instructions, serum samples were measured at 510 nm, 520 nm, 505 nm, and 505 nm, respectively.
Bone marrow smears. The bone marrow extracted from the right femur of the animals was smeared onto slides and stained with May–Grünwald Giemsa. The fixed smears were stained with May–Grünwald for 5 min. The smears were then directly put into Giemsa without washing for 30 min. The smears were then washed in distilled water and air‐dried. The stained blood cells were observed under a light microscope. Myelosuppression was evaluated as + (scored as 1 point). More plus signs indicated higher degrees of bone marrow hyperplasia.
Transfection. GRP58 siRNA was from Ambion (Austin, TX, USA). The GRP58 siRNA targeting sequence was: sense 5′‐GGAAUAGUCCCAUUAGCAATT‐3′. Transfection of GRP58 siRNA was performed by following the instructions in the kit (siPORT NeoFX kit; Ambion). Cells were diluted in normal growth medium to 1 × 105 cells/mL. Five μL siPORT NeoFX was diluted in OPTI‐MEM I medium (serum free) to 100 μL and incubated for 10 min at room temperature. GRP58 siRNA (2 μm) was then diluted with OPTI‐MEM I medium to a final concentration of 10 nm. The siRNA and the diluted siPORT NeoFX were then mixed, incubated for 10 min at room temperature, and dispensed into a culture plate. Cells were gently resuspended and dispensed into six wells containing transfection complexes. The transfected cells were incubated in normal cell culture conditions.
Western blot analyses. Briefly, cell pellets were resuspended in a lysis buffer containing 2 m sodium chloride, 10% NP‐40, 10% SDS, 1 m/L Tris‐Cl, 1 g/L phenyl‐methylsulfonyl fluoride (PMSF), 0.1 g/L aprotinin, and 0.01 g/L leupeptin, and incubated at 4°C for 30 min. After 13 400 g centrifugation for 5 min at 4°C, the protein content of the supernatant was determined using protein assay reagent (Bio‐Rad, Hercules, CA, USA). Proteins were separated on a 10% SDS‐PAGE gel and blotted onto PVDF membrane. The membrane was blocked with bovine serum albumin (BSA) for about 1 h at room temperature. Protein expressions were detected using primary antibody (GRP58, 1:1000) and secondary antibody (1:800) conjugated with horseradish peroxidase and ECL reagents (Pharmacia, Buckinghamshire, UK). Quantitative analyses of western blots were carried out using Alpha Ease FC (FluorChem FC2; Medford, NJ, USA) software. The density ratio of proteins to GAPDH as spot density was calculated using the analysis tools.
Mitomycin C (MMC)‐induced DNA cross‐linking. MMC‐induced DNA cross‐linking assays were performed as described.( 19 , 20 ) Briefly, two strands of 23 base pairs of oligonucleotides containing the MMC binding site were synthesized and used for the cross‐linking experiments. The nucleotide sequence of the 23 base pair oligonucleotide was: sense strand 5′‐CTA CAT CGT GTC ATG CAC AGG AT‐3′ and anti‐sense strand 5′‐A GAT CCT GTG CAT GAC ACG ATG T‐3′. The complementary strands were mixed in equal amounts and annealed by heating to 70°C for 15 min and slowly cooling to room temperature. The 3′‐end of the top strand was selectively labeled with DNA polymerase I large (Klenow) fragment (Promega, Madison, WI, USA) and [α‐32P] dCTP (NEN, Boston, MA, USA). The labeled oligonucleotides were purified on a 15% non‐denaturing polyacrylamide gel and concentrated by ethanol precipitation. 32P‐labeled oligonucleotides were used to detect MMC‐induced DNA cross‐linking by incubating with 0.5 mg of cytosolic proteins, 100 mm phosphate buffer (pH 5.8), 1 mm NADH, 150 μm mitomycin C, 5 μm FAD, 0.01% Tween 20, and 0.18 mg BSA. Reactions were incubated for 1 h at 37°C and terminated by the addition of ethanol and1.5‐m ammonium acetate. The samples were frozen at −70°C and DNA was pelleted by centrifugation at 17 200 g for 30 min at 4°C. The supernatant was removed and the precipitated oligonucleotides were centrifuged in Speed‐Vac until dry. The precipitated oligonucleotides were resuspended in DNA sequencing dye‐containing formamide and denatured by heating at 95°C for 15 min, and then rapidly cooled on ice. The samples were analyzed on a 15% denaturing polyacrylamide gel containing 8 m urea, and cross‐linked and unmodified (free) oligonucleotides were detected by autoradiography performed on Kodak Bio‐max MS films (Kodak, Rochester, WY, USA) using intensifying screens at −70°C for 18 h.
