Abstract
Ras proteins regulate signaling pathways that control many cellular responses, such as proliferation, survival, and differentiation. However, there are intriguing questions about the relationship between the developmental timing of specific mutations and the resultant phenotypes in individual cells. In this study, we used the Cre/loxP system for maintaining transgenic zebrafish lines harboring oncogenic KrasV12 under the nestin promoter, and investigated the developmental effects of Ras activation in neural progenitor cells. Activated human KrasV12 was induced within pDSNesLCherryLEGFPKRasV12 transgenic fish by Cre mRNA injection. Cre‐mediated gene excision was confirmed by polymerase chain reaction, and the injected embryos were investigated for KrasV12 effects using the hemotoxylin–eosin staining, terminal deoxynucleotidyl transferase‐mediated digoxigenin‐dUTP nick‐end labeling assay, and in situ hybridization. pDSNesLCherryLEGFPKRasV12 transgenic embryos normally expressed mCherry in their central nervous system throughout the developmental stage. However, Cre mRNA injection efficiently excised the flanking stop sequence, and the injected embryos expressed enhanced green fluorescent protein in their brain with severe edema. Brain histology showed that neuronal cell differentiation could occur in spite of oncogenic KrasV12 overexpression, but massive apoptosis and brain edema caused early embryonal death. In summary, the overexpression of KrasV12 induces extensive apoptosis of neural progenitor cells followed by severe edema of the brain. However, some neural progenitor cells are resistant to KrasV12 and can retain their ability to differentiate into neurons. Finally, our transgenic model demonstrates the inability of KrasV12 alone to induce brain tumors at the early stage of development. (Cancer Sci 2009)
In recent years, transgenic technology has been extensively used in the zebrafish (Danio rerio) model to investigate a wide range of studies from developmental biology and genetics. 1 , 2 , 3 , 4 , 5 , 6 , 7 Although possible in mouse models, large‐scale, whole‐genome, unbiased genetic screens designed to identify genetic modifiers of oncogene‐induced malignancies are costly and require a large number of animals. 8 , 9 , 10 In contrast, live zebrafish allow relatively inexpensive large‐scale genetic and chemical screens. 11 Currently, transgenic approaches are beginning to be widely used to generate zebrafish disease models, 4 , 12 , 13 , 14 but one of the major challenges in the field is developing conditional gene expression models. 3 , 6 , 7 For this purpose, several approaches have been developed, including the use of an inducible TetR‐based system, 15 GAL4/UAS system, 16 LexA‐based system, 17 Cre/loxP system, and FLP/FRT system. 18 Among them, the Cre/loxP system was shown to be effective for maintaining transgenic fish lines harboring functional genes that may affect the survival and reproduction of the transgenic host. 3 , 6 , 19
Ras gene mutations are highly prevalent in human pancreatic (>80%), colorectal (40–50%), endometrial (40%), lung (30%), and cervical cancers (20–30%), as well as in myeloid malignancies (20–40%). 20 , 21 Because Ras proteins regulate signaling pathways that control many cellular responses, such as proliferation, survival, and differentiation, 22 the degree and duration of Ras activation has important effects on developmental programs. 20 The aberrant overexpression of Ras during the developmental stage rarely develops malignant tumors, which is in marked contrast to the highly penetrant cancer phenotypes seen in individuals with germline mutations of tumor‐suppressor genes, such as TP53, RB1, and BRCA1. One potential explanation for this observation is that the degree and/or duration of Ras activation are insufficient to initiate tumorigenesis in most tissues. Recent observations also raise intriguing questions about the relationship between the developmental timing of specific mutations and the resultant phenotypes in individual cells. 23 However, there has been no report about the developmental effects of Ras activation in neural progenitor cells.
