Abstract
The influence of three high frequency ABCB1 polymorphisms (c.1236C>T, c.2677G>A/T, and c.3435C>T) and the ABCG2 c.421C>A polymorphism on the disposition of doxorubicin in Asian breast cancer patients receiving adjuvant chemotherapy was investigated in the present study. The allelic frequency of the ABCB1 c.1236T, c.2677T, c.2677A, and c.3435T variants were 60%, 38%, 7%, and 22%, respectively, and the frequency of the ABCG2 c.421A allele was 23%. Pairwise analysis showed increased exposure levels to doxorubicin in patients harboring at least one ABCB1 c.1236T allele (P = 0.03). Patients homozygous for the CC‐GG‐CC genotype had significantly lower doxorubicin exposure levels compared to the patients who had CT‐GT‐CT (P = 0.02) and TT‐TT‐TT genotypes (P = 0.03). Significantly increased clearance of doxorubicin was also observed in patients harboring CC‐GG‐CC genotypes when compared to patients harboring the CT‐GT‐CT genotype (P = 0.01). Patients harboring the CC‐GG‐CC genotypes had significantly lower peak plasma concentrations of doxorubicinol compared to patients who had TT‐TT‐TT genotypes (P = 0.03). No significant influences on doxorubicin pharmacokinetic parameters were observed in relation to the ABCG2 c.421C>A polymorphism. In conclusion, the present exploratory study suggests that the three high frequency linked polymorphisms in the ABCB1 gene might be functionally important with regards to the altered pharmacokinetics of doxorubicin in Asian breast cancer patients, resulting in significantly increased exposure levels and reduced clearance. (Cancer Sci 2008; 99: 816–823)
P‐glycoprotein (P‐gp), encoded by the multidrug resistance gene ABCB1 (MDR1), is the best‐characterized ATP‐binding cassette (ABC) efflux transporter. It was isolated in 1976.( 1 ) Human P‐gp is a phosphorylated and glycosylated protein with 1280 amino acids and consists of two homologous halves of six hydrophobic transmembrane segments, each with its own intracellular ATP binding site. Since the report by Hoffmeyer et al.( 2 ) that the exon 26 c.3435C>T polymorphism in the ABCB1 gene is associated with lower ABCB1 expression, resulting in 50% higher plasma concentration of digoxin, studies in different populations and patient subgroups have suggested that this synonymous transition is associated with altered ABCB1 functionality and bioavailability of ABCB1 substrates.( 3 , 4 ) Studies suggested reduced exposure of digoxin in c.3435TT subjects, whereas other reports indicated both increased digoxin exposure in c.3435T carriers and also a lack of influence on digoxin pharmacokinetics.( 5 , 6 ) The alterations in the pharmacokinetics of other P‐gp drug substrates such as fexofenadine,( 7 , 8 , 9 ) and cyclosporine,( 10 , 11 ) also showed conflicting patterns with regards to the c.3435C>T polymorphism. In the search for potential causal mechanisms involved, Kim et al.( 7 ) reported that the c.3435C>T polymorphism is linked to a synonymous c.1236C>T (exon 12) and a non‐synonymous c.2677G>T/A (Ala893Ser) (exon 21) polymorphism. Independent studies showed that the c.2677G>T variant had substrate‐dependent effects in cellular systems, caused alterations in mRNA levels, and increased drug efflux,( 4 , 12 , 13 ) whereas patients carrying the c.1236CC reference genotype showed lower peak drug concentrations and reduced drug exposure levels compared with mutated allele carriers.( 14 )
Several groups have since highlighted the strong linkage disequilibrium associated with ABCB1 polymorphisms at exons 12, 21, and 26 and identified haplotypes revealing ethnicity‐specific patterns.( 7 , 15 , 16 ) Consequently, homozygosity for the variant forms at the three loci was associated with reduced P‐gp activity and higher exposure levels of drug substrates.( 15 , 17 , 18 , 19 ) The 2677G/3435T haplotype was shown to be associated with significantly higher bioavailability of digoxin, whereas the 2677G/3435C haplotype correlated with lower exposure levels to digoxin.( 17 ) In another study, however, 1236T/2677G/3435T carriers showed higher P‐gp activity when compared with non‐carriers.( 7 ) Johne et al.( 17 ) recently reported that analysis of ABCB1 haplotypes is superior to unphased single nucleotide polymorphism analysis for predicting ABCB1 phenotypes and suggested that the 2677GT/3435TT genotype is key to describing interindividual differences in drug disposition.
ABCG2, (breast cancer‐resistant protein or mitoxantrone‐resistant protein [MRP]), is the second member of the G‐subfamily of ABC transporters. The ABCG2 gene is located at chromosome 4q22 and encodes a 72‐kDa membrane protein composed of 655 amino acids. Unlike ABCB1, ABCG2 protein is a half molecule consisting of one transmembrane domain with a single ATP‐binding site requiring homodimerization for substrate transport. ABCG2 is expressed in a variety of normal tissues such as the placenta, intestine, liver, ovary, testis, and hematopoeitic stem cells.( 20 , 21 ) Overexpression of ABCG2 has been shown to mediate resistance to several anticancer agents such as mitoxantrone, SN‐38, flavopiridol, and doxorubicin,( 22 , 23 , 24 ) and has been postulated to limit the effectiveness of some of these therapeutic agents. The ABCG2 c.421C>A (Glyn141Lys) (exon 5) polymorphism reported by Imai et al.( 25 ) was shown to result in markedly decreased protein expression and low‐level drug resistance compared with ABCG2 transfected cells carrying the reference genotype. The wide ethnic variations in allele frequency distribution of the ABCG2 c.421C>A polymorphism suggest that interindividual variability in the disposition of candidate drug substrates might be due, in part, to its functional influence.( 26 , 27 )
The anticancer agent doxorubicin is widely used as a single agent or as part of combination regimens for curative, adjuvant, and palliative treatment in cancer chemotherapy, particularly for the treatment of solid tumors including breast cancer. Doxorubicin is a substrate of both ABCB1 and ABCG2 and overexpression of these transporters has been associated with increased resistance to doxorubicin.( 21 , 22 ) There is currently a lack of studies in cancer patients that investigate the contribution of ABCB1 and ABCG2 polymorphisms in interindividual differences in pharmacokinetics and pharmacodynamics of doxorubicin and its metabolites. The aim of the present study was to examine the influence of three high frequency ABCB1 polymorphisms [c.1236C>T, c.2677G>A/T, and c.3435C>T] and the ABCG2 c.421C>A polymorphism on the pharmacokinetics of doxorubicin in Asian breast cancer patients receiving adjuvant chemotherapy.