Statistical analyses. All data were expressed as mean ± SD. Comparisons among groups were performed by Student’s t‐test and one‐way anova analysis. The signification level was set at P < 0.05.
Results
Curcumin and MMC combination treatment inhibited MCF‐7 cell growth. MCF‐7 cells were exposed to MMC (2.5 μm/L), curcumin, or a combination of MMC and a range of concentrations of curcumin. Cell viability was examined by MTT assay. Figure 1(A) shows that curcumin inhibited cell growth in a dose‐dependent manner when cells were treated for 48 h. MMC with 5 or 10 μm/L of curcumin did not increase cell growth inhibition compared with MMC alone. Addition of curcumin at 20, 40, and 80 μm/L significantly inhibited cell growth compared with MMC alone. Figure 1(B) shows that MMC in combination with curcumin at 20, 40, and 80 μm/L inhibited cell growth compared with MMC alone by about 10%, 20%, and 32% at 24 h; 10%, 32%, and 45% at 48 h; and 3%, 13%, and 24% at 72 h, respectively. These data showed that curcumin at 5 or 10 μm/L reduced the antiproliferating effect of MMC but at higher concentrations, curcumin enhanced the inhibitory effect of MMC.
Figure 1.
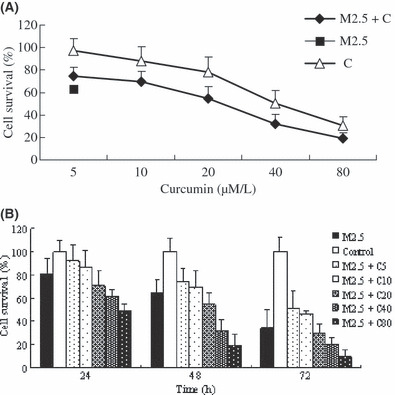
Growth inhibitory effect of mitomycin (MMC), curcumin and their combination at different concentrations and time‐points. The inhibition ratio of drugs was observed by MTT assay. (A) MCF‐7 cells were treated with curcumin 5, 10, 20, 40, and 80 μm/L and MMC 2.5 μm/L and their combination at 48 h. (B) MCF‐7 cells were treated with MMC 2.5 μm/L plus curcumin 5, 10, 20, 40, and 80 μm/L separately or MMC 2.5 μm/L alone at 24, 48, and 72 h. Values are means ± SD from three independent experiments.
Curcumin and MMC combination treatment inhibited tumor growth and reduced the side effects of MMC in MCF‐7 breast cancer xenografts. We next used MCF‐7 breast cancer xenografts to examine the tumor growth–inhibitory effect. Animals were given saline, MMC (2 mg/kg i.p.), curcumin (100 mg/kg i.p.), or MMC and curcumin for 4 weeks. No deaths were observed between curcumin‐ or combination‐treated animals, but half of the MMC‐treated animals (4/8) died. No tumors were found in the remaining animals with MMC treatment. However, the combination treatment reduced tumor weight by 70% and 36% compared with the saline‐ and curcumin‐treated groups, respectively. A significant difference was observed not only between the combination treatment and saline treatment, but also between the combination treatment and curcumin alone (P < 0.01) (Fig. 2A).
Figure 2.