In the present study, we developed a Cre/loxP‐mediated transgenic approach, in which activated zebrafish KrasV12 can be induced within transgenic animals by Cre mRNA injection. Transgenic zebrafish expressing oncogenic KrasV12 in the neural progenitor cells could not develop any neoplastic changes; they could develop some normal differentiated neuronal cell layers, but finally went to apoptosis. Embryos showed early deaths because of massive apoptosis and consequent cerebral edema in the brain. Our results describe the effects of ectopic KrasV12 expression in developing neural progenitor cells. In addition, we have shown the feasibility of our transgenic model to investigate further mechanisms about the relationships of Ras and neural cell differentiation.
Materials and Methods
Zebrafish and maintenance. Adult wild‐type zebrafish were originally purchased from a local aquarium farm and maintained in the laboratory facility. Breeding and maintenance were conducted in accordance with the guidelines established by the Seoul National University Institutional Animal Care and Use Committee (Approval No. SNU 050418‐2).
Plasmid construct. The zebrafish promoter of nestin was amplified from genomic DNA with forward primer AGAGATGCATTGGGCGTATCACGGCTCAGG and reverse primer AGAGCCTAGGCGAGAGATATGAAGTGAAATCTCACTAGAGG. In total, 930 base pair fragments containing the nestin promoter and the partial 5′ untranslated region of nestin was cloned into NsiI/AvrII sites of plasmid pMDS6, 24 containing loxed mCherry with lox2272 sites for Cre recombinase in front of the zebrafish Kras2B with modified codon 12 into valine (KrasV12) N‐terminally fused with enhanced green fluorescent protein (EGFP) 17 (pDSNesLCherryLEGFPKrasV12, Fig. 1A). To exclude premature activation translation from ATG located in the loxP site and for a more convenient cloning procedure, we used the lox2272 sequence 25 instead of the wild‐type loxP sequence.
Figure 1.
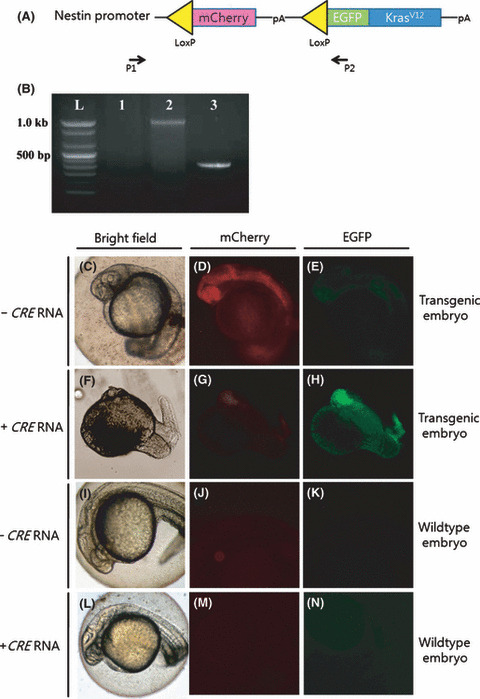
Cre mRNA‐mediated gene recombination in the pDSNesLCherryLEGFPKrasV12 transgenic embryos. (A) pDSNesLCherryLEGFPKrasV12 construct. P1 and P2 are the forward and reverse primers for genotyping, respectively. (B) Excision polymerase chain reaction (PCR) showing gene recombination after Cre mRNA injection in DNA extracted from zebrafish embryos 48 h post‐fertilization. P1 and P2 primers amplified the mCherry containing the 1.1‐kb fragment from genomic DNA of transgenic embryos without Cre mRNA injection (lane 2) and also amplified the 0.4‐kb fragment, which resulted when Cre mRNA was injected into the transgenic embryos (lane 3). Lane 1 is the PCR result of wild‐type embryos as the negative control. (C–N) Fluorescent images of 24 h post‐fertilization transgenic embryos without (C–E) or with Cre mRNA (F–H; 50 ng/μL) at one cell stage, and wild‐type control embryos (I–N). (C,F,I,L) Bright‐field (D,G,J,M) mCherry, and (E,H,K,N) enhanced green fluorescent protein (EGFP) images of embryos. Anterior is to the left, and dorsal is towards the top.