Materials and Methods
Breast cancer patients. Patients who had histologically confirmed invasive breast cancer (n = 62) and were receiving adjuvant chemotherapy with doxorubicin were recruited for the study. Informed consent was obtained from all patients and the protocol was approved by the institutional ethics committee at the National Cancer Center, Singapore. Inclusion criteria required patients to have adequate bone marrow (absolute neutrophil count >1500/µL, platelet count >100 000/µL), hepatic (aspartate amino transferase [AST] and alanine amino transferase [ALT] levels ≤ 2.5 and total bilirubin < 2.0 times upper limit of normal), and renal functions (serum creatinine <140 mmol/L) documented at the time of enrolment. Patients with serious comorbidities, including poorly controlled diabetes mellitus, ischemic heart disease, uncontrolled hypertension, active infection, or performance status score of ≥2 on the Eastern Cooperative Oncology Group scale, were excluded from the study. Pregnancy and use of growth factors during the cycle of chemotherapy were also considered as criteria for exclusion. No forms of endocrine therapy, immunotherapy, or biologic response modifiers were allowed during the period of chemotherapy.
ABCB1 and ABCG2 genotyping. Purified genomic DNA was isolated from peripheral blood samples (5 mL) and hepatic tissues using the phenol–chloroform extraction method. The liver tissues were obtained from colon cancer patients undergoing hepatectomy for liver metastasis. Genotyping for the ABCB1 (GenBank accession number NM_000927) c.1236C>T, c.2677G>T/A, and c.3435C>T polymorphisms was carried out using restriction enzymes Eco01091, MboI, and BanI/RsaI, respectively, as described in Chowbay et al.( 15 ) Genotyping for the ABCG2 (GenBank accession number NM_004827) c.421C>A polymorphism was carried out by polymerase chain reaction followed by restriction fragment length polymorphism using MseI.( 27 ) Digested fragments were analyzed by electrophoretic separation on a 40% polyacrylamide gel, followed by direct visualization under ultraviolet light.
Doxorubicin treatment and pharmacokinetic analysis. Doxorubicin was given at a dose of 60 mg/m2 intravenously (i.v.) over 20 min, once every 3‐week cycle after standard premedications with i.v. dexamethasone 10 mg, diphenhydramine 50 mg, cimetidine 300 mg, or ranitidine 50 mg. All patients received concomitant i.v. cyclophosphamide 600 mg/m2 given over 30 min once every 3 weeks. Hematological parameters including hemoglobin, total leukocyte count, platelet count, and the absolute neutrophil count were measured after the start of treatment (days 7 and 14) and prior to the start of each cycle. New treatment cycles were started only if the absolute neutrophil count was ≥1500/µL and the platelet count was ≥100 000/µL. Blood samples for pharmacokinetic analysis were obtained at the following time points after the start of infusion on the first day of the first cycle of chemotherapy: at predose and 5 min; postinfusion at 15 and 30 min; and at 1, 4, 8, and 24 h. Blood samples were collected in plain ethylenediamine tetraacetic acid containing vacutainer glass tubes and immediately centrifuged at 1000 g for 10 min; the plasma fraction was collected and stored at –20°C until analysis.
Plasma concentrations of doxorubicin and its major metabolite doxorubicinol were estimated by reversed‐phase high performance liquid chromatography with fluorescence detection.( 28 ) Briefly, following a single protein precipitation step, chromatographic separation was accomplished using a C‐18 column with a mobile phase consisting of 50 mM sodium phosphate buffer–acetonitrile–1‐propanol (65:25:2, v/v), pH 2.0. The analytes were measured by fluorescence detection with an excitation wavelength of 480 nm and emission wavelength of 560 nm. The lower limits of quantitation were 10 ng/mL for doxorubicin and 5 ng/mL for doxorubicinol. The calibration curves were linear over a concentration range of 10–2500 ng/mL for doxorubicin and 5–1250 ng/mL for doxorubicinol. The average recoveries were greater than 89% for all analytes and within‐day and between‐day coefficients of variation was less than 13%.
Pharmacokinetic parameters were determined using a non‐linear regression program WinNonLin version 2.1 (Pharsight, Mountain View, CA, USA). The area under the plasma concentration–time curve (AUC) was calculated from time 0 to the time (t) of the last detectable concentration (AUC0→t) using the trapezoidal rule. The area was extrapolated to infinity (AUC0‐∞) by adding Ct/λz to AUC0→t, where Ct was the last detectable plasma concentration and λz is the elimination rate constant. Peak plasma concentrations (Cmax) were directly identified from individual subject concentration–time curves.
Statistical analysis. The χ2 analysis was used to test for departure of genotype frequencies from Hardy–Weinberg expectations. The possible contribution of subject characteristics (ethnicity, age, height, and body surface area [BSA]) on pharmacokinetics of doxorubicin and doxorubicinol were determined using univariate linear regression analysis. Other independent variables investigated to evaluate their relative contribution to the variability in pharmacokinetic parameters of doxorubicin and doxorubicinol included patient biochemical parameters such as albumin, total protein, and markers of hepatic (AST, ALT, alkaline phosphatase, and bilirubin) and renal function (serum creatinine). The non‐parametric Mann–Whitney U‐test was used for pairwise comparisons between genotype groups. Pharmacokinetic parameters are reported as median values unless otherwise indicated. The minimum level of statistical significance was set at P = 0.05 for pairwise comparisons. Linkage analysis between the ABCB1 polymorphisms was carried out using Haploview software version 3.32 (http://www.broad.mit.edu/mpg/haploview). All statistical analyses were carried out using Stata Statistical Software release 7.0 (Stata Corporation, TX, USA).
Results
Subject demographics. Most of the breast cancer patients belonged to the Chinese ethnic group (74%), followed by Malays (18%), and Indians (8%). The median age, height, and BSA of the patients were 51 years (range, 29–73 years), 154 cm (range, 144–168 cm), and 1.52 m2 (range, 1.23–1.95 m2), respectively.