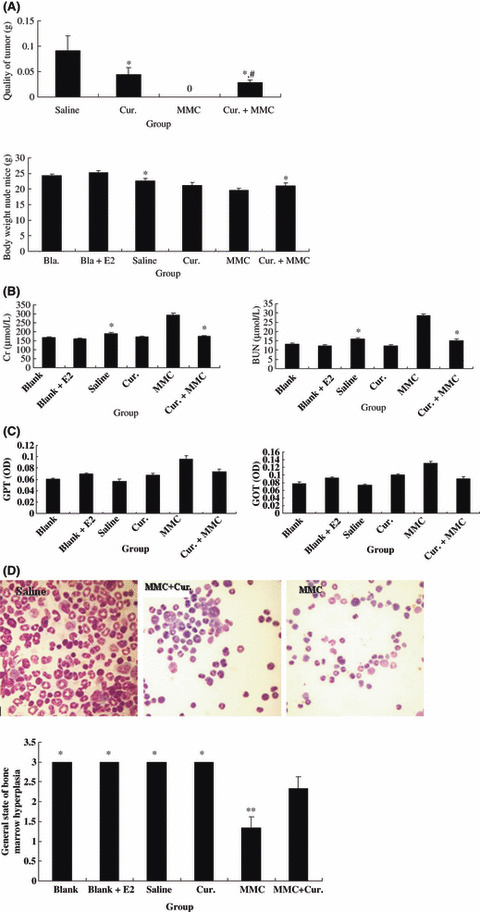
Tumor growth–inhibitory effect and side effects in all groups of MCF‐7 xenografts. (A) Tumor and body weight. Animals were treated with or without mitomycin (MMC) (2 mg/kg), curcumin (Cur.) (100 mg/kg), and their combination for 4 weeks. Tumor weights were measured. *Indicates P < 0.01 as compared with saline treatment. #Indicates P < 0.01 as compared with curcumin treatment; body weights of animals with different treatment were measured. *Indicates P < 0.01 as compared with MMC treatment. (B,C) Kidney and liver function. The levels of serum creatinine (Cr) and blood urea nitrogen (BUN), and the levels of serum glutamic pyruvic transaminase (GPT) and glutamic oxalacetic transaminase (GOT) were measured by the colorimetric method in treatment groups. *Indicates P < 0.01 as compared with MMC treatment. (D) Bone marrow smear. Bone marrow hyperplasia was measured by bone marrow smear in treatment groups. *Indicates P > 0.05; **indicates P < 0.01 as compared with combination treatment. Representative photographs are on the upper (×400).
There were inappetence, weight loss (Fig. 2A), and pre‐death behaviors such as pale skin and cachexia in the remaining animals with MMC treatment. As shown in Figure 2(B), the levels of Cr and BUN were increased significantly by MMC treatment compared with saline treatment. The levels of Cr and BUN were decreased by 60% and 53%, respectively, by the combination treatment compared with MMC alone. There was a significant difference between MMC alone and the combination treatment (P < 0.01). There was no significant difference in GPT and GOT among all groups (Fig. 2C). Moreover, the combination treatment reduced myelosuppression (Fig. 2D). The general state of bone marrow hyperplasia decreased by 58% with MMC treatment, but it only decreased by 25% with the combination treatment. There was a significant difference between the MMC‐treated group and the combination‐treated group (P < 0.01). These data suggested that the combination treatment inhibited tumor growth and that curcumin improved MMC‐induced weight loss, disorder of kidney function, and myelosuppression in MCF‐7 xenografts.
Curcumin and MMC combination treatment inhibited GRP58‐mediated DNA cross‐linking in MCF‐7 cells, It has been reported that MMC‐induced DNA cross‐linking and toxicity was mediated by GRP58.( 14 ) To confirm a role for GRP58 in the combination treatment, GRP58 siRNA transfection and an MMC‐induced DNA cross‐linking assay were performed in MCF‐7 cells. As shown in Figure 3, cross‐linked DNA was decreased by 91% by GRP58 siRNA. The cross‐linked DNA increased 2‐fold when cells were treated by 10 μm/L MMC compared with the untreated control. The cross‐linked DNA was unchanged by 160 μm/L of curcumin, but it was decreased by 39% by the combination treatment. After GRP58 siRNA was transfected, the expression of cross‐linked DNA was not affected by treatment with MMC, curcumin, or their combination, suggesting that DNA cross‐linking was abolished by GRP58 siRNA. These results suggested that curcumin and MMC together inhibited GRP58‐mediated DNA cross‐linking in MCF‐7 cells.