Generation of transgenic fish. The transgenic fish containing the loxed oncogene was created with a co‐injection of pDSNesLCherryLEGFPKrasV12 and Ac‐transposase. 24 The F1 generation of transgenic fish was selected according to the mCherry expression in the central nervous system. One of the lines containing a single insert of the transgene was selected for further experiments. We confirmed the single insertion site integrated in the chromosome 18 disks as the large‐associated protein 1 (DAP‐1) gene using thermal asymmetric interlaced PCR (TAIL)–polymerase chain reaction (PCR) as described previously. 26
Cre mRNA injection and the development of embryos. One batch of embryos from stable transgenic zebrafish was divided into two partitions. The embryos in one partition were injected with 50 ng/μL nuclear localization signal (nls)Cre mRNA. The modified Cre recombinase, containing SV40 nls was cloned into plasmid p64T and used for the in vitro transcription synthesis of Cre mRNA with SP6 polymerase (pSP6nlsCre; Ambion, Austin, Texas, USA). Three groups were selected to examine the survival rates: EGFP‐positive transgenic embryos and EGFP‐negative, wild‐type embryos were selected from the Cre‐injected batch; the mCherry‐positive transgenic embryo group was selected in the Cre‐uninjected batch. The inspected numbers of embryos for the survival curve were 28, 41, and 19, respectively. Fluorescence was observed under an Olympus IX70 microscope (Tokyo, Japan). EGFP was observed under a blue filter (450–490 nm), and mCherry under a yellow filter (546 nm).
Detection of Cre/loxP excision by PCR. To detect the excision of floxed mCherry in the pDSNesLCherryLEGFPKrasV12 transgenic embryos, primers were designed and used for PCR as follows: forward primer, 5′‐CCTTCTCTCGCATTCAGTCC, against the zebrafish nestin promoter, and reverse primer, 5′‐AAGTCGTGCTGCTTCATGTG, designed from the EGFP coding region. The amplification number was 35, which could fully expand any remnant mCherry sequence, with a melting temperature of 55°C. There were a total of 10 embryos per group made up of wild‐type control embryos, Cre‐uninjected mCherry‐expressing transgenic embryos, and Cre‐injected EGFP‐expressing transgenic embryos, which were collected at 2 days post‐fertilization (dpf). DNA was extracted, and PCR was carried out as described by Pan et al. 6
Hematoxylin–eosin staining, terminal deoxynucleotidyl transferase‐mediated digoxigenin‐dUTP nick‐end labeling, and in situ hybridization. Zebrafish embryos at 3 dpf were fixed in 4% paraformaldehyde for 2 h and processed into an alcohol–xylene series followed by paraffin embedding. For the histological examination, 3‐μm‐thick sections were stained with hematoxylin–eosin (HE). For the terminal deoxynucleotidyl transferase‐mediated digoxigenin‐dUTP nick‐end labeling (TUNEL) assay, sectioned slides were followed according to the manufacturer’s instructions (In situ cell death detection kit; Roche Diagnostics, Basel, Germany). For in situ hybridization, Cre‐injected transgenic embryos were isolated and fixed in 4% paraformaldehyde at 4°C overnight. A Digoxigenin (DIG)‐labeled EGFP antisense RNA probe was used 27 for staining KrasV12–EGFP fusion mRNA. The EGFP sense strand was used as the control.