Doxorubicin pharmacokinetics. The pharmacokinetic parameters of doxorubicin and its metabolite doxorubicinol in Asian breast cancer patients are summarized in Table 1. Plasma samples for pharmacokinetic analysis were available in 52 of the 62 patients and a high degree of interpatient variability was observed with regards to the pharmacokinetic parameters of doxorubicin and doxorubicinol. The clearance (CL) of doxorubicin was 22.9 L/h/m2 and was within the range reported in published studies.( 29 ) Approximately six‐fold (median, 22.9; range, 8.7–55.7 L/h/m2) variation in CL and 13‐fold (median, 237.5; range, 53.9–703.7/Lm2) variations in volume of distribution at steady state (Vss) of doxorubicin were observed among the cancer patients. Interpatient variability was also high with regards to doxorubicinol pharmacokinetic parameters with more than six‐fold variation observed in its AUC0‐∞ (median, 9.6; range, 3.7–24.3/hm5). Univariate analysis revealed patient's height and weight to be significant covariates influencing the AUC0‐∞(P < 0.015 and P < 0.0001, respectively) and Cmax of doxorubicin (P < 0.028 and P < 0.0001, respectively). Age and ethnicity were identified as significant covariates in relation to the t1/2 (h) of doxorubicin (P < 0.018). The half‐life of doxorubicin was analyzed in patients following stratification according to their age (below and above 60 years). The t1/2 (h) of doxorubicin was significantly higher in patients above 60 years of age (median, 15.73; range 4.7–24.6) compared with patients below 60 years of age (median, 12.54; range, 8.1–17.8; P < 0.02). No significant influence of covariates, including markers of hepatic (AST, ALT, alkaline phosphatase, bilirubin) or renal (serum creatinine) functions, were observed on doxorubicin pharmacokinetic parameters.
Table 1.
Summary of doxorubicin pharmacokinetic parameters in Asian breast cancer patients (n = 52)
| Pharmacokinetic parameters | Median (range) |
|---|---|
| Doxorubicin | |
| Cmax/dose/BSA (/m5) | 34.01 (4.50–97.70) |
| AUC0‐∞/dose/BSA (h/m5) | 17.50 (6.20–67.20) |
| t1/2 (h) | 15.40 (4.70–24.60) |
| CL/BSA (Lh−1/m2) | 22.90 (8.70–55.70) |
| Vss/BSA (/Lm2) | 237.50 (53.90–703.70) |
| Doxorubicinol | |
| Cmax/dose/BSA (/m5) | 0.33 (0.15–2.00) |
| AUC0‐∞/dose/BSA (h/m5) | 9.60 (3.70–24.30) |
| t1/2 (h) | 27.50 (6.30–112.40) |
AUC0‐∞/dose/BSA, area under plasma concentration–time curve from time zero to infinity normalized by dose and body surface area; CL/BSA; plasma clearance normalized by body surface area; Cmax/dose/BSA, peak plasma concentration normalized by dose and body surface area; t1/2; half‐life; Vss/BSA; volume of distribution at steady‐state normalized by body surface area.
ABCB1 and ABCG2 genotype distribution. Table 2 shows the genotype and allele frequencies of the ABCB1 [c.1236C>T, c.2677G>T/A, and c.3435C>T] and the ABCG2 c.421C>A polymorphisms in Asian breast cancer patients. The observed genotype distribution in both genes conformed to Hardy–Weinberg proportions. The allelic frequency of the ABCB1 c.1236T, c.2677T, and c.2677A, and ABCB2 c.3435T variants among Asian breast cancer patients were 60%, 38%, 7%, and 22%, respectively, and were in agreement with previously published reports in Asian healthy populations.( 15 ) The frequency of the ABCG2 c.421 A allele was 23% and patients homozygous for the variant allele were absent in the cancer patients. The allele frequency of the c.421C>A polymorphism was comparable to earlier reports in the Asian population.( 30 ) Linkage analysis showed a moderate degree of linkage between all three ABCB1 polymorphisms (c.2677G>T/A and c.3435C>T,|D′| = 0.63; c.1236C>T and c.3435C>T, |D′| = 0.56; and c.1236C>T and c.2677G>T/A, |D′| = 0.57] (Fig. 1).
Table 2.
Genotype and allele frequencies of ABCB1 and ABCG2 polymorphisms in Asian breast cancer patients
| Polymorphisms | Genotypes; n (%) | Allele frequencies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ABCB1 | |||||||||
| c.1236C>T | CC | CT | TT | C | T | ||||
| 11 (17.7) | 27 (43.5) | 24 (38.7) | 0.40 | 0.60 | |||||
| c.2677G>T/A | GG | GT | GA | AT | AA | TT | G | A | T |
| 21 (33.9) | 20 (32.3) | 6 (9.7) | 1 (1.6) | 1 (1.6) | 13 (21.0) | 0.55 | 0.07 | 0.38 | |
| c.3435C>T | CC | CT | TT | C | T | ||||
| 32 (51.6) | 17 (27.4) | 13 (21.0) | 0.78 | 0.22 | |||||
| ABCG2 | |||||||||
| c.421C>A | CC | CA | AA | C | A | ||||
| 28 (45.2) | 34 (54.8) | 0 | 0.77 | 0.23 | |||||
Figure 1.
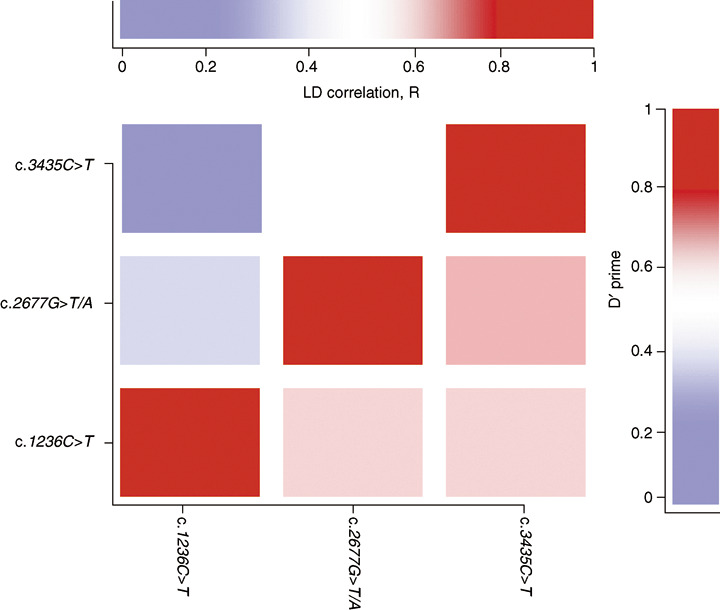
Pairwise linkage disequilibrium (LD) between the multidrug resistance gene ABCB1 polymorphisms c.1236C>T, c.2677G>T/A, and c.3435C>T in Asian cancer patients. The intensity of the colors indicates the linkage between polymorphisms.