Figure 3.
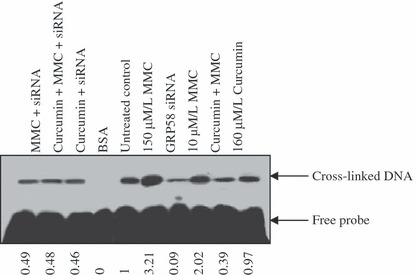
Effect on glucose regulatory protein (GRP58)‐associated with mitomycin (MMC)‐induced DNA cross‐linking. With or without transfection of GRP58 siRNA, cytosolic samples containing 0.5 mg of proteins from control and curcumin and/or MMC were incubated with 32P‐labeled 23 base pairs of oligonucleotide containing the mitomycin C binding site, and the DNA‐cross‐linking assay was performed as described in ‘Material and Methods’. The samples were analyzed on a 15% denaturing polyacrylamide gel, and cross‐linked and unmodified (free) oligonucleotides were detected by autoradiography. BSA, as negative control. MMC 150 μm/L, as positive control.
Curcumin and MMC combination treatment inhibited GRP58 via the ERK/p38 MAPK pathway in MCF‐7 cells. To determine the relationship among ERK, p38 MAPK, and GRP58 in MCF‐7cells, cells were treated with ERK specific inhibitor PD98059 80 μm/L and p38 MAPK inhibitor SB203580 15 μm/L, respectively, after they were transfected with GRP58 siRNA. As shown in Figure 4(A,B), GRP58 siRNA down‐regulated the level of GRP58 expression, and both PD98059 and SB203580 increased the level of GRP58 expression with about 4‐fold improvement as compared with GRP58 siRNA transfection only. The inhibitory effect of GRP58 siRNA was abolished by PD98059 and SB203580. These results suggested that the regulation of GRP58 involved ERK/p38 MAPK pathways.
Figure 4.
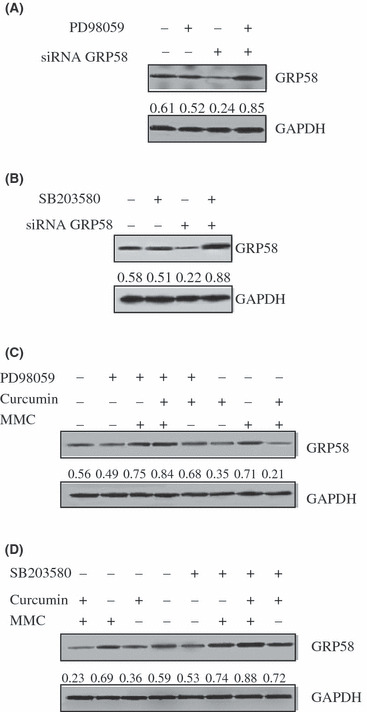
Effect on the level of glucose regulatory protein (GRP58) expression by curcumin, mitomycin (MMC), or their combination with or without SB203580 and PD98059. (A) Relationship between ERK and GRP58. After GRP58 siRNA was transfected, MCF‐7 cells were treated with PD98059 80 μm/L for 48 h. (B) Relation between p38 MAPK and GRP58. After GRP58 siRNA was transfected, the level of GRP58 expression was observed by SB203580 15 μm/L treatment for 48 h. (C) The level of GRP58 expression was regulated by combination treatment via the ERK pathway. The level of GRP58 expression was observed by MMC, curcumin, and their combination treatment with or without PD98059 in MCF‐7 cells. After MCF‐7 cells were pretreated with or without PD98059 80 μm/L for 2 h, cells were treated with MMC 2.5 μm/L, curcumin 40 μm/L, and MMC plus curcumin for 48 h. (D) The level of GRP58 expression was regulated by combination treatment via the p38 MAPK pathway. After MCF‐7 cells were pretreated with or without SB203580 15 μm/L for 2 h, cells were treated with MMC 2.5 μm/L, curcumin 40 μm/L, and MMC plus curcumin for 48 h. Total cellular protein was extracted and western blotting analysis was performed. The density ratio of proteins to GAPDH is shown as relative expression.