Results
Cre mRNA‐mediated gene recombination in the pDSNesLCherryLEGFPKrasV12 transgenic embryos. First, we confirmed our transgenic zebrafish line, which could stably express the mCherry protein under the nestin promoter using the adult transgenic zebrafish brain, at the F4 generation (Fig. S1). Zebrafish embryos at the F5 generation expressed mCherry during the developmental stage throughout the central nervous system and along the spinal cord (Fig. 1D). They did not show any EGFP fluorescence (Fig. 1E). When Cre mRNA was injected into one‐cell stage transgenic embryos, the EGFP signal in the central nervous system was correlated with abnormal morphological phenotypes (Fig. 1H). To detect gene recombination in the transgenic embryos, we selected one primer pair (P1 and P2) that expands exogenous sequences from the nestin promoter to the EGFP sequence (Fig. 1A). These primers produce 1100 base pairs products in the transgenic embryos, but 400 base pairs under Cre recombinase‐mediated gene recombination. Excision PCR showed gene recombination by Cre recombinase in the transgenic embryos (Fig. 1B).
Early expression of KrasV12 causes embryonic death. The survival rates of Cre‐injected, wild‐type embryos are normally around 80% as shown in Figure 2A. mCherry expression in the central nervous system of stable transgenic embryos did not affect the overall survival rates. However, when Cre recombinase was injected and oncogenic KrasV12 was expressed under the nestin promoter, the survival rate decreased to 20% at 3 dpf. Brain edema in the EGFP‐expressing transgenic embryos was irreversible and started from 3∼4 dpf (Fig. 2B).
Figure 2.
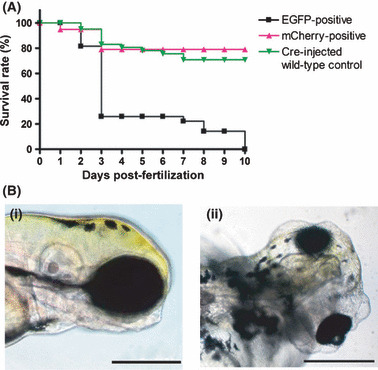
Cre mRNA injection into stable transgenic pDSNesLCherryLEGFPKrasV12 fish leads to embryonic early death. (A) Survival curve of the transgenic embryos with or without Cre mRNA injection. Transgenic embryos could not survive over 10 days post‐fertilization when injected with Cre mRNA at the one cell stage. (B) Cre mRNA‐injected transgenic zebrafish at 4 days post‐fertilization. (i) Cre mRNA‐injected wild‐type embryo. Compared to control, Cre mRNA‐injected transgenic embryo shows severe brain edema (ii). Brain parenchyma inside seems to be relatively smaller than that of the control. Fish oriented with anterior to the right and dorsal to the top. Bar = 200 μm.
Neural cell differentiation with brain edema in Cre mRNA‐injected transgenic embryos. Although Cre mRNA‐injected embryos showed abnormal brain phenotypes, brain histology revealed that oncogenic KrasV12 overexpression in the neural progenitor cells could not disturb neuronal cell differentiation completely. HE staining with wild‐type control (Fig. 3A) and Cre‐injected transgenic embryos (Fig. 3B) at 3 dpf showed that the differentiated neuronal zone is clearly reduced and the abnormal cerebral fold could be seen in the latter group. However, Cre mRNA‐injected embryos could develop normal architecture, including in the tectum opticum, mesencephalon, rhombencephalon, and cerebellum. Main proliferating zones, post‐mitotic mantle zone, and neuropilar marginal zone also existed. However, the formed structures were much smaller in size than normal. Intercellular edema was obvious, as shown in Figure 3B (arrow). Between the edematous intercellular spaces, apoptotic cells were observed.
Figure 3.
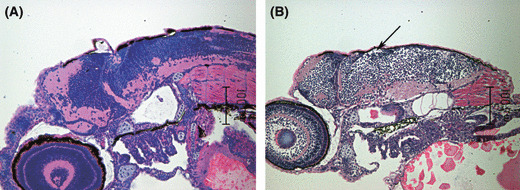
Brain edema during neural cell development in Cre mRNA‐injected pDSNesLCherryLEGFPKrasV12 transgenic embryos. Sagittal section of zebrafish embryo brain at 3 days post‐fertilization. Hematoxylin–eosin staining with wild‐type control (A) and Cre mRNA‐injected transgenic embryos (B) oriented with anterior to the left and dorsal to the top. Note that differentiated neuronal zone is clearly reduced, and abnormal cerebral fold, as well as intercellular edema (arrow), can be seen in the latter group.