Genotypic–phenotypic correlates. The influence of ABCB1 polymorphisms on the pharmacokinetic parameters of doxorubicin in Asian breast cancer patients are summarized in Table 3. Significant correlations of doxorubicin pharmacokinetic parameters with ABCB1 c.1236C>T polymorphism were observed. As no significant differences were observed in pharmacokinetic parameters between cancer patients carrying one or two variant alleles at the ABCB1 1236 locus, patients harboring the c.1236CT and c.1236TT genotypes were grouped together and compared with the reference genotype. Breast cancer patients carrying a least one c.1236T allele had significantly higher (69%) peak plasma concentrations of doxorubicin (Cmax/dose/BSA (/m5), median, 36.8; range, 10.4–97.7) when compared to the patients carrying the reference genotype (Cmax/dose/BSA (/m5), median, 21.8; range: 4.6–57.9; P = 0.03). The exposure level of doxorubicin was 42% higher in patients who carried at least one c.1236T allele (AUC0‐∞/dose/BSA (h/m5): median, 18.8; range, 8.2–38.0) compared to patients who were homozygous for the reference genotype (AUC0‐∞/dose/BSA (h/m5), median, 13.2; range, 6.2–67.2; P = 0.07). The clearance of doxorubicin was approximately 25% lower in patients carrying at least one variant T allele compared with patients carrying the reference genotype (P = 0.14).
Table 3.
ABCB1 and ABCG2 polymorphisms and pharmacokinetics of doxorubicin in Asian breast cancer patients
| Pharmacokinetic parametersc.1236C>T | ABCB1 genotypes † | Pairwise P‐valueCC versus CT + TT | |
|---|---|---|---|
| CC (n = 9) | CT + TT (n = 43) | ||
| Doxorubicin | |||
| AUC0‐∞/dose/BSA (h/m5) | 13.20 (6.20–67.20) | 18.8 (8.20–38.00) | 0.07 |
| Cmax/dose/BSA (/m5) | 21.80 (4.60–57.90) | 36.8 (10.40–97.70) | 0.03 |
| CL/BSA (Lh−1/m2) | 29.90 (8.70–49.40) | 22.5 (13.80–55.70) | 0.14 |
| Doxorubicinol | |||
| AUC0‐∞/dose/BSA (h/m5) | 8.40 (3.70–13.70) | 9.9 (5.80–24.30) | 0.08 |
| Cmax/dose/BSA (/m5) | 0.31 (0.16–0.76) | 0.36 (0.15–2.00) | 0.26 |
| c.2677G>T/A | GG (n = 15) | GA + GT (n = 19) | AA + TT (n = 9) | GG versus GA + GT | GA + GT versus AA + TT | GG versus AA + TT |
|---|---|---|---|---|---|---|
| Doxorubicin | ||||||
| AUC0‐∞/dose/BSA (h/m5) | 15.2 (7.10–67.20) | 19.8 (6.20–38.00) | 13.2 (8.20–30.20) | 0.12 | 0.08 | 0.55 |
| Cmax/dose/BSA (/m5) | 36.2 (7.00–77.40) | 35.8 (4.50–97.70) | 23.3 (13.60–80.70) | 0.34 | 0.19 | 0.70 |
| CL/BSA (Lh−1/m2) | 22.3 (8.70–36.90) | 21.7 (15.00–49.40) | 30.3 (13.80–55.70) | 0.46 | 0.01 | 0.04 |
| Doxorubicinol | ||||||
| AUC0‐∞/dose/BSA (h/m5) | 9.1 (3.70–21.70) | 10.5 (5.60–24.30) | 8.9 (6.10–20.60) | 0.14 | 0.67 | 0.57 |
| Cmax/dose/BSA (/m5) | 0.34 (0.16–1.49) | 0.37 (0.18–2.00) | 0.31 (0.15–0.57) | 0.56 | 0.19 | 0.30 |
| c.3435C>T | CC (n = 28) | CT + TT (n = 24) | CC versus CT + TT |
|---|---|---|---|
| Doxorubicin | |||
| AUC0‐∞/dose/BSA (h/m5) | 16.90 (6.20–38.00) | 18.2 (9.00–67.20) | 0.59 |
| Cmax/dose/BSA (/m5) | 35.50 (4.60–97.70) | 33.1 (10.40–80.70) | 0.72 |
| CL/BSA (Lh−1/m2) | 22.40 (16.40–49.40) | 22.4 (8.70–55.70) | 0.89 |
| Doxorubicinol | |||
| AUC0‐∞/dose/BSA (h/m5) | 9.20 (3.70–24.30) | 11.04 (6.30–18.70) | 0.12 |
| Cmax/dose/BSA (/m5) | 0.33 (0.16–1.50) | 0.38 (0.15–2.00) | 0.58 |
| c.421C>A | ABCG2 genotypes † | CC versus CA | |
|---|---|---|---|
| CC (n = 23) | CA (n = 29) | ||
| Doxorubicin | |||
| AUC0‐∞/dose/BSA (h/m5) | 17.40 (6.20–67.20) | 17.6 (9.80–29.30) | 0.97 |
| Cmax/dose/BSA (/m5) | 32.90 (4.60–81.20) | 34.8 (10.40–97.70) | 1.00 |
| CL/BSA (Lh−1/m2) | 21.90 (8.70–55.70) | 23.27 (19.20–33.60) | 0.47 |
| Doxorubicinol | |||
| AUC0‐∞/dose/BSA (h/m5) | 9.8 (3.70–21.70) | 9.6 (5.40–24.30) | 0.63 |
| Cmax/dose/BSA (/m5) | 0.3 (0.15–1.20) | 0.38 (0.16–2.00) | 0.15 |
Values are expressed as median (range). P < 0.05 considered statistically significant. AUC0‐∞/dose/BSA, area under plasma concentration‐time curve from time zero to infinity normalized by dose and body surface area; CL/BSA, plasma clearance normalized by body surface area; Cmax/dose/BSA, peak plasma concentration normalized by dose and body surface area.