To assess the role of ERK and p38 MAPK in the combination treatment, cells were stimulated with PD98059 and SB203580 alone or PD98059 or SB203580 plus different drugs. As shown in Figure 4(C,D), the level of GRP58 expression was decreased by 63% by MMC and curcumin combination treatment compared with the untreated control. However, in the presence of PD98059, the level of GRP58 expression was increased about 4‐fold compared with the combination treatment without PD98059. The level of GRP58 expression was markedly increased 3.8‐fold by SB203580 plus the combination of curcumin and MMC, compared with the combination of curcumin and MMC. These results suggested that the combination treatment of curcumin and MMC inhibited GRP58 via the ERK/p38 MAPK pathway in MCF‐7 cells.
Discussion
Chemotherapeutic agents can significantly inhibit tumor growth, but it is often accompanied by severe side effects such as emesis,( 21 ) leucopenia,( 22 ) and even severe bone marrow depression( 23 ) and renal toxicity.( 24 ) These side effects impact the viability and quality of life in patients with cancer. It has been reported that a plant‐derived compound curcumin( 25 ) not only sensitizes cancer cells to cisplatin,( 26 ) but also reduces the effect on cisplatin‐induced nephrotoxicity.( 27 ) In this study, to determine whether the toxicity of MMC could be decreased by curcumin, we examined its safety by using the dosage of clinical treatment in MCF‐7 xenografts, and explored its mechanisms.
As a third‐line chemotherapeutic agent, the side effects of MMC cannot be ignored. In this study, our experimental results showed that MMC completely inhibited the tumor growth in MCF‐7 breast cancer xenografts. However, lethal toxicity was observed. Half of the tested animals died and there were distinct side effects in the remaining animals with MMC treatment. Interestingly, although the tumor growth‐inhibitory effect was not as good as MMC alone, the combination of MMC and curcumin did not show lethal toxicity in treated animals. The inhibitory effect was enhanced more significantly by the combination treatment than by curcumin alone in MCF‐7 breast cancer xenografts. Moreover, compared with MMC treatment, the combination treatment significantly decreased the levels of serum BUN and Cr, reduced the degree of myelosuppression, and improved the survival quality of experimental animals. Therefore, these results suggest that the combination therapy of MMC and curcumin may be helpful not only in tumor growth control, but also in improvement of the survival rate and quality of life in patients with breast cancer. The potential applications should be of great interest.
It has been reported that MMC induces cytotoxicity via DNA cross‐linking and breaking( 3 , 14 ) and GRP58 mediates MMC‐induced DNA cross‐linking.( 12 , 14 ) In this study, we demonstrated that GRP58‐mediated cytotoxcity of MMC that led to DNA cross‐linking was inhibited by the combination treatment (Fig. 3). Moreover, the combination treatment alleviated the side effects of MMC in the MCF‐7 xenografts. Therefore, the data suggested that the side effects of MMC were depressed by curcumin through the inhibition of GRP58‐mediated DNA cross‐linking.
MAPK p38 is involved in regulating cellular responses to stress and cytokines.( 28 , 29 ) It has been reported that cell survival and apoptosis are regulated through ERK/MAPK in various cancer cells such as human pancreatic cancer cells( 30 ) and human endometrial cancer cells.( 31 ) To explore whether GRP58 was regulated by ERK and p38 MAPK, ERK‐specific inhibitor PD98059 and p38 inhibitor SB203580 were used. As shown in Figure 4(A,B), the expression of GRP58 was decreased by GRP58 siRNA, but this inhibitory effect was abolished by SB203580 and PD98059. These results suggested that the expression of GRP58 requires the p38 MAPK and ERK pathways. Therefore, GRP58 may be regulated by ERK/p38 MAPK in MCF‐7 cells. Furthermore, the level of GRP58 expression was decreased by the combination treatment in the absence of SB203580 and PD98059, while it was increased by the combination treatment in the presence of PD98059 and SB203580 (Fig. 4C,D). These results suggested that the combination treatment decreased the level of GRP58 expression through the ERK/p38 MAPK pathway.