KrasV12 overexpression causes apoptosis of neural progenitor cells. The brain of a 3 dpf, wild‐type embryo showed compact proliferating neural progenitor cells in the cerebellum and adjacent hindbrain area (Fig. 4A). The fully‐differentiated neuronal cell layer was well demarcated from the above proliferating zone. Cre mRNA‐injected embryo brains also showed both layers, but the massive apoptotic cells were obvious between these two layers (Fig. 4B). To investigate the relationship between apoptosis and edema, we conducted an in situ hybridization for EGFP mRNA, as well as TUNEL assay. Strong EGFP mRNA was seen in the active proliferating zone in the cerebellum and hindbrain area (Fig. 4C), but not in the below differentiated zones. Below the strong signaled layer, weak positive signals were shown. Apoptotic cells were well matched with the weakly‐expressing cells (Fig. 4D).
Figure 4.
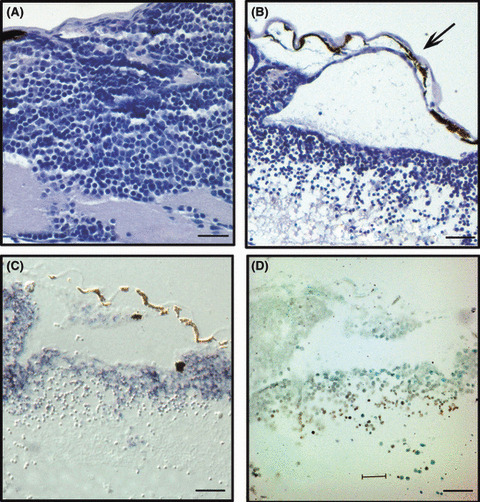
Enhanced green fluorescent protein (EGFP)‐expressing neural progenitor cells go through oncogene‐induced massive apoptosis. (A) Hematoxylin–eosin (HE) staining of 3 days post‐fertilization (dpf) wild‐type embryo brain shows normal neural progenitor and a differentiated neuronal cell layer, as well as a normal cerebral fold. (B–D) Representative images of serial sections of Cre mRNA‐injected pDSNesLCherryLEGFPKrasV12 transgenic 3 dpf embryos. (B) HE staining shows severe brain edema (arrow) and loosely scattered apoptotic cells below the progenital cell layer. (C) In situ hybridization for EGFP shows that only the neural progenitor cell layer expresses EGFP‐positive signals. (D) Note that cells with weak EGFP signals are well matched with terminal deoxynucleotidyl transferase‐mediated digoxigenin‐dUTP nick‐end labeling‐positive cells. Anterior is to the left, and dorsal is towards the top. Bar = 12.5 μm.
Discussion
We developed transgenic zebrafish that can conditionally express oncogenic KrasV12 in neural progenitor cells only in presence of Cre mRNA. The expression of oncogenic KrasV12 under the nestin promoter did not develop any neoplastic changes in the developmental stage of zebrafish embryos. However, the embryos showed massive apoptosis in the brain and died before 10 dpf.
Nestin is an intermediate filament protein that has been used as a marker to label potential neural stem cells. 28 Nestin is normally detected in the subgranular and subventricular zones 29 , 30 in the adult zebrafish brain. 31 In mice, nestin is currently the best‐known precursor cell marker in vivo, and the development of transgenic mice expressing EGFP under the control of the nestin regulatory regions has been very helpful in the identification of precursor cells and the visualization of neurogenesis throughout the life of the animal. 32 Our transgenic zebrafish line showed a high level of expression of mCherry from 12 h post‐fertilization and during adult life (Fig. 1, Fig. S1). Because neural stem cells are good candidates for investigating tissue regeneration and cancer initiation, 33 our model can be used in many further studies. As Lam et al. mentioned, 31 this transgenic line will provide a means for the identification and manipulation of neural stem cells in vivo.