As depicted in Table 3, patients who were heterozygous for the c.2677G>T/A polymorphism showed a trend towards higher doxorubicin exposure levels (AUC0‐∞/dose/BSA (h/m5), median, 19.8; range, 6.2–38.0) compared to patients homozygous for the reference allele (AUC0‐∞/dose/BSA (h/m5), median, 15.2; range, 7.1–67.2, P > 0.05). The clearance of doxorubicin was significantly higher among patients who harbored the homozygous variant alleles for the c.2677G>T/A polymorphism (CL/BSA (Lh−1/m2), median, 30.3; range, 13.8–55.7] when compared to patients who were heterozygous (CL/BSA (Lh−1/m2), median, 21.7; range, 15.0–49.4, P = 0.01] and homozygous for the reference allele (CL/BSA (Lh−1/m2), median, 22.3; range, 8.7–36.9, P = 0.04). Pairwise analysis failed to reveal any significant influences of the c.3435C>T polymorphism on pharmacokinetic parameters of doxorubicin. No significant influences of ABCB1 polymorphisms on the pharmacokinetic parameters of doxorubicinol were observed in the present study.
Table 4 shows the doxorubicin pharmacokinetic parameters by the three major genotypes of ABCB1 exon 12, 21, and 26 polymorphisms in Asian breast cancer patients. Patients homozygous for the CC‐GG‐CC genotype had significantly lower doxorubicin exposure levels (AUC0‐∞/dose/BSA (h/m5), median, 11.13; range, 7.12–13.21) compared to the patients who had heterozygous (CT‐GT‐CT) (AUC0‐∞/dose/BSA (h/m5), median, 19.0; range, 11.88–31.6, P = 0.02) and homozygous (TT‐TT‐TT) genotypes (AUC0‐∞/dose/BSA (h/m5), median, 17.59; range, 12.55–30.16; P = 0.03) at the three loci (Fig. 2a). Patients harboring the CC‐GG‐CC genotypes also had significantly lower peak plasma concentrations of doxorubicin (Cmax/dose/BSA (/m5), median, 14.63; range, 7.0–22.23) compared to patients who harbored the CT‐GT‐CT (Cmax/dose/BSA (/m5), median, 32.99; range, 18.3–73.5, P = 0.02) and TT‐TT‐TT genotypes (Cmax/dose/BSA (/m5), median, 37.98; range, 20.9–80.7; P = 0.03). The significantly lower exposure levels of doxorubicin in patients harboring CC‐GG‐CC genotypes was also associated with a significantly increased clearance of doxorubicin (CL/BSA (Lh−1/m2), median, 31.73; range, 24.8–36.9) when compared to patients harboring the CT‐GT‐CT genotype (CL/BSA (Lh−1/m2), median, 21.9; range, 15.0–28.5; P = 0.01) (Fig. 2b). With regards to doxorubicinol, patients harboring the CC‐GG‐CC genotypes had significantly lower peak plasma concentrations of doxorubicinol (Cmax/dose/BSA (/m5), median, 0.22; range, 0.16–0.32) compared to the patients who had TT‐TT‐TT genotypes (Cmax/dose/BSA (/m5), median: 0.39; range: 0.31–0.57, P = 0.03].
Table 4.
Influence of ABCB1‐linked polymorphisms on the pharmacokinetics of doxorubicin and doxorubicinol in Asian breast cancer patients
| Pharmacokinetic parameters | CC‐GG‐CC (n = 4) † | CT‐GT‐CT (n = 7) † | TT‐TT‐TT (n = 5) † | Pairwise P‐values | ||
|---|---|---|---|---|---|---|
| CT‐GT‐CT versus CT‐GT‐CT | CC‐GG‐CC versus TT‐TT‐TT | CC‐GG‐CC versus TT‐TT‐TT | ||||
| Doxorubicin | ||||||
| AUC0‐∞/dose/BSA (h/m5) | 11.130 (7.120–13.210) | 19.00 (11.88–31.60) | 17.59 (12.55–30.16) | 0.020 | 1.000 | 0.030 |
| Cmax/dose/BSA (/m5) | 14.630 (7.000–22.230) | 32.99 (18.30–73.50) | 37.98 (20.90–80.70) | 0.020 | 0.760 | 0.030 |
| CL/BSA (Lh−1/m2) | 31.730 (24.800–36.900) | 21.90 (15.00–28.50) | 24.98 (13.80–40.90) | 0.010 | 0.200 | 0.910 |
| Doxorubicinol | ||||||
| AUC0‐∞/dose/BSA (h/m5) | 7.213 (3.700–10.800) | 11.85 (7.9–18.70) | 11.64 (6.60–15.60) | 0.067 | 0.790 | 0.064 |
| Cmax/dose/BSA (/m5) | 0.220 (0.160–0.320) | 0.28 (0.18–0.59) | 0.39 (0.31–0.57) | 0.352 | 0.178 | 0.032 |
Values are expressed as median (range). P < 0.05 considered statistically significant. AUC0‐∞/dose/BSA, area under plasma concentration‐time curve from time zero to infinity normalized by dose and body surface area; CL/BSA, plasma clearance normalized by body surface area; Cmax/dose/BSA, peak plasma concentration normalized by dose and body surface area.
Figure 2.

Influence of the multidrug resistance gene ABCB1 genotype on the exposure levels (a) and clearance (b) of doxorubicin in Asian breast cancer patients. AUC0‐∞/dose/BSA, area under plasma concentration–time curve from time zero to infinity normalized by dose and body surface area; CL dose/BSA; plasma clearance normalized by dose and body surface area; NS, not significant.
Pairwise comparisons failed to show any significant influences of the ABCG2 c.421C>A polymorphism on the pharmacokinetic parameters of doxorubicin and doxorubicinol in the present study (Table 3).