Currently, we are trying to find proper drug concentrations in the combination therapy. Studies are under way to explore the safety and efficacy of the combination therapy, which may prove to be more effective than monotherapy in suppressing tumor growth, and may decrease the side effects caused by chemotherapies.
In conclusion, the combination of MMC and curcumin reduced the side effects of MMC in MCF‐7 xenografts. The mechanism of the combination treatment was related to the down‐regulation of GRP58, which mediated MMC‐induced DNA cross‐linking. Moreover, the inhibitory effect of GRP58‐mediated DNA cross‐linking by the combination treatment may involve the ERK/p38 MAPK pathway.
Acknowledgments
This work was supported by MOST of China (no. 2006BAI08B0206), the Shanghai Municipal Education Commission Project (no. 08YZ54), and E‐institutes of the Shanghai Municipal Education Commission (no. E03008).
References
- 1. Jemal A, Clegg LX, Ward E et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 2004; 101: 3–27. [DOI] [PubMed] [Google Scholar]
- 2. Linos E, Spanos D, Rosner BA et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst 2008; 100: 1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Volpato M, Seargent J, Loadman PM, Phillips RM. Formation of DNA interstrand cross‐links as a marker of Mitomycin C bioreductive activation and chemosensitivity. Eur J Cancer 2005; 41: 1331–8. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani P, Kurtin S, Dragovich T. Response to a third‐line mitomycin C (MMC)‐based chemotherapy in a patient with metastatic pancreatic adenocarcinoma carrying germline BRCA2 mutation. JOP 2008; 9: 305–8. [PubMed] [Google Scholar]
- 5. Wu HI, Brown JA, Dorie MJ, Lazzeroni L, Brown JM. Genome‐wide identification of genes conferring resistance to the anticancer agents cisplatin, oxaliplatin, and mitomycin C. Cancer Res 2004; 64: 3940–8. [DOI] [PubMed] [Google Scholar]
- 6. Verwey J, De Vries J, Pinedo HM. Mitomycin C‐induced renal toxicity, a dose‐dependent side effect? Eur J Cancer Clin Oncol 1987; 23: 195–9. [DOI] [PubMed] [Google Scholar]
- 7. Campbell FC, Collett GP. Chemopreventive properties of curcumin. Future Oncol 2005; 1: 405–14. [DOI] [PubMed] [Google Scholar]
- 8. Vis I, Sriganth P, Premalatha B. Dietary curcumin with cisplatin administration modulates tumour marker indices in experimental fibrosarcoma. Pharmacol Res 1999; 39: 175–9. [DOI] [PubMed] [Google Scholar]
- 9. Antoniou AN, Ford S, Alphey M, Osborne A, Elliot T, Powis SJ. The oxidoreductase ERp57 efficiently reduces partially folded in preference to fully folded MHC class I molecules. EMBO J 2002; 21: 2655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coppari S, Altieri F, Ferraro A, Chichiarelli S, Eufemi M, Turano C. Nuclear localization and DNA interaction of protein disulfide isomerase ERp57 in mammalian cells. J Cell Biochem 2002; 85: 325–33. [DOI] [PubMed] [Google Scholar]
- 11. Ferraro A, Altieri F, Coppari S, Eufemi M, Chichiarelli S, Turano C. Binding of the protein disulfide isomerase isoform ERp60 to the nuclear matrix‐associated regions of DNA. J Cell Biochem 1999; 72: 528–39. [DOI] [PubMed] [Google Scholar]
- 12. Adikesavan AK, Jaiswal AK. Thioredoxin‐like domains required for glucose regulatory protein 58‐mediated reductive activation of mitomycin C leading to DNA cross‐linking. Mol Cancer Ther 2007; 6: 2719–27. [DOI] [PubMed] [Google Scholar]