Many applications of the Cre/loxP system in the zebrafish have been reported, but now researchers can regulate gene expression in a site‐ and time‐specific manner using the Cre/loxP system. For example, the Cre/loxP‐regulated transgenic zebrafish model developed conditional myc‐induced, T‐cell acute lymphoblastic leukemia, 3 and heat shock‐inducible Cre/loxP approaches induce diverse types of tumors and hyperplasia in transgenic zebrafish. 5 Gong et al. also reported that oocytes specifically expressed Cre transgenic zebrafish. 34 We are now trying to create Cre transgenic lines that can be induced by exogenous mifepristone treatment so that the oncogenic Ras effects in neural progenitor cells are regulated at conditional time points.
Many in vivo models expressing the Kras oncogene have been described. 9 , 35 , 36 , 37 Because Ras genes are one of the most widely studied cancer‐related genes due to their frequent activation in human tumors, 8 researchers have used Kras to induce tumors under specific tissue promoters. The effects of Ras expression is highly variable and depends on the cell type. Activated human Kras in nestin‐positive pancreatic progenitor cells have been found to develop solid tumors, 2 whereas KrasD12 alone failed to induce glioblastoma in mice. 38 The most sensitive target site to be transformed was the lungs. 8 Ras also affected progenitor cell differentiation, 39 and in this case, only a high concentration of Ras expression could inhibit thyroid cell differentiation. In the present study, some KrasV12‐expressing neural progenitor cells became apoptotic without any malignant signs. Interestingly, some neural progenitor cells normally can differentiate into marginal neural zones (Fig. 3), but others do not survive because of Ras‐induced apoptosis and edema. Although we confirmed efficient gene excision after Cre mRNA injection by PCR, we could not show exactly whether some cells still contain the mCherry sequence. As shown in Figure 4, most cells in the proliferating zones express EGFP–KrasV12, but not all the cells become apoptotic and some do survive. These cells might escape gene excision, however, even if the same level of KrasV12 is expressed in the same group of cells, the results could be different between cells. A strong observation from our results is that KrasV12 overexpression in neural progenitor cells during the developmental stage does not transform cells, but induces massive apoptosis, which can cause severe edema. Our results also showed that in Cre‐injected zebrafish, the telencephalon size decreased, which could be due to the premature cellular senescence of neural progenitor cells. It has been demonstrated that supraphysiological expression levels of oncogenic H‐RasV12 could induce cellular senescence, 40 while endogenous expression leads to tumorigenesis 10 in mouse models. Our data, using the teleost model, are well matched with these vertebrate models, because transgenic zebrafish in this study have one copy number of oncogenic KrasV12 under the nestin promoter, thus driving the overexpression of oncogenes in neural progenitor cells and inducing cellular senescence followed by apoptosis. By modulating the gene excision time point in our model, we are now trying to develop another brain tumor model by modulating the gene excision time point in our model.
In summary, we established the transgenic zebrafish system to express oncogenic KrasV12 under Cre mRNA‐mediated gene recombination in neural progenitor cells. This system allowed us to examine the effects of KrasV12 in developmental neural progenitor cells by simply injecting Cre mRNA into one cell stage of zebrafish embryos. This system enables the maintenance of transgenic zebrafish lines and the study the mechanisms of oncogenic Kras in the central nervous system. We found that the overexpression of KrasV12 in the early stage could not develop any malignant signs, but induced extensive apoptosis of neural progenitor cells followed by severe edema of the brain. Interestingly, some neural progenitor cells resisted oncogenic KrasV12 and could differentiate into neurons. It might be possible that the nestin promoter drives different levels of transcripts at each neural progenitor cell, or each cells having nestin expression might have different resistance to oncogenic KrasV12.