Discussion
Several studies have reported higher bioavailability of ABCB1 drug substrates in carriers of the T allele at the ABCB1 c.3435 locus, attributed to lower functional activity of the protein or reduced ABCB1 expression.( 31 ) The search for causal mechanisms has confirmed varying degrees of contribution from the ABCB1 c.1236C>T and c.2677G>T/A polymorphisms. The c.1236C>T, c.2677G>T/A, and c.3435C>T polymorphisms are known to be in linkage disequilibrium, and population studies in different ethnic groups have revealed significant heterogeneity in the patterns of haplotype structures. Although several studies have been published, there is no consensus regarding a definitive influence of major ABCB1 polymorphisms on drug disposition either independently or as part of different haplotypes.( 7 , 32 , 33 )
In the present study, pairwise analysis suggested that the ABCB1 c.1236C>T polymorphism might have a functional influence on doxorubicin pharmacokinetics resulting in increased exposure levels to doxorubicin in patients harboring at least one c.1236T allele. This finding was similar to an earlier report of the effect of the c.1236C>T polymorphism on the pharmacokinetics of cyclosporinee where subjects with the c.1236CC genotype had slight but significantly lower dose‐adjusted Cmax and AUC0–4 h of cyclosporinee compared with mutated allele carriers.( 14 ) With regards to the influence of the c.2677G >T/A polymorphism, a trend of higher exposure levels and significantly decreased clearance of doxorubicin was observed in c.2677G>T/A heterozygote patients when compared to patients who were homozygous for the c.2677T and c.2677 A alleles. The non‐synonymous c.2677G>T/A polymorphism has been previously shown to be associated with alterations in saturation kinetics of vincristine,( 32 ) and the bioavailability of other ABCB1 substrates, but most often has been analyzed in conjunction with the c.3435C>T polymorphism. The possible mechanistic roles of naturally occurring synonymous variations was highlighted in a recent report investigating the ABCB1 c.3435C>T polymorphism showing alterations in conformation following changes in translation kinetics due to the rare codon usage at c.3435T, resulting in variations in the structure of substrate and inhibitor binding sites.( 34 ) Our results, however, failed to reveal any significant influence of the ABCB1 c.3435C>T polymorphism on doxorubicin disposition in the present study. This could be due to the minimal phenotypic influence of the c.3435C>T polymorphism, or due to its effects being masked by the multiple determinants of doxorubicin disposition including those from other transporter proteins and metabolizing enzymes.
Recent studies have suggested that the association of the ABCB1 polymorphisms with phenotypic correlates might be better explained by considering the composite influence of genetic information from the ABCB1 1236, 2677, and 3435 loci.( 15 , 17 ) Haplotype analysis of ABCB1 gene blocks in the Japanese population have shown a genotype‐dependent renal clearance of irinotecan.( 35 ) Haplotype–phenotype evaluations of the drug metabolizing enzyme CYP2C8 have also been shown to delineate the subset of patients having altered pharmacokinetics of paclitaxel,( 36 ) suggesting that haplotype analysis of candidate gene polymorphisms might have significant roles in understanding interindividual variations in the pharmacokinetics of antineoplastic agents. In order to observe the net effect of all three ABCB1 polymorphisms on the pharmacokinetics of doxorubicin and doxorubicinol, we analyzed the contribution of these polymorphisms using the genotype information from the ABCB1 1236‐2677‐3435 loci. Patients with the CC‐GG‐CC genotype constitution were associated with lower exposure levels and increased clearance of doxorubicin when compared to the subjects harboring the TT‐TT‐TT genotype. The observation of reduced peak plasma concentrations of doxorubicin in these subjects who were homozygous for the reference allele at all the three loci is similar to earlier reports on the influence of ABCB1 haplotypes on cyclosporine levels.( 15 ) In a study investigating the influence of ABCB1 haplotypes on cyclosporinee disposition in heart transplant recipients, it was observed that patients homozygous for the CC‐GG‐CC genotypes had lower peak and trough cyclosporine concentrations as well AUC0–4 h and AUC0–12 h, whereas higher levels were observed in patients carrying mutations at all three loci. The study also showed that the C allele at exon 12 was associated with G and A allele at the 2677 loci, which in turn linked significantly with the c.3435C allele. Homozygosity at all three ABCB1 loci was associated with reduced cyclosporinee exposure levels. The tight linkage of the ABCB1 c.1236C>T polymorphism with the c.2677G>T/A and c.3435C>T polymorphisms has also been shown to serve as an important marker influencing irinotecan clearance in Japanese cancer patients.( 35 ) Taken together, our results suggest that linkage between ABCB1 polymorphisms might be important in influencing doxorubicin disposition. Our study, however, does not address the possibility of doxorubicin disposition being subject to the influence of other ABCB1 polymorphisms that have been shown to be part of important haplotype structures in different populations.( 16 ) Given the modest sample size of patients analyzed, the findings should be considered exploratory in nature, and larger studies with a higher sample size must be carried out to arrive at definitive conclusions.
ABCG2 has been associated with drug resistance and cell lines overexpressing the protein have been shown to be several‐fold resistant to doxorubicin. In earlier studies, the ABCG2 c.421C>A polymorphism resulted in markedly decreased protein expression and low‐level drug resistance compared to ABCG2 transfected cells carrying the reference genotype.( 25 ) The c.421C>A polymorphism occurs with a higher frequency among Asians (30–60%) when compared to Caucasians and black Americans (5–10%), and has been associated with low protein expression and subsequent sensitivity to anticancer agents.( 37 ) The wide ethnic variations in allele frequency distribution of the ABCG2 c.421C>A polymorphism suggests their potential contribution to interindividual variability in drug disposition.( 26 , 38 ) Our study, however, failed to show any influence of the c.421C>A polymorphism on pharmacokinetic parameters of doxorubicin and doxorubicinol in Asian breast cancer patients. Further studies are required to identify mechanisms behind the observed disparate pharmacokinetic modulation of drugs that have similar substrate specificities for ABCB1 and ABCG2 transporters.( 39 ) It is also possible that the multiplicity of transporters involved in the transport of doxorubicin across membranes could mask the contribution, if any, of the ABCG2 c.421C>A polymorphism. Further studies are required to conclusively ascertain the relationship of ABCG2 c.421C>A polymorphism to doxorubicin disposition.