- 13. Celli CM, Jaiswal AK. Role of GRP58 in mitomycin C‐induced DNA cross‐linking. Cancer Res 2003; 63: 6016–25. [PubMed] [Google Scholar]
- 14. Su S, Adikesavan AK, Jaiswal AK. Si RNA inhibition of GRP58 associated with decrease in mitomycin C‐induced DNA cross‐linking and cytotoxicity. Chem Biol Interact 2006; 162: 81–7. [DOI] [PubMed] [Google Scholar]
- 15. Makin G, Dive C. Modulating sensitivity to drug‐induced apoptosis: the future for chemotherapy? Breast Cancer Res 2001; 3: 150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res 1998; 74: 49–139. [DOI] [PubMed] [Google Scholar]
- 17. Schaeffer HJ, Weber MJ. Mitogen‐activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 1999; 19: 2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK‐p38 MAP kinases on apoptosis. Science 1995; 270: 1326–31. [DOI] [PubMed] [Google Scholar]
- 19. Joseph P, Xu Y, Jaiswal AK. Non‐enzymatic and enzymatic activation of mitomycin C: identification of a unique cytosolic activity. Int J Cancer 1996; 65: 263–71. [DOI] [PubMed] [Google Scholar]
- 20. Joseph P, Xu Y, Jaiswal AK. A unique cytosolic activity related but distinct from NQO1 catalyzes metabolic activation of mitomycin C. Br J Cancer 2000; 82: 1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abali H, Celik I. Tropisetron, ondansetron, and granisetron for control of chemotherapy‐induced emesis in Turkish cancer patients: a comparison of efficacy, side‐effect profile, and cost. Cancer Invest 2007; 25: 135–9. [DOI] [PubMed] [Google Scholar]
- 22. Breidenbach M, Rein DT, Schöndorf T et al. Hematological side‐effect profiles of individualized chemotherapy regimen for recurrent ovarian cancer. Anticancer Drugs 2003; 14: 341–6. [DOI] [PubMed] [Google Scholar]
- 23. Hofstra LS, Kristensen GB, Willemse PH et al. Randomized trial of recombinant human interleukin‐3 versus placebo in prevention of bone marrow depression during first‐line chemotherapy for ovarian carcinoma. J Clin Oncol 1998; 16: 3335–44. [DOI] [PubMed] [Google Scholar]
- 24. Welz S, Hehr T, Kollmannsberger C, Bokemeyer C, Belka C, Budach W. Renal toxicity of adjuvant chemoradiotherapy with cisplatin in gastric cancer. Int J Radiat Oncol Biol Phys 2007; 69: 1429–35. [DOI] [PubMed] [Google Scholar]
- 25. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett 2007; 255: 170–81. [DOI] [PubMed] [Google Scholar]
- 26. Chanvorachote P, Pongrakhananon V, Wannachaiyasit S, Luanpitpong S, Rojanasakul Y, Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin‐induced apoptosis through superoxide anion‐mediated Bcl‐2 degradation. Cancer Invest 2009; 27: 624–35. [DOI] [PubMed] [Google Scholar]
- 27. Antunes LM, Darin JD, Bianchi Nde L. Effects of the antioxidants curcumin or selenium on cisplatin‐induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res 2001; 43: 145–50. [DOI] [PubMed] [Google Scholar]
- 28. Lee JC, Laydon JT, McDonnell PC et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994; 372: 739–46. [DOI] [PubMed] [Google Scholar]
- 29. Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994; 265: 808–11. [DOI] [PubMed] [Google Scholar]
- 30. Lee KH, Hyun MS, Kim JR. Growth factor‐dependent activation of the MAPK pathway in human pancreatic cancer: MEK/ERK and p38 MAP kinase interaction in uPA synthesis. Clin Exp Metastasis 2003; 20: 499–505. [DOI] [PubMed] [Google Scholar]
- 31. Gao J, Tian J, Lv Y et al. Leptin induces functional activation of cyclooxygenase‐2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci 2009; 100: 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