Supporting information
Fig. S1. Fluorescent images of stable transgenic zebrafish brain (a–c), Fli mutant zebrafish brain as the enhanced green fluorescent protein (EGFP)‐positive control (d–f), and wild‐type negative control (g–i). Bright‐field (a,d,g), mCherry (b,e,h), and EGFP images (c,f,i) of adult zebrafish brain 20 days post‐fertilization. mCherry expression is maintained in the transgenic adult brain without Cre mRNA injection. Anterior is to the left, and dorsal is towards the top. Bar=25 μm.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
We gratefully acknowledge financial support from a Korea Research Foundation grant (No. KRF‐005‐E00077) and additional financial support from the BK21 Program for Veterinary Science.
References
- 1. Covassin LD, Siekmann AF, Kacergis MC et al. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Dev Biol 2009; 329: 212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davison JM, Woo Park S, Rhee JM, Leach SD. Characterization of Kras‐mediated pancreatic tumorigenesis in zebrafish. Methods Enzymol 2008; 438: 391–417. [DOI] [PubMed] [Google Scholar]
- 3. Langenau DM, Feng H, Berghmans S, Kanki JP, Kutok JL, Look AT. Cre/lox‐regulated transgenic zebrafish model with conditional myc‐induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2005; 102: 6068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langenau DM, Traver D, Ferrando AA et al. Myc‐induced T cell leukemia in transgenic zebrafish. Science 2003; 299: 887–90. [DOI] [PubMed] [Google Scholar]
- 5. Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, Zon LI. Heat shock‐inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci U S A 2007; 104: 9410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan X, Wan H, Chia W, Tong Y, Gong Z. Demonstration of site‐directed recombination in transgenic zebrafish using the Cre/loxP system. Transgenic Res 2005; 14: 217–23. [DOI] [PubMed] [Google Scholar]
- 7. Thummel R, Burket CT, Brewer JL et al. Cre‐mediated site‐specific recombination in zebrafish embryos. Dev Dyn 2005; 233: 1366–77. [DOI] [PubMed] [Google Scholar]
- 8. Guerra C, Mijimolle N, Dhawahir A et al. Tumor induction by an endogenous K‐ras oncogene is highly dependent on cellular context. Cancer Cell 2003; 4: 111–20. [DOI] [PubMed] [Google Scholar]
- 9. Jackson EL, Willis N, Mercer K et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K‐ras. Genes Dev 2001; 15: 3243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tuveson DA, Shaw AT, Willis NA et al. Endogenous oncogenic K‐ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 2004; 5: 375–87. [DOI] [PubMed] [Google Scholar]
- 11. Stern HM, Murphey RD, Shepard JL et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol 2005; 1: 366–70. [DOI] [PubMed] [Google Scholar]
- 12. Patton EE, Widlund HR, Kutok JL et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol 2005; 15: 249–54. [DOI] [PubMed] [Google Scholar]
- 13. Sabaawy HE, Azuma M, Embree LJ, Tsai HJ, Starost MF, Hickstein DD. TEL‐AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2006; 103: 15166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang HW, Kutok JL, Lee NH et al. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res 2004; 64: 7256–62. [DOI] [PubMed] [Google Scholar]
- 15. Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline‐responsive promoters. Proc Natl Acad Sci U S A 1992; 89: 5547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature 1988; 332: 853–6. [DOI] [PubMed] [Google Scholar]
- 17. Emelyanov A, Parinov S. Mifepristone‐inducible LexPR system to drive and control gene expression in transgenic zebrafish. Dev Biol 2008; 320: 113–21. [DOI] [PubMed] [Google Scholar]
- 18. O’Gorman S, Fox DT, Wahl GM. Recombinase‐mediated gene activation and site‐specific integration in mammalian cells. Science 1991; 251: 1351–5. [DOI] [PubMed] [Google Scholar]