The pharmacokinetics of doxorubicin could also be influenced by polymorphic variations in genes encoding other doxorubicin transporting proteins and metabolizing enzymes in the doxorubicin biochemical pathway. We had earlier reported on the higher exposure levels of doxorubicin in Asian breast cancer patients homozygous for the highly linked c.146A>G and c.312T>C polymorphisms in influx transporter SLC22A16 gene.( 40 ) Further studies are required to investigate polymorphisms in genes encoding the efflux transporters ABCB5, ABCC5, and the Ral‐interacting protein RLIP76 which have been shown to confer in vitro doxorubicin resistance.( 41 , 42 , 43 ) In vitro studies in doxorubicin selected cell lines suggested a role for MRP1 (ABCC1) induction in resistance to treatment in myeloid leukemia.( 44 ) Naturally occurring non‐synonymous mutations (Arg433Ser) in the MRP1 cytoplasmic domain, leading to doxorubicin resistance,( 45 ) also needs further investigation in terms of its role in altering doxorubicin disposition in cancer patients.
The biotransformation of doxorubicin to doxorubicinol occurs primarily in the liver, by the cytoplasmic carbonyl reductases (CBR1 and CBR3) and aldo‐keto reductase metabolizing enzymes.( 46 ) Subsequent metabolism by hydrolytic glycosidic cleavage and reduction leads to production of doxorubicinone and 7‐deoxydoxorubicinone from doxorubicin, and doxorubicinolone and 7‐deoxydoxorubicinolone from doxorubicinol. Polymorphisms in CBR1 and CBR3 genes have been shown to have significant functional consequences,( 47 , 48 ) and might influence doxorubicin disposition. The CBR1 c.262G>A (V88I) polymorphism results in isoforms with distinct kinetic and thermodynamic properties whereas the CBR3 c.730G>A (V244M) polymorphism has been shown to result in higher enzyme catalytic efficiency. Further population‐based studies are required to understand the contribution of polymorphisms in genes encoding CBR1 and CBR3 to the observed variations in pharmacokinetics of doxorubicin and doxorubicinol in patients receiving chemotherapy.
In conclusion, the present exploratory study suggests that polymorphisms in the ABCB1 gene could be functionally important with regards to the altered pharmacokinetics of doxorubicin. The linked ABCB1 1236‐2677‐3435 genotypes were found to significantly influence the disposition of doxorubicin in Asian breast cancer patients, resulting in significantly increased exposure levels, peak plasma concentrations, and reduced clearance in patients who were homozygous for the variant allele at the three loci. No significant influences on doxorubicin pharmacokinetic parameters were observed in relation to the ABCG2 c.421C>A polymorphism. Further studies that also investigate other pharmacogenetic candidates across the doxorubicin biochemical pathway are warranted in cancer patients of other ethnic groups to conclusively ascertain the identified genotypic–phenotypic relationships in the present study.
Acknowledgments
This study was supported by grants from the Singhealth Research Fund (SRF‐SU110/2004) and Singapore Cancer Syndicate (SCS‐PS0023).
References
- 1. Juliano RL, Ling VA. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976; 455: 152–62. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmeyer S, Burk O, Von Richter O et al . Functional polymorphisms of the human multidrug‐resistance gene: multiple sequence variations and correlation of one allele with P‐glycoprotein expression and activity in vivo . Proc Natl Acad Sci USA 2000; 97: 3473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics 2005; 6: 115–38. [DOI] [PubMed] [Google Scholar]
- 4. Pauli‐Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1 ). Pharm Res 2004; 21: 904–13. [DOI] [PubMed] [Google Scholar]
- 5. Morita Y, Sakaeda T, Horinouchi M et al . MDR1 genotype‐related duodenal absorption rate of digoxin in healthy Japanese subjects. Pharm Res 2003; 20: 552–6. [DOI] [PubMed] [Google Scholar]
- 6. Sakaeda T, Nakamura T, Okumura K. Pharmacogenetics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics 2003; 4: 397–410. [DOI] [PubMed] [Google Scholar]
- 7. Kim RB, Leake BF, Choo EF et al . Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 2001; 70: 189–99. [DOI] [PubMed] [Google Scholar]
- 8. Drescher S, Schaeffeler E, Hitzl M et al . MDR1 gene polymorphisms and disposition of the P‐glycoprotein substrate fexofenadine. Br J Clin Pharmacol 2002; 53: 526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi SY, Hong KS, Lim HS et al . A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther 2004; 76: 418–27. [DOI] [PubMed] [Google Scholar]
- 10. Szekeres T, Haushofer A. Clinical pharmacogenetics of immunosuppressive drugs in organ transplantation. Pharmacogenomics 2005; 6: 163–8. [DOI] [PubMed] [Google Scholar]
- 11. Hauser IA, Schaeffeler E, Gauer S et al . ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine‐related nephrotoxicity after renal transplantation. J Am Soc Nephrol 2005; 16: 1501–11. [DOI] [PubMed] [Google Scholar]
- 12. Salama NN, Yang Z, Bui T, Ho RJ. MDR1 haplotypes significantly minimize intracellular uptake and transcellular P‐gp substrate transport in recombinant LLC‐PK1 cells. J Pharm Sci 2006; 95: 2293–308. [DOI] [PubMed] [Google Scholar]
- 13. Meissner K, Jedlitschky G, Meyer zu Schwabedissen H et al . Modulation of multidrug resistance P‐glycoprotein 1 (ABCB1) expression in human heart by hereditary polymorphisms. Pharmacogenetics 2004; 14: 381–5. [DOI] [PubMed] [Google Scholar]
- 14. Anglicheau D, Thervet E, Etienne I et al . CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther 2004; 75: 422–33. [DOI] [PubMed] [Google Scholar]
- 15. Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJ. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics 2003; 13: 89–95. [DOI] [PubMed] [Google Scholar]
- 16. Kroetz DL, Pauli‐Magnus C, Hodges LM et al . Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics 2003; 13: 481–94. [DOI] [PubMed] [Google Scholar]
- 17. Johne A, Köpke K, Gerloff T et al . Modulation of steady‐state kinetics of digoxin by haplotypes of the P‐glycoprotein MDR1 gene. Clin Pharmacol Ther 2002; 72: 584–94. [DOI] [PubMed] [Google Scholar]
- 18. Kurata Y, Ieiri I, Kimura M et al . Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P‐glycoprotein. Clin Pharmacol Ther 2002; 72: 209–19. [DOI] [PubMed] [Google Scholar]
- 19. Wong M, Evans S, Rivory LP et al . Hepatic technetium Tc 99m‐labeled sestamibi elimination rate and ABCB1 (MDR1) genotype as indicators of ABCB1 (P‐glycoprotein) activity in patients with cancer. Clin Pharmacol Ther 2005; 77: 33–42. [DOI] [PubMed] [Google Scholar]
- 20. Maliepaard M, Scheffer GL, Faneyte IF et al . Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001; 61: 3458–64. [PubMed] [Google Scholar]
- 21. Zhou S, Schuetz JD, Bunting KD et al . The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat Med 2001; 7: 1028–34. [DOI] [PubMed] [Google Scholar]
- 22. Miyake K, Mickley L, Litman T et al . Molecular cloning of cDNAs which is highly overexpressed in mitoxantrone‐resistant cells: demonstration of homology to ABC transport genes. Cancer Res 1999; 59: 8–13. [PubMed] [Google Scholar]
- 23. Kawabata S, Oka M, Shiozawa K et al . Breast cancer resistance protein directly confers SN‐38 resistance of lung cancer cells. Biochem Biophys Res Commun 2001; 280: 1216–23. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Furukawa T, Nitanda T et al . Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV‐1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol 2003; 63: 65–72. [DOI] [PubMed] [Google Scholar]
- 25. Imai Y, Nakane M, Kage K et al . C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low‐level drug resistance. Mol Cancer Ther 2002; 1: 611–6. [PubMed] [Google Scholar]
- 26. Sparreboom A, Gelderblom H, Marsh S et al . Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther 2004; 76: 38–44. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Q, Sparreboom A, Tan EH et al . Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol 2005; 59: 415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Q, Chowbay B. Determination of doxorubicin and its metabolites in rat serum and bile by LC: application to preclinical pharmacokinetic studies. J Pharm Biomed Anal 2002; 30: 1063–74. [DOI] [PubMed] [Google Scholar]
- 29. Speth PA, Van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet 1998; 15: 5–31. [DOI] [PubMed] [Google Scholar]
- 30. Jada SR, Lim R, Wong CI et al . Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan‐induced neutropenia in Asian cancer patients. Cancer Sci 2007; 98: 1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C4T affects mRNA stability. Pharmacogenet Genomics 2005; 15: 693–704. [PubMed] [Google Scholar]
- 32. Kioka N, Tsubota J, Kakehi Y et al . P‐glycoprotein gene (MDR1) cDNA from human adrenal: normal P‐glycoprotein carries Gly185 with an altered pattern of multidrug resistance. Biochem Biophys Res Commun 1989; 162: 224–31. [DOI] [PubMed] [Google Scholar]
- 33. Illmer T, Schuler US, Thiede C et al . MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients. Cancer Res 2002; 62: 4955–62. [PubMed] [Google Scholar]
- 34. Kimchi‐Sarfaty C, Oh JM, Kim IW et al . A ‘silent’ polymorphism in the MDR1 gene changes substrate specificity. Science 2007; 315: 525–8. [DOI] [PubMed] [Google Scholar]
- 35. Sai K, Kaniwa N, Itoda M et al . Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype‐dependent renal clearance of irinotecan. Pharmacogenetics 2003; 13: 741–57. [DOI] [PubMed] [Google Scholar]
- 36. Rodríguez‐Antona C, Niemi M, Backman JT et al . Characterization of novel CYP2C8 haplotypes and their contribution to paclitaxel and repaglinide metabolism. Pharmacogenomics J 2007 October 9: [Epub ahead of print]. [DOI] [PubMed]
- 37. Kobayashi D, Ieiri I, Hirota T et al . Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos 2005; 33: 94–101. [DOI] [PubMed] [Google Scholar]
- 38. De Jong FA, Marsh S, Mathijssen RH et al . ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res 2004; 10: 5889–94. [DOI] [PubMed] [Google Scholar]
- 39. Han JY, Lim HS, Yoo YK et al . Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan‐pharmacokinetics and clinical outcome in patients with advanced non‐small cell lung cancer. Cancer 2007; 110: 138–47. [DOI] [PubMed] [Google Scholar]
- 40. Lal S, Wong ZW, Jada SR et al . Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics 2007; 6: 567–75. [DOI] [PubMed] [Google Scholar]
- 41. Frank NY, Margaryan A, Huang Y et al . ABCB5‐mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res 2005; 65: 4320–33. [DOI] [PubMed] [Google Scholar]
- 42. Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W 3rd, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5‐fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther 2005; 4: 855–63. [DOI] [PubMed] [Google Scholar]
- 43. Awasthi S, Cheng J, Singhal SS et al . Novel function of human RLIP76: ATP‐dependent transport of glutathione conjugates and doxorubicin. Biochemistry 2000; 39: 9327–34. [DOI] [PubMed] [Google Scholar]
- 44. Slapak CA, Mizunuma N, Kufe DW. Expression of the multidrug resistance associated protein and P‐glycoprotein in doxorubicin‐selected human myeloid leukemia cells. Blood 1994; 84: 3113–21. [PubMed] [Google Scholar]
- 45. Zhang DW, Cole SP, Deeley RG. Identification of an amino acid residue in multidrug resistance protein 1 critical for conferring resistance to anthracyclines. J Biol Chem 2001; 276: 13231–9. [DOI] [PubMed] [Google Scholar]
- 46. Takanashi S, Bachur NR. Adriamycin metabolism in man. Evidence from urinary metabolites. Drug Metab Dispos 1976; 4: 79–87. [PubMed] [Google Scholar]
- 47. Gonzalez‐Covarrubias V, Ghosh D, Lakhman SS, Pendyala L, Blanco JG. A functional genetic polymorphism on human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic activity and NADPH binding affinity. Drug Metab Dispos 2007; 35: 973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lakhman SS, Ghosh D, Blanco JG. Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3). Drug Metab Dispos 2005; 33: 254–7. [DOI] [PubMed] [Google Scholar]