- 19. Langenau DM, Jette C, Berghmans S et al. Suppression of apoptosis by bcl‐2 overexpression in lymphoid cells of transgenic zebrafish. Blood 2005; 105: 3278–85. [DOI] [PubMed] [Google Scholar]
- 20. Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007; 7: 295–308. [DOI] [PubMed] [Google Scholar]
- 21. Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: new tricks for an old dog. Curr Opin Genet Dev 2007; 17: 15–22. [DOI] [PubMed] [Google Scholar]
- 22. Koera K, Nakamura K, Nakao K et al. K‐ras is essential for the development of the mouse embryo. Oncogene 1997; 15: 1151–9. [DOI] [PubMed] [Google Scholar]
- 23. Carta C, Pantaleoni F, Bocchinfuso G et al. Germline missense mutations affecting KRAS Isoform B are associated with a severe Noonan syndrome phenotype. Am J Hum Genet 2006; 79: 129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emelyanov A, Gao Y, Naqvi NI, Parinov S. Trans‐kingdom transposition of the maize dissociation element. Genetics 2006; 174: 1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee G, Saito I. Role of nucleotide sequences of loxP spacer region in Cre‐mediated recombination. Gene 1998; 216: 55–65. [DOI] [PubMed] [Google Scholar]
- 26. Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon‐mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn 2004; 231: 449–59. [DOI] [PubMed] [Google Scholar]
- 27. Gong Z, Ju B, Wang X et al. Green fluorescent protein expression in germ‐line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8. Dev Dyn 2002; 223: 204–15. [DOI] [PubMed] [Google Scholar]
- 28. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 1990; 60: 585–95. [DOI] [PubMed] [Google Scholar]
- 29. Doetsch F, Garcia‐Verdugo JM, Alvarez‐Buylla A. Cellular composition and three‐dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997; 17: 5046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 2004; 27: 447–52. [DOI] [PubMed] [Google Scholar]
- 31. Lam CS, Marz M, Strahle U. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev Dyn 2009; 238: 475–86. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter‐GFP transgenic mice. Neuroreport 2000; 11: 1991–6. [DOI] [PubMed] [Google Scholar]
- 33. Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell 2003; 12: 889–901. [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Li Z, Emelyanov A, Parinov S, Gong Z. Generation of oocyte‐specifically expressed cre transgenic zebrafish for female germline excision of loxP‐flanked transgene. Dev Dyn 2008; 237: 2955–62. [DOI] [PubMed] [Google Scholar]
- 35. Fisher GH, Wellen SL, Klimstra D et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K‐Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev 2001; 15: 3249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson L, Mercer K, Greenbaum D et al. Somatic activation of the K‐ras oncogene causes early onset lung cancer in mice. Nature 2001; 410: 1111–6. [DOI] [PubMed] [Google Scholar]
- 37. Meuwissen R, Linn SC, Van Der Valk M, Mooi WJ, Berns A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K‐Ras oncogene. Oncogene 2001; 20: 6551–8. [DOI] [PubMed] [Google Scholar]
- 38. Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 2000; 25: 55–7. [DOI] [PubMed] [Google Scholar]
- 39. De Vita G, Bauer L, Da Costa VM et al. Dose‐dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol 2005; 19: 76–89. [DOI] [PubMed] [Google Scholar]
- 40. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88: 593–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Fluorescent images of stable transgenic zebrafish brain (a–c), Fli mutant zebrafish brain as the enhanced green fluorescent protein (EGFP)‐positive control (d–f), and wild‐type negative control (g–i). Bright‐field (a,d,g), mCherry (b,e,h), and EGFP images (c,f,i) of adult zebrafish brain 20 days post‐fertilization. mCherry expression is maintained in the transgenic adult brain without Cre mRNA injection. Anterior is to the left, and dorsal is towards the top. Bar=25 μm.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


